Abstract
Lipids play a vital role in the health and functioning of neurons and interest in the physiological role of neuronal lipids is certainly increasing. One neuronal function in which neuronal lipids appears to play key roles in neurotransmission. Our understanding of the role of lipids in the synaptic vesicle cycle and neurotransmitter release is becoming increasingly more important. Much of the initial research in this area has highlighted the major roles played by the phosphoinositides (PtdIns), diacylglycerol (DAG), and phosphatidic acid (PtdOH). Of these, PtdOH has not received as much attention as the other lipids although its role and metabolism appears to be extremely important. This lipid has been shown to play a role in modulating both exocytosis and endocytosis although its precise role in either process is not well defined. The currently evidence suggest this lipid likely participates in key processes by altering membrane architecture necessary for membrane fusion, mediating the penetration of membrane proteins, serving as a precursor for other important SV cycling lipids, or activating essential enzymes. In this review, we address the sources of PtdOH, the enzymes involved in its production, the regulation of these enzymes, and its potential roles in neurotransmission in the central nervous system.
Introduction
One of the more exciting developments in understanding regulated neurotransmission has been the increasing interest in the role of lipids (Davletov and Montecucco, 2010, Gauthier-Kemper et al., 2015, Huttner and Schmidt, 2000, Koch and Holt, 2012, Lauwers et al., 2016, Lim and Wenk, 2009, Milovanovic and Jahn, 2015, Posor et al., 2015, Puchkov and Haucke, 2013, Rohrbough and Broadie, 2005, Stutz and Horvath, 2015, Wen et al., 2012). Proper neuronal function depends on neurotransmission to occur in a rapid and reliable manner (Farsad and De Camilli, 2002, Fernandez-Alfonso and Ryan, 2006, Haucke et al., 2011, Kavalali, 2006). It is recognized that this is largely accomplished via a coordinated mechanism at the presynaptic bouton referred to as the synaptic vesicle (SV) cycle where exocytosis of neurotransmitter loaded vesicles is followed by a synchronized endocytosis of empty vesicles, which are refilled and subsequently re-released (Falcieri et al., 1992, Royle and Lagnado, 2010, Schweizer and Ryan, 2006, Sudhof, 2004). Many previous studies led to the discovery of an array of proteins responsible for regulating this cycle and it is now recognized that lipids are also important mediators for SV recycling (Ammar et al., 2013, Cremona and De, 2001, Cremona et al., 1999b, Huttner and Schmidt, 2000, Lim and Wenk, 2009, Puchkov and Haucke, 2013, Rohrbough and Broadie, 2005, Tu-Sekine et al., 2015).
As a result of the research to examine the role of lipid involvement in neurotransmission, it is now clear that phosphoinositides (PtdIns), diacylglycerol (DAG), and phosphatidic acid (PtdOH), play key roles (Davletov and Montecucco, 2010, Lauwers, Goodchild, 2016, Lim and Wenk, 2009, Rohrbough and Broadie, 2005, Schwarz et al., 2011, Tu-Sekine, Goldschmidt, 2015, Tu-Sekine and Raben, 2011, Yuan et al., 2015). Given their popularity, it is understandable that PtdIns(4,5)P2 and PtdIns(3,4,5)P3 have received a great deal of attention (see (Cremona et al., 1999a, Davletov and Montecucco, 2010, Di Paolo and De Camilli, 2006, Di Paolo et al., 2004, Lauwers, Goodchild, 2016, Lim and Wenk, 2009, Martin, 2015, Micheva et al., 2001, Puchkov and Haucke, 2013). In addition to these lipids, now there is also rather convincing evidence for the roles DAG plays in evoked exocytosis of SV vesicles, largely via its well-documented ability to activate PKCs (Antal and Newton, 2014) or recruit munc-13 (Betz et al., 1998, Rhee et al., 2002). New data suggest that a gradient of this lipid may also be involved in modulating evoked endocytosis (Yuan, Liu, 2015).
While not receiving a lot of the initial attention, there are now compelling data implicating PtdOH in modulating exocytosis and endocytosis (Antonescu et al., 2010, Burger et al., 2000, Chasserot-Golaz et al., 2010, Lim and Wenk, 2009, Paillart et al., 2003, Puchkov and Haucke, 2013, Rohrbough and Broadie, 2005, Schwarz, Natarajan, 2011, Takei et al., 1998, Xie et al., 2015) although its precise role is not well defined. This lipid could play roles by altering membrane architecture necessary for membrane fusion (see (Lauwers, Goodchild, 2016, Rohrbough and Broadie, 2005)), mediating membrane penetration of proteins such as dynamin (Burger, Demel, 2000, Takei, Haucke, 1998); (see (Doherty and McMahon, 2009, Ferguson and De Camilli, 2012, Kokotos and Cousin, 2015)), serving as a precursor for other important SV cycling lipids such as PtdIns(4,5)P2, or activating enzymes such as a PI kinase (Jenkins et al., 1994, Moritz et al., 1992) needed for the synthesis of this lipid (Thieman et al., 2009, Volpicelli-Daley et al., 2010). In this review, we address the sources of PtdOH, the enzymes involved in its production, the regulation of these enzymes, and its potential roles in neurotransmission in the central nervous system.
Sources and Enzymes
There are four major routes of cellular PtdOH production (Figure 1): a) de novo synthesis, b) phospholipase D (PLD)-mediated hydrolysis of a phospholipid, most likely phosphatidylcholine (PtdCho), c) a diacylglycerol kinase (DGK) catalyzed phosphorylation of diacylglycerol (DAG), or d) an acylation of lysoPtdOH catalyzed by lysoPtdOH acyl transferase (LPAAT). All four will be discussed and their role in generating PtdOH to modulate the SV cycle will be addressed.
Figure 1. Sources of Phosphatidic Acid.
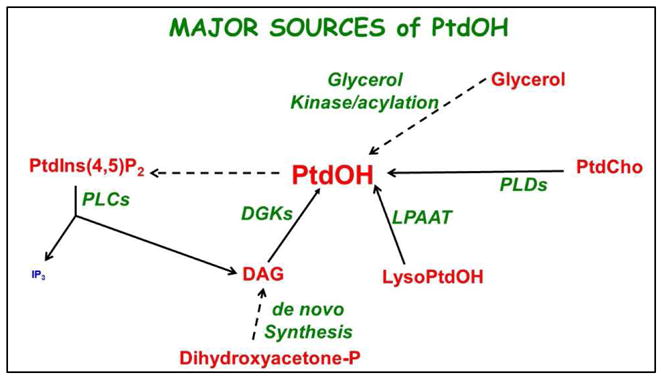
PtdIns(4,5)P2 = phosphatidylinositol-4,5-bisphosphate; PtdOH = phosphatidic acid; lysPtdOH = lysophosphatidic acid; PtdCho = phosphatidylcholine; DGK = diacyglycerol kinase; PLD = phospholipase D; PLC = phospholipase C; LPAAT = lysophosphatidic acid acyltransferase.
In neurons, as in most tissues, PtdOH is an intermediate in the synthesis of phospholipids. PtdOH is generated via the acylation of glycerol-3-phosphate. This metabolic intermediate can be generated via reduction of dihydroxyacetone-3-phosphate or via the phosphorylation of glycerol catalyzed by glycerol kinase (except in adipocytes which lack this enzyme). While this is a well-studied process, there is no evidence that the de novo synthesis of PtdOH plays a role in SV cycling.
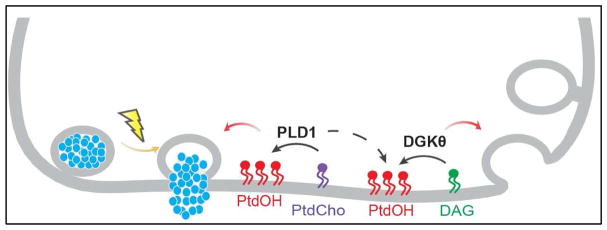
Six isoforms of PLDs have been identified based on sequence homology (Frohman, 2015). Of these, PLD1 and PLD2 are the major isoforms found in neuronal tissues (Kim et al., 2007). Their precise roles in SV cycling and neurotransmitter release remains uncertain. PLD2 appears to play a role in the endocytosis of some receptors (Bhattacharya et al., 2004, Du et al., 2004, Koch et al., 2006, Padron et al., 2006, Rankovic et al., 2009) but more data are needed to suggest it plays a role in SV cycling. In contrast, there is evidence that PLD1 is involved in neurotransmitter release (see (Humeau et al., 2001, Klein, 2005, Rohrbough and Broadie, 2005, Sun et al., 2013)) and in dendritic branching? PLD1 is a mammalian synaptosomal protein (Chalifa et al., 1990) that interacts with synaptosome-specific proteins (Chung et al., 1997, Lee et al., 1997). More directly, Humeau et al. showed PLD1, but not PLD2, induced neurotransmitter release from Aplysia californica neurons (Humeau, Vitale, 2001). Additionally, this isoform has been implicated in the sensitivity to pain in response to prenatal stress (Sun, Gooding, 2013). PLD2, on the other hand, has been implicated in regulating the glutamate transporter (Mateos et al., 2012) the internalization of mGluR (Bhattacharya, Babwah, 2004) and the synaptotoxic action of the Abeta protein in Alzheimer’s disease (AD). Interestingly, ablation of PLD2 rescues memory deficits and provides synaptic protection in an AD transgenic mouse model (SwAPP) (Oliveira et al., 2010). A nice review of the role of PLD2 in neurological dysfunctions has been recently presented (Ghim et al., 2016).
Another class of enzymes implicated in generating PtdOH in neurons is the DGKs. Of the ten known DGKs, all are found in the brain with nine of them in neurons. Only DGK-α is not found in neurons but in glia type cells (Ishisaka and Hara, 2014, Tu-Sekine and Raben, 2011). These enzymes are particularly interesting as their substrate, DAG, is a product of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) hydrolysis which is also an important neuronal lipid. This suggests a scenario whereby DAG generated by a phosphoinositide specific phospholipase C (PtdIns-PLC)-mediated hydrolysis of PtdIns(4,5)P2 serves as the substrate for a DGK-mediated production of PtdOH. In a recent study, we showed one isoform, DGK-θ, is important for modulating evoked SV endocytosis. Ablation of DGK-θ suppressed evoked endocytosis, which is accentuated with increasing strength and duration of action potentials. This defect is rescued by catalytically active DGK-θ but not an inactive enzyme (Goldschmidt et al., 2016). These data demonstrate that the conversion of DAG to PtdOH catalyzed by DGK-θ plays a role in SV cycling. Another DGK isoform implicated in neurotransmission is DGK-κ. Yang et al. provided data suggesting this isoform plays a role in mGluR-dependent long-term depression (Yang et al., 2011). The authors of this report indicated that DGK-κ ablation affects presynaptic release probability. This effect, however, was only observed when a number of data sets were pooled. Furthermore, induced (DHPG, dihydroxyphenylglycine) levels of PtdOH were not significantly altered in the DGK-κ KO neurons. While intriguing, more work is required to define the role of DGK-κ in neurons. DGK-ε has also been implicated in modulating long-term potentiation and seizure susceptibility (Cole-Edwards and Bazan, 2005, Goldschmidt, Tu-Sekine, 2016, Musto and Bazan, 2006, Rodriguez de Turco et al., 2001), and may play a role in Huntington disease (Zhang et al., 2012) but the molecular mechanisms remain obscure. Finally, a recent report suggest DGK-κ associates with the fragile X mental retardation protein (FMRP) in neurons and suggest this enzyme may indirectly impact on the pathology of fragile X syndrome (Tabet et al., 2016).
Another route for PtdOH production in neurons is via the acylation of a lysoPtdOH catalyzed by a lysoPtdOH acyltransferase (LPAAT). This enzyme appears to play an important role in ribbon synapses (Schwarz, Natarajan, 2011). These synapses are found in certain sensory neurons requiring large amount of vesicle recycling in a fast and efficient manner; photoreceptor cells, retinal bipolar cells, and inner ear hair cells. One of the distinguishing features of these neurons is their high rates of synaptic vesicle exocytosis and vesicle release (Wan and Heidelberger, 2011). The name of these synapses derives from a rather unique presynaptic structure that is perpendicular to the plasma membrane, called the synaptic ribbon, on which are found a large number of synaptic vesicles. These vesicles are primed for exocytosis and represent a readily releasable pool of synaptic vesicles. One of the critical proteins in this ribbon is termed RIBEYE. This protein has both a structural and enzymatic role. Its enzymatic activity was shown to be an LPAAT generating PtdOH important for exocytosis at these synapses (Schwarz, Natarajan, 2011). We note that endophilin I, a protein involved in endocytosis that interacts with synaptojanin and dynamin, was once believed to possess LPAAT activity (Schmidt et al., 1999). This conclusion, however, is at best controversial as this activity was later shown to be an artifact (Farsad et al., 2001, Gallop et al., 2005).
Regulation of Phosphatidic Acid Producing Enzymes
PtdOH may play a variety of roles in the nervous system (below). Given this, it is becoming more important to understand the factors controlling the localization and regulation of the enzymes involved in its production. Regulated de novo synthesis or LPAAT activity (above) have not been addressed and the regulation of these PtdOH pathways does not appear to be involved in neurons. There are data, on the other hand, supporting roles for the regulation of PLDs and DGKs in neurons. A recent review outlines some of regulatory roles of these enzymes are outlined in a recent review (Tu-Sekine, Goldschmidt, 2015).
The observations that PLD and DGK (Davletov and Montecucco, 2010, Humeau, Vitale, 2001, Liu et al., 2005, Rohrbough and Broadie, 2005, Waring et al., 1999)(Almena and Merida, 2011, Kanoh et al., 2002, Merida et al., 2008, van Blitterswijk and Houssa, 2000)) play roles in neurotransmitter release strongly implicate this class of enzymes in modulating the levels of PtdOH in neurons (Cremona, Di, 1999b, Davletov and Montecucco, 2010, Goldschmidt, Tu-Sekine, 2016, Huttner and Schmidt, 2000, Lauwers, Goodchild, 2016, Lim and Wenk, 2009, Puchkov and Haucke, 2013, Rohrbough and Broadie, 2005, Schwarz, Natarajan, 2011, Tu-Sekine, Goldschmidt, 2015, Tu-Sekine and Raben, 2011, Yuan, Liu, 2015). Much has been written about the regulation of PLDs and has been the subject of numerous other reviews (Bruntz et al., 2014, Exton, 2002a, b, Klein, 2005, Lauwers, Goodchild, 2016, Lee, Kang, 1997, Lee et al., 2000, Sarri et al., 1998, Selvy et al., 2011, Vitale et al., 2005, Waring, Drappatz, 1999, Wenk and De Camilli, 2004). With respect to neurons, the regulated metabolism of PtdIns(4,5)P2 is involved in neurotransmission and this metabolism is also involved in the regulation of a PLD (PLD1) (Powner and Wakelam, 2002). It is also interesting that a number of synaptic vesicle proteins are also implicated in regulating PLDs and this was nicely summarized in a review by Bruntz et al (Bruntz, Lindsley, 2014). At the present time, however, there are no compelling data regarding the precise proteins or mechanisms by which these proteins regulate a PLD in a physiological manner. In addition to the synaptic proteins, other proteins have been shown to regulate PLDs. PLD1, for example, is activated by small GTP-binding proteins such as ARF and Rho proteins (Exton, 1999, 2000, 2002a, b, Frohman, 2015, Powner and Wakelam, 2002). A number of proteins have also been shown to interact with PLD2 (Gomez-Cambronero, 2011) and phosphorylation may also play a role in regulating PLD1 and PLD2 (Henkels et al., 2010).
