Abstract
In late 2015, The National Cancer Institute (NCI) Division of Cancer Prevention convened cancer prevention research experts and stakeholders to discuss the current state of cancer prevention research, identify key prevention research priorities for the NCI, and identify studies that could be conducted within the NCI Community Oncology Research Program (NCORP).
Goals included identifying cancer prevention research opportunities offering the highest return on investment, exploring the concept of precision prevention and what is needed to advance this area of research, and identifying possible targets for prevention.
Four study populations were considered for cancer prevention research: healthy people; those at increased risk for a specific cancer; people with preneoplastic lesions; and children, adolescents, and young adults.
Priorities that emerged include screening (e.g., surveillance intervals, tomosynthesis vs. digital mammography), a pre-cancer genome atlas (PreTCGA), HPV vaccines, immunoprevention of non-infectious origins, and overdiagnosis. Challenges exist, as the priority list is ambitious and potentially expensive. Clinical trials need to be carefully designed to include and maximize prospective tissue collection. Exploring existing co-funding mechanisms will likely be necessary. Finally, relationships with a new generation of physician specialists will need to be cultivated in order to reach the target populations.
Keywords: cancer prevention research, community cancer research, early detection research, NCI, NCORP
Introduction
The National Cancer Institute (NCI) Division of Cancer Prevention invited experts and stakeholders in cancer prevention research in November 2015 to discuss the current state of cancer prevention research, and to identify key prevention research priorities for the National Cancer Institute (NCI). The focus of this Think Tank was to identify studies that could be conducted within the NCI Community Oncology Research Program (NCORP)(http://ncorp.cancer.gov).
The overall goals for the Think Tank were to: (1) identify areas where cancer prevention research opportunities offer the most likely return on investment; (2) explore the concept of precision prevention and determine what is needed to advance this area of research; (3) identify possible targets for prevention where potential opportunities for gains in cancer research exist; and, (4) rebalance the NCORP study portfolio by introducing new cancer prevention research clinical trials.
An additional perspective was to consider cancer prevention ideas over four study populations: healthy people, those at increased risk for a specific cancer, people with preneoplastic lesions, and children, adolescents, and young adults.
Clinical Trials Network Background
Recognizing that clinical trials must keep pace with advances in the scientific understanding of cancer, the Institute of Medicine (IOM) (1) issued a report in 2010 that outlined necessary, systematic changes to more efficiently design, review, and conduct clinical trials. Based on that report, sweeping changes to the NCI clinical trials program were implemented starting in 2014, with the creation of the NCI National Clinical Trials Network (NCTN). The network was organized to take advantage of the opportunities afforded by the improved understanding of tumor biology and improved efficiencies of centralizing and streamlining critical functions.
Working in parallel to preserve and enhance prevention and cancer control clinical research and community-based research, the NCI integrated two prior networks, the NCI Community Clinical Oncology/Minority-Based Community Clinical Oncology Program and the NCI Community Cancer Centers Program, into NCORP, also in 2014. NCORP is a national network of investigators, cancer care providers, academic institutions, and other organizations that conducts multi-site cancer clinical trials and studies in diverse populations in community-based practices and healthcare systems across the United States and Puerto Rico. The overall goal of NCORP is to bring cancer clinical trials (cancer control, prevention, screening, treatment, and imaