Background
Seborrheic Dermatitis (SD) is a common inflammatory skin disorder. It is chronic and relapsing, affecting 1–3% of the general adult population [1]. SD presents as pink to red greasy-looking skin with yellowish scales in seborrheic areas such as the scalp, face (nasolabial folds, upper lip, eyelids and eyebrows), retro-auricular area, and the upper chest. It can be pruritic and socially embarrassing. Current treatment includes antifungals, anti-inflammatory, and immune modulators, but treatment in some patients may cause adverse effects such as skin atrophy and telangiectasia if used long term. Sebaceous secretion, yeast Malassezia infection, and individual susceptibilities such as host immunity and epidermal barrier integrity have all been identified as predisposing factors, and may work together to worsen disease. In 2006, Birnbaum et al demonstrated that a frame-shift mutation in ZNF750, a zinc finger transcription factor and master regulator of epidermal differentiation, causes early onset (<10 years of age) autosomal dominant seborrhea-like psoriasiform dermatitis with 100% penetrance (OMIM #610227) [2]. Yet, how ZNF750 dysfunction causes the SD phenotype remains unknown.
We have previously generated knockout mice to determine MPZL3 (Myelin Protein Zero-like 3) function in the skin. Mpzl3 encodes an immunoglobulin protein and is expressed in the suprabasal layers of mouse epidermis, the sebaceous gland, and anagen hair follicles [3,4]. Not surprisingly, Mpzl3 knockout mice showed various skin abnormalities, including epidermal and sebaceous hyperplasia and hair loss [3–5]. Interestingly, these mice also developed early onset skin inflammation with dandruff-like flakes [4]. Importantly, ZNF750 was recently shown to directly bind to MPZL3 promoter and activate its transcription in cultured human keratinocytes [6]. Therefore, Mpzl3 knockout mice can serve as a useful model to understand SD pathogenesis caused by ZNF750 dysfunction.
Questions addressed
In this study, we further investigated the inflammatory skin phenotype of Mpzl3 knockout mice to better understand the pathobiology of SD.
Experimental design
Results
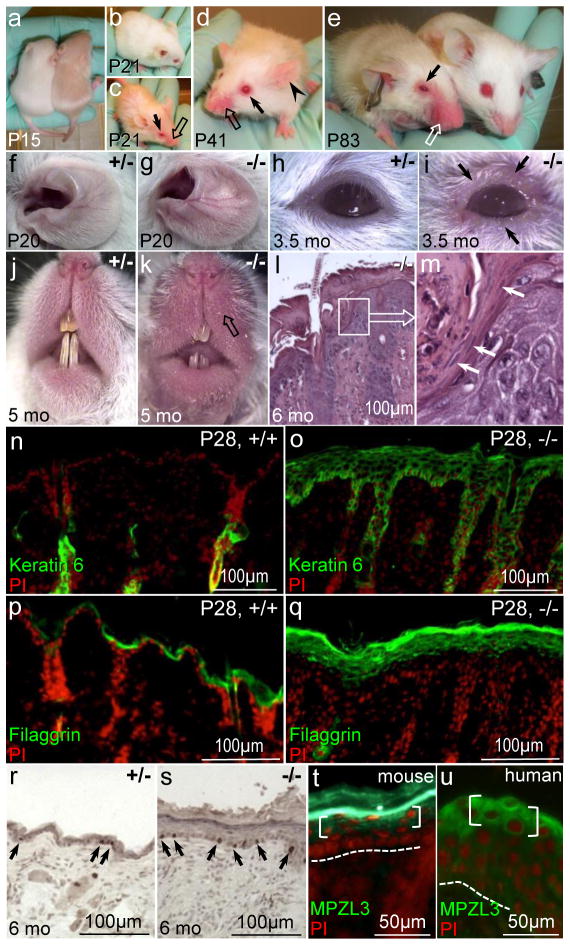
To investigate the effects of loss of MPZL3 function in skin inflammation, we examined homozygous Mpzl3 knockout (−/−) mice and their wild type (+/+) or heterozygous (+/−) littermates. Skin inflammation became apparent in Mpzl3−/− mice within 3 weeks after birth, with 100% penetrance, when areas of alopecia on the animals’ face, eyelid, and upper chest appeared red and swollen (Fig. 1a–k). Histology of affected skin in Mpzl3−/− mice showed epidermal hyperplasia, hyperkeratosis, infiltrates including neutrophils, and with occasional parakeratosis at the hair canal opening (“shoulder parakeratosis”) (Fig. 1l, m). Spongiosis typical of SD, especially during the acute phase, was seen only occasionally. Interestingly, spongiosis was not detected in the family with SD and the ZNF750 mutation [2].
Figure 1.
Early onset skin inflammation with features of seborrheic dermatitis in Mpzl3−/− mice (a–s) and MPZL3 protein expression in human epidermis (t). (a–k) Mpzl3−/− mice start to show greasy hair 2 weeks after birth, and develop skin inflammation by 3 weeks of age that persists. They show red, swollen skin on the vibrissae area (block arrows) and margin of the eyelid (arrows), accompanied by hair loss, and dilated blood vessels in the ears (arrowhead). (l, m) H&E staining of Mpzl3−/− skin shows epidermal hyperplasia, hyperkeratosis, parakeratosis at the hair canal opening (“shoulder parakeratosis”) (white arrows), infiltrates, and occasional spongiosis. Image in (m) is enlarged from inset in (l). (n–q) Aberrant Keratin 6 expression (n, o) and increased Filaggrin expression (p, q) in hyperplastic Mpzl3−/− epidermis. (r, s) Immunohistochemistry of PCNA expression (brown nuclei, arrows) in Mpzl3 +/− and −/− epidermis. (t,u) MPZL3 protein expression in the granular layers (brackets) of mouse (t) and human (u) epidermis. Signal in mouse stratum corneum (t) is staining artifact (Leiva et al, 2014).
Prior to apparent skin inflammation, significant levels of aberrant keratin 6 expression were already detected in the hyperplastic epidermis of Mpzl3−/− mice 12 days after birth (P12) (Fig. S1). Expression of filaggrin was also increased in Mpzl3−/− epidermis (Fig. 1p, q). PCNA immunohistochemistry demonstrated increased basal cell proliferation in Mpzl3−/− epidermis (Fig. 1r, s). We also detected MPZL3 protein expression in the granular layers of human epidermis (Fig. 1u), consistent with previous reports in mouse (Fig. 1t) [4] and human skin [6].
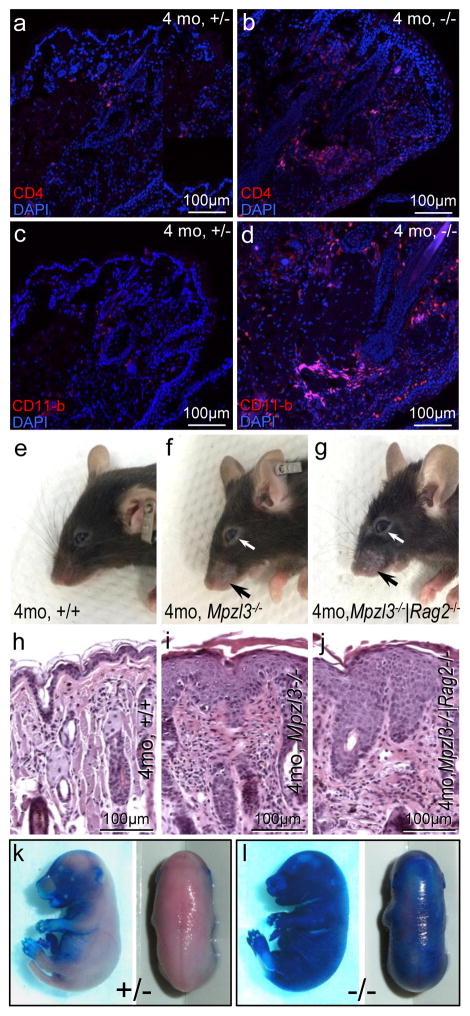
Immunofluorescent staining demonstrated increased T-helper cells (CD4+) and macrophages (CD11-b+) (Fig. 2a–d), but not cytotoxic T cells (CD8+) or B cells (CD19+) (Fig. S1g–j), in the dermal infiltrates of Mpzl3−/− eyelids. To determine the role of adaptive immunity in skin inflammation, Mpzl3−/− mice were bred with Rag2−/− mice, which lack mature B and T lymphocytes [7]. Mpzl3−/−|Rag2−/− double knockout mice developed skin inflammation and alopecia similar to that observed in Mpzl3−/− mice (Fig. 2e–j). These observations suggest that while adaptive immunity participated in skin inflammation (increased CD4+ cell infiltrates) in the Mpzl3−/− mice, it was not required for initiation (inflammation developed in the absence of adaptive immunity in the Rag2−/− background).
Figure 2.
Skin inflammation and epidermal barrier defects in the Mpzl3−/− mice. (a–d) Increased T-helper cell (CD4 positive) (a, b) and macrophage (CD11-b positive) (c, d) infiltrates in the dermis of Mpzl3−/− eyelids. (e–j) Gross phenotype (e–g) and hematoxylin and eosin staining of skin sections (h–j) showing skin inflammation and alopecia (arrows) in Mpzl3−/−|Rag2−/− mice (g, j) similar to that of Mpzl3−/− mice (f, i), compared with wild type mice (e, h). (k, l) Increased toluidine blue dye permeability +/− in E17.0 Mpzl3−/− embryos.