In 2011, we provided a perspective regarding the localization, function and regulation of neuronal DGKs (Tu-Sekine and Raben, 2011). Very little progress has been made regarding the neuronal roles and regulation of catalytic activity of these enzymes with the exception of DGK-θ. Shortly after this review was published, we provided evidence for a polybasic protein in regulating DGK-θ (Tu-Sekine and Raben, 2012), but the precise protein(s) in neurons has not been identified. Interestingly, DGK-θ’s activity is also increased in the presence of its product PtdOH (Tu-Sekine et al., 2007, Tu-Sekine and Raben, 2012), suggesting a feed-forward regulation in neurons.
As noted above, Rho proteins, such as RhoA, are known to activate PLD1 (Exton, 1999, 2000, 2002a, b, Frohman, 2015, Powner and Wakelam, 2002). It is particularly interesting that RhoA also modulates DGK-θ by inhibiting its activity (Houssa et al., 1999). This tempts the suggestion that RhoA may reciprocally regulate PLD1 and DGK-θ in neurons although this hypothesis has not been fully tested. In support of this notion, however, RhoA is also implicated in regulating neurotransmission (Hiley et al., 2006, McMullan et al., 2006, Wang et al., 2005), and associates with PLD1 in a light-dependent manner in photoreceptor rod outer segments (Salvador and Giusto, 2006).
Neuronal Roles of Phosphatidic Acid
Perhaps the most challenging question with regard to PtdOH in neurotransmission pertains to defining its precise role. The role of this lipid in neurotransmission is often ascribed to its reported ability to serve as a “fusogenic” lipid in that it alters membrane architecture to assist in membrane fusion. PtdOH, however, may also affect the localization and activities of proteins. While a full review of these properties is beyond the scope of this review, studies by Kooijman and colleagues have provided some of the most exciting studies and reviews on this topic (Kooijman and Burger, 2009, Kooijman et al., 2005a, Kooijman et al., 2003, Kooijman et al., 2005b, Kooijman et al., 2007, Loew et al., 2013) and the reader is referred to these articles for a more in depth discussion.
The notion that PtdOH represents a fusogenic lipid has probably attracted the most attention with respect to neurotransmitter release. This property of PtdOH is largely ascribed to its well-documented tendency to generate non-bilayer, hexagonal II phase, membrane domains particularly in the presence of calcium ions (Jouhet, 2013). The ability of PtdOH to be fusogenic is in part due to the fact that it is one of the cone-shaped lipids known to promote negative membrane curvature and fusion. This is important for neurotransmission where vesicles have a large positive curvature and the formation of non-bilayer phases may help to lower the energy needed for fusion (Kozlovsky et al., 2002).
PtdOH also plays an important role in neurotransmission by binding specific proteins Further, some of proteins essential for the exocytosis/endocytosis of synaptic vesicles localize to regions of membrane curvature (e.g. see (Jung et al., 2010, Martens, 2010, Milosevic et al., 2011, Shen et al., 2014)). PtdOH mediates the membrane penetration of dynamin (Burger, Demel, 2000, Takei, Haucke, 1998); a protein involved in SV endocytosis (see (Doherty and McMahon, 2009, Ferguson and De Camilli, 2012, Kokotos and Cousin, 2015)) and binds to syntaxin1A, a protein known to be involved in vesicle exocytosis. This protein binds and actually recruits PtdOH to vesicle release sites (Aalmo et al., 1984). A functional interaction between PtOH and syntaxin1A is believed to be necessary for regulating the energetics of membrane fusion (Lam et al., 2008). Indeed, mutations that disrupt the recruitment of PtdOH suppress vesicle secretion in PC12 cells and overexpression of PLD1 overcomes the secretory defect (Lam et al., 2008). As indicated above, PtdOH activates a PI kinase (Jenkins, Fisette, 1994, Moritz, De Graan, 1992) needed for the synthesis of another lipid important for neurotransmission- PtdIns(4,5)P2 (Thieman, Mishra, 2009, Volpicelli-Daley, Lucast, 2010).
Summary
One of the captivating aspects of scientific research is the well-known phenomenon that as we discover answers we illuminate more questions. Our recognition regarding the role of PtdIns(4,5)P2 in neurons has led to question the roles of its metabolites DAG and PtdOH. Given the popularity of PtdIns(4,5)P2 turnover and the generation of DAG we’ve learned how important both of these lipids are to neurotransmission. We are now also increasing our appreciation for the potential role of another metabolite PtdOH and becoming more interested in the other pathways responsible for generating and regulating the levels of this lipid. PtdOH plays a number of roles in establishing important membrane architectural changes, binding specific proteins, and modulating essential enzyme activities. Both DGKs and PLDs appear to play important roles in neuronal functions such as modulating the SV cycle (Figure 2), while LPAAT may play a role in very specialized neurons such as sensory neurons with ribbon synapses. Clearly, this field is ripe for further discoveries.
Figure 2.
Proposed Roles of PLD1 and DGK-θ Generated PtdOH in the SV Exocytosis and Endocytosis.
Acknowledgments
The work from DMR was supported by grant RO1 NS077923
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalmo K, Hansen L, Hough E, Jynge K, Krane J, Little C, et al. An anion binding site in the active centre of phospholipase C from Bacillus cereus. BiochemInt. 1984;8:27–33. [PubMed] [Google Scholar]
- Almena M, Merida I. Shaping up the membrane: diacylglycerol coordinates spatial orientation of signaling. Trends in biochemical sciences. 2011;36:593–603. doi: 10.1016/j.tibs.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Ammar MR, Kassas N, Chasserot-Golaz S, Bader MF, Vitale N. Lipids in Regulated Exocytosis: What are They Doing? Frontiers in endocrinology. 2013;4:125. doi: 10.3389/fendo.2013.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Newton AC. Tuning the signalling output of protein kinase C. Biochem Soc Trans. 2014;42:1477–83. doi: 10.1042/BST20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonescu CN, Danuser G, Schmid SL. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol Biol Cell. 2010;21:2944–52. doi: 10.1091/mbc.E10-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, et al. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–36. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Godin C, Anborgh PH, Dale LB, Poulter MO, et al. Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8752–61. doi: 10.1523/JNEUROSCI.3155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruntz RC, Lindsley CW, Brown HA. Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer. Pharmacological Reviews. 2014;66:1033–79. doi: 10.1124/pr.114.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KN, Demel RA, Schmid SL, de Kruijff B. Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry. 2000;39:12485–93. doi: 10.1021/bi000971r. [DOI] [PubMed] [Google Scholar]
- Chalifa V, Mohn H, Liscovitch M. A neutral phospholipase D activity from rat brain synaptic plasma membranes. Identification and partial characterization. JBiolChem. 1990;265:17512–9. [PubMed] [Google Scholar]
- Chasserot-Golaz S, Coorssen JR, Meunier FA, Vitale N. Lipid dynamics in exocytosis. Cellular and molecular neurobiology. 2010;30:1335–42. doi: 10.1007/s10571-010-9577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JK, Sekiya F, Kang HS, Lee C, Han JS, Kim SR, et al. Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate. JBiolChem. 1997;272:15980–5. doi: 10.1074/jbc.272.25.15980. [DOI] [PubMed] [Google Scholar]
- Cole-Edwards KK, Bazan NG. Lipid signaling in experimental epilepsy. NeurochemRes. 2005;30:847–53. doi: 10.1007/s11064-005-6878-4. [DOI] [PubMed] [Google Scholar]
- Cremona O, De CP. Phosphoinositides in membrane traffic at the synapse. JCell Sci. 2001;114:1041–52. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999a;99:179–88. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Cremona O, Di PG, Wenk MR, Luthi A, Kim WT, Takei K, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999b;99:179–88. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- Davletov B, Montecucco C. Lipid function at synapses. CurrOpinNeurobiol. 2010;20:543–9. doi: 10.1016/j.conb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, et al. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–22. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Du G, Huang P, Liang BT, Frohman MA. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol BiolCell. 2004;15:1024–30. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. BiochimBiophysActa. 1999;1439:121–33. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- Exton JH, Phospholipase D. AnnNYAcadSci. 2000;905:61–8. doi: 10.1111/j.1749-6632.2000.tb06538.x. [DOI] [PubMed] [Google Scholar]
- Exton JH. Phospholipase D-structure, regulation and function. RevPhysiol BiochemPharmacol. 2002a;144:1–94. doi: 10.1007/BFb0116585. [DOI] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. FEBS Lett. 2002b;531:58–61. doi: 10.1016/s0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- Falcieri E, Gobbi P, Sabatelli P, Santi S, Farabegoli F, Rana R, et al. A combined ultrastructural approach to the study of nuclear matrix thermal stabilization. Histochemistry. 1992;98:121–9. doi: 10.1007/BF00717003. [DOI] [PubMed] [Google Scholar]
- Farsad K, De Camilli P. Neurotransmission and the synaptic vesicle cycle. The Yale journal of biology and medicine. 2002;75:261–84. [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. The Journal of cell biology. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alfonso T, Ryan TA. The efficiency of the synaptic vesicle cycle at central nervous system synapses. Trends in cell biology. 2006;16:413–20. doi: 10.1016/j.tcb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Frohman MA. The phospholipase D superfamily as therapeutic targets. Trends in pharmacological sciences. 2015;36:137–44. doi: 10.1016/j.tips.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–8. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- Gauthier-Kemper A, Kahms M, Klingauf J. Restoring synaptic vesicles during compensatory endocytosis. Essays in biochemistry. 2015;57:121–34. doi: 10.1042/bse0570121. [DOI] [PubMed] [Google Scholar]