Toluidine blue permeability assay detected increased dye permeability of Mpzl3−/− skin, suggestive of a defective epidermal barrier (Fig. 2k, l). As expected, gene expression profiling detected correlated changes in genes related to barrier function and inflammation (Table S1), including small proline-rich protein (Sprr) genes, keratins 6 and 16, inflammatory mediators and antimicrobial peptides (defensins and lipocalin), chemokines (ccls), and inflammatory cytokines and receptors (interleukins and receptors) (Table S1) [S1–S3]. These observations suggest a two-fold consequence of loss of MPZL3 function in the skin: defective barrier and recruitment of immune cells.
Conclusions
In this study, we demonstrated seborrheic dermatitis-like clinical features with associated histology in the Mpzl3 knockout mice. Mpzl3−/− skin showed increased macrophage and CD4+ lymphocyte infiltration, however adaptive immunity was not required for the onset of skin inflammation. Furthermore, we detected epidermal barrier defects as suggested by increased dye permeability and altered gene expression in Mpzl3−/− embryos and pup skin. Taken together, these findings suggest MPZL3 plays an essential role in epidermal differentiation and barrier function, and underscore the interplay between epidermal barrier and immunity in SD.
Previous studies of SD have focused on Malassezia yeast colonization and the immunologic abnormalities [Note, 1, S4–S8]. Our data suggest that epidermal barrier defects and the ensuing innate immune response likely initiated SD-like phenotype in Mpzl3−/− mice. In toto, our results suggest a more primary role of barrier maintenance in the pathogenesis of SD.
The SD-like clinical and histologic features in the Mpzl3−/− mice also resemble those in patients carrying a frame-shift mutation in ZNF750, a key regulator of epidermal differentiation and a transcriptional activator of the MPZL3 gene [2, 6]. Therefore, we conclude that the ZNF750/MPZL3 pathway plays a critical role in the pathogenesis of SD, and a better understanding of skin inflammation and barrier restoration in the Mpzl3−/− mice will provide insight into the pathogenesis and treatment/prevention of recurrent SD.
Supplementary Material
Acknowledgments
This work was supported by AR059907 from NIH/NIAMS; the Brian V. Jegasothy, M.D., Basic Science Research Award; and Dermatology Gift Fund from the Department of Dermatology & Cutaneous Surgery, University of Miami Miller School of Medicine (T.C.W.). We thank Drs. Jie Li, Marjana Tomic-Canic, Evangelos Badiavas, Mariya Miteva, Paolo Romanelli, Gabriel Villada, and Mina Zarei for helpful discussions.
Footnotes
Author contribution
V.L. Perez and T.C. Wikramanayake designed the study and experiments, T.C. Wikramanayake, L.J. Borda, Y. Wang, S. Duffort, A. Reyes-Capo, M. Urbieta and A. Barsam performed the experiments and data analysis, T.C. Wikramanayake and R.S. Kirsner wrote the paper and other authors reviewed/revised the paper.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investigat Dermatol. 2015;3(2):10. doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum RY, et al. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nat Genet. 2006;38(7):749–51. doi: 10.1038/ng1813. [DOI] [PubMed] [Google Scholar]
- 3.Cao T, et al. Mutation in Mpzl3, a novel [corrected] gene encoding a predicted [corrected] adhesion protein, in the rough coat (rc) mice with severe skin and hair abnormalities. J Invest Dermatol. 2007;127(6):1375–86. doi: 10.1038/sj.jid.5700706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiva AG, et al. Loss of Mpzl3 function causes various skin abnormalities and greatly reduced adipose depots. J Invest Dermatol. 2014;134(7):1817–27. doi: 10.1038/jid.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czyzyk TA, et al. Genetic ablation of myelin protein zero-like 3 in mice increases energy expenditure, improves glycemic control, and reduces hepatic lipid synthesis. Am J Physiol Endocrinol Metab. 2013;305(2):E282–92. doi: 10.1152/ajpendo.00228.2013. [DOI] [PubMed] [Google Scholar]
- 6.Bhaduri A, et al. Network Analysis Identifies Mitochondrial Regulation of Epidermal Differentiation by MPZL3 and FDXR. Dev Cell. 2015;35(4):444–57. doi: 10.1016/j.devcel.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 8.Oble DA, et al. A novel T cell receptor transgenic animal model of seborrheic dermatitis-like skin disease. J Invest Dermatol. 2005;124(1):151–9. doi: 10.1111/j.0022-202X.2004.23565.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.