- Ghim J, Chelakkot C, Bae YS, Suh PG, Ryu SH. Accumulating insights into the role of phospholipase D2 in human diseases. Advances in biological regulation. 2016;61:42–6. doi: 10.1016/j.jbior.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Goldschmidt HL, Tu-Sekine B, Volk L, Anggono V, Huganir RL, Raben DM. DGKtheta Catalytic Activity Is Required for Efficient Recycling of Presynaptic Vesicles at Excitatory Synapses. Cell reports. 2016;14:200–7. doi: 10.1016/j.celrep.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J. The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (and a surprise discovery: PLD2 is a GEF) Cellular signalling. 2011;23:1885–95. doi: 10.1016/j.cellsig.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nature reviews Neuroscience. 2011;12:127–38. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- Henkels KM, Peng HJ, Frondorf K, Gomez-Cambronero J. A comprehensive model that explains the regulation of phospholipase D2 activity by phosphorylation-dephosphorylation. Mol Cell Biol. 2010;30:2251–63. doi: 10.1128/MCB.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley E, McMullan R, Nurrish SJ. The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 2006;25:5884–95. doi: 10.1038/sj.emboj.7601458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssa B, de Widt J, Kranenburg O, Moolenaar WH, van Blitterswijk WJ. Diacylglycerol kinase theta binds to and is negatively regulated by active RhoA. JBiolChem. 1999;274:6820–2. doi: 10.1074/jbc.274.11.6820. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Dupont JL, Du G, Frohman MA, et al. A role for phospholipase D1 in neurotransmitter release. ProcNatlAcadSciUSA. 2001;98:15300–5. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schmidt A. Lipids, lipid modification and lipid-protein interaction in membrane budding and fission--insights from the roles of endophilin A1 and synaptophysin in synaptic vesicle endocytosis. CurrOpinNeurobiol. 2000;10:543–51. doi: 10.1016/s0959-4388(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Ishisaka M, Hara H. The roles of diacylglycerol kinases in the central nervous system: review of genetic studies in mice. Journal of pharmacological sciences. 2014;124:336–43. doi: 10.1254/jphs.13r07cr. [DOI] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. JBiolChem. 1994;269:11547–54. [PubMed] [Google Scholar]
- Jouhet J. Importance of the hexagonal lipid phase in biological membrane organization. Frontiers in plant science. 2013;4:494. doi: 10.3389/fpls.2013.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AG, Labarrera C, Jansen AM, Qvortrup K, Wild K, Kjaerulff O. A mutational analysis of the endophilin-A N-BAR domain performed in living flies. PloS one. 2010;5:e9492. doi: 10.1371/journal.pone.0009492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H, Yamada K, Sakane F. Diacylglycerol kinases: emerging downstream regulators in cell signaling systems. JBiochem(Tokyo) 2002;131:629–33. doi: 10.1093/oxfordjournals.jbchem.a003144. [DOI] [PubMed] [Google Scholar]
- Kavalali ET. Synaptic vesicle reuse and its implications. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2006;12:57–66. doi: 10.1177/1073858405281852. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee J, Kim S, Shin MK, Min dS, Shin T. Differential expression of phospholipases D1 and D2 in mouse tissues. Cell BiolInt. 2007;31:148–55. doi: 10.1016/j.cellbi.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Klein J. Functions and pathophysiological roles of phospholipase D in the brain. J Neurochem. 2005;94:1473–87. doi: 10.1111/j.1471-4159.2005.03315.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Holt M. Coupling exo- and endocytosis: an essential role for PIP(2) at the synapse. Biochimica et biophysica acta. 2012;1821:1114–32. doi: 10.1016/j.bbalip.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Koch T, Wu DF, Yang LQ, Brandenburg LO, Hollt V. Role of phospholipase D2 in the agonist-induced and constitutive endocytosis of G-protein coupled receptors. J Neurochem. 2006;97:365–72. doi: 10.1111/j.1471-4159.2006.03736.x. [DOI] [PubMed] [Google Scholar]
- Kokotos AC, Cousin MA. Synaptic vesicle generation from central nerve terminal endosomes. Traffic. 2015;16:229–40. doi: 10.1111/tra.12235. [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Burger KN. Biophysics and function of phosphatidic acid: a molecular perspective. Biochimica et biophysica acta. 2009;1791:881–8. doi: 10.1016/j.bbalip.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Carter KM, van Laar EG, Chupin V, Burger KN, De Kruijff B. What makes the bioactive lipids phosphatidic acid and lysophosphatidic acid so special? Biochemistry. 2005a;44:17007–15. doi: 10.1021/bi0518794. [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Chupin V, De Kruijff B, Burger KN. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003;4:162–74. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Chupin V, Fuller NL, Kozlov MM, De Kruijff B, Burger KN, et al. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005b;44:2097–102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DT, Burger KN, et al. An electrostatic/hydrogen bond switch as basis for the specific interaction of phosphatidic acid with proteins. JBiolChem. 2007 doi: 10.1074/jbc.M609737200. [DOI] [PubMed] [Google Scholar]
- Kozlovsky Y, Chernomordik LV, Kozlov MM. Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophys J. 2002;83:2634–51. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol Biol Cell. 2008;19:485–97. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers E, Goodchild R, Verstreken P. Membrane Lipids in Presynaptic Function and Disease. Neuron. 2016;90:11–25. doi: 10.1016/j.neuron.2016.02.033. [DOI] [PubMed] [Google Scholar]
- Lee C, Kang HS, Chung JK, Sekiya F, Kim JR, Han JS, et al. Inhibition of phospholipase D by clathrin assembly protein 3 (AP3) JBiolChem. 1997;272:15986–92. doi: 10.1074/jbc.272.25.15986. [DOI] [PubMed] [Google Scholar]
- Lee C, Kim SR, Chung JK, Frohman MA, Kilimann MW, Rhee SG. Inhibition of phospholipase D by amphiphysins. The Journal of biological chemistry. 2000;275:18751–8. doi: 10.1074/jbc.M001695200. [DOI] [PubMed] [Google Scholar]
- Lim L, Wenk M. Neuronal Membrane Lipids-Their Role in the Synaptic Vesicle Cycle. In: Lajtha A, Tettamanti G, Goracci G, editors. Handbook of Neurochemistry and Molecular Neurobiology; Neural Lipids. 3. Springer; 2009. pp. 223–38. [Google Scholar]
- Liu L, Liao H, Castle A, Zhang J, Casanova J, Szabo G, et al. SCAMP2 interacts with Arf6 and phospholipase D1 and links their function to exocytotic fusion pore formation in PC12 cells. Mol Biol Cell. 2005;16:4463–72. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew S, Kooijman EE, May S. Increased pH-sensitivity of protein binding to lipid membranes through the electrostatic-hydrogen bond switch. Chemistry and physics of lipids. 2013;169:9–18. doi: 10.1016/j.chemphyslip.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Martens S. Role of C2 domain proteins during synaptic vesicle exocytosis. Biochem Soc Trans. 2010;38:213–6. doi: 10.1042/BST0380213. [DOI] [PubMed] [Google Scholar]
- Martin TF. PI(4,5)P(2)-binding effector proteins for vesicle exocytosis. Biochimica et biophysica acta. 2015;1851:785–93. doi: 10.1016/j.bbalip.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos MV, Giusto NM, Salvador GA. Distinctive roles of PLD signaling elicited by oxidative stress in synaptic endings from adult and aged rats. Biochimica et biophysica acta. 2012;1823:2136–48. doi: 10.1016/j.bbamcr.2012.09.005. [DOI] [PubMed] [Google Scholar]
- McMullan R, Hiley E, Morrison P, Nurrish SJ. Rho is a presynaptic activator of neurotransmitter release at pre-existing synapses in C. elegans. Genes Dev. 2006;20:65–76. doi: 10.1101/gad.359706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. BiochemJ. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Holz RW, Smith SJ. Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. The Journal of cell biology. 2001;154:355–68. doi: 10.1083/jcb.200102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Jahn R. Organization and dynamics of SNARE proteins in the presynaptic membrane. Frontiers in physiology. 2015;6:89. doi: 10.3389/fphys.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A, De Graan PN, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. JBiolChem. 1992;267:7207–10. [PubMed] [Google Scholar]
- Musto A, Bazan NG. Diacylglycerol kinase epsilon modulates rapid kindling epileptogenesis. Epilepsia. 2006;47:267–76. doi: 10.1111/j.1528-1167.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Oliveira TG, Chan RB, Tian H, Laredo M, Shui G, Staniszewski A, et al. Phospholipase d2 ablation ameliorates Alzheimer's disease-linked synaptic dysfunction and cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16419–28. doi: 10.1523/JNEUROSCI.3317-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron D, Tall RD, Roth MG. Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors. Mol Biol Cell. 2006;17:598–606. doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4092–9. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posor Y, Eichhorn-Grunig M, Haucke V. Phosphoinositides in endocytosis. Biochimica et biophysica acta. 2015;1851:794–804. doi: 10.1016/j.bbalip.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Powner DJ, Wakelam MJ. The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett. 2002;531:62–4. doi: 10.1016/s0014-5793(02)03410-5. [DOI] [PubMed] [Google Scholar]
- Puchkov D, Haucke V. Greasing the synaptic vesicle cycle by membrane lipids. Trends in cell biology. 2013;23:493–503. doi: 10.1016/j.tcb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Rankovic M, Jacob L, Rankovic V, Brandenburg LO, Schroder H, Hollt V, et al. ADP-ribosylation factor 6 regulates mu-opioid receptor trafficking and signaling via activation of phospholipase D2. Cellular signalling. 2009;21:1784–93. doi: 10.1016/j.cellsig.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–33. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, et al. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. ProcNatlAcadSciUSA. 2001;98:4740–5. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K. Lipid regulation of the synaptic vesicle cycle. NatRevNeurosci. 2005;6:139–50. doi: 10.1038/nrn1608. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Clathrin-mediated endocytosis at the synaptic terminal: bridging the gap between physiology and molecules. Traffic. 2010;11:1489–97. doi: 10.1111/j.1600-0854.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador GA, Giusto NM. Phospholipase D from photoreceptor rod outer segments is a downstream effector of RhoA: evidence of a light-dependent mechanism. Experimental eye research. 2006;83:202–11. doi: 10.1016/j.exer.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Sarri E, Bockmann I, Kempter U, Valeva A, von Eichel-Streiber C, Weichel O, et al. Regulation of phospholipase D activity in synaptosomes permeabilized with Staphylococcus aureus alpha-toxin. FEBS Lett. 1998;440:287–90. doi: 10.1016/s0014-5793(98)01479-3. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV, et al. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–41. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Natarajan S, Kassas N, Vitale N, Schmitz F. The synaptic ribbon is a site of phosphatidic acid generation in ribbon synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15996–6011. doi: 10.1523/JNEUROSCI.2965-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer FE, Ryan TA. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr Opin Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Selvy PE, Lavieri RR, Lindsley CW, Brown HA. Phospholipase D: enzymology, functionality, and chemical modulation. Chemical reviews. 2011;111:6064–119. doi: 10.1021/cr200296t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Giordano F, Wu Y, Chan J, Zhu C, Milosevic I, et al. Coupling between endocytosis and sphingosine kinase 1 recruitment. Nature cell biology. 2014;16:652–62. doi: 10.1038/ncb2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz B, Horvath TL. Synaptic lipids in cortical function and psychiatric disorders. EMBO molecular medicine. 2015;8:3–5. doi: 10.15252/emmm.201505749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. AnnuRevNeurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sun L, Gooding HL, Brunton PJ, Russell JA, Mitchell R, Fleetwood-Walker S. Phospholipase D-mediated hypersensitivity at central synapses is associated with abnormal behaviours and pain sensitivity in rats exposed to prenatal stress. The international journal of biochemistry & cell biology. 2013;45:2706–12. doi: 10.1016/j.biocel.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Tabet R, Moutin E, Becker JA, Heintz D, Fouillen L, Flatter E, et al. Fragile X Mental Retardation Protein (FMRP) controls diacylglycerol kinase activity in neurons. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1522631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–41. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- Thieman JR, Mishra SK, Ling K, Doray B, Anderson RA, Traub LM. Clathrin regulates the association of PIPKIgamma661 with the AP-2 adaptor beta2 appendage. The Journal of biological chemistry. 2009;284:13924–39. doi: 10.1074/jbc.M901017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu-Sekine B, Goldschmidt H, Raben DM. Diacylglycerol, phosphatidic acid, and their metabolic enzymes in synaptic vesicle recycling. Advances in biological regulation. 2015;57:147–52. doi: 10.1016/j.jbior.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu-Sekine B, Ostroski M, Raben DM. Modulation of diacylglycerol kinase theta activity by alpha-thrombin and phospholipids. Biochemistry. 2007;46:924–32. doi: 10.1021/bi061170c. [DOI] [PubMed] [Google Scholar]
- Tu-Sekine B, Raben DM. Regulation and roles of neuronal diacylglycerol kinases: a lipid perspective. Crit RevBiochem Mol Biol. 2011;46:353–64. doi: 10.3109/10409238.2011.577761. [DOI] [PubMed] [Google Scholar]
- Tu-Sekine B, Raben DM. Dual regulation of diacylglycerol kinase (DGK)-theta: polybasic proteins promote activation by phospholipids and increase substrate affinity. The Journal of biological chemistry. 2012;287:41619–27. doi: 10.1074/jbc.M112.404855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Houssa B. Properties and functions of diacylglycerol kinases. Cell Signal. 2000;12:595–605. doi: 10.1016/s0898-6568(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Vitale N, Mawet J, Camonis J, Regazzi R, Bader MF, Chasserot-Golaz S. The Small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. The Journal of biological chemistry. 2005;280:29921–8. doi: 10.1074/jbc.M413748200. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Lucast L, Gong LW, Liu L, Sasaki J, Sasaki T, et al. Phosphatidylinositol-4-phosphate 5-kinases and phosphatidylinositol 4,5-bisphosphate synthesis in the brain. The Journal of biological chemistry. 2010;285:28708–14. doi: 10.1074/jbc.M110.132191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan QF, Heidelberger R. Synaptic release at mammalian bipolar cell terminals. Vis Neurosci. 2011;28:109–19. doi: 10.1017/S0952523810000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, et al. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Waring M, Drappatz J, Weichel O, Seimetz P, Sarri E, Bockmann I, et al. Modulation of neuronal phospholipase D activity under depolarizing conditions. Febs Letters. 1999;464:21–4. doi: 10.1016/s0014-5793(99)01669-5. [DOI] [PubMed] [Google Scholar]
- Wen PJ, Osborne SL, Meunier FA. Phosphoinositides in neuroexocytosis and neuronal diseases. Current topics in microbiology and immunology. 2012;362:87–98. doi: 10.1007/978-94-007-5025-8_4. [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci U S A. 2004;101:8262–9. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Naslavsky N, Caplan S. Diacylglycerol kinases in membrane trafficking. Cellular logistics. 2015;5:e1078431. doi: 10.1080/21592799.2015.1078431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Seo J, Nair R, Han S, Jang S, Kim K, et al. DGKiota regulates presynaptic release during mGluR-dependent LTD. EMBO J. 2011;30:165–80. doi: 10.1038/emboj.2010.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Liu L, Zhang Y, Wei L, Zhao S, Zheng X, et al. Diacylglycerol Guides the Hopping of Clathrin-Coated Pits along Microtubules for Exo-Endocytosis Coupling. Dev Cell. 2015;35:120–30. doi: 10.1016/j.devcel.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang N, Li B, Al-Ramahi I, Cong X, Held JM, Kim E, et al. Inhibition of lipid signaling enzyme diacylglycerol kinase epsilon attenuates mutant huntingtin toxicity. The Journal of biological chemistry. 2012;287:21204–13. doi: 10.1074/jbc.M111.321661. [DOI] [PMC free article] [PubMed] [Google Scholar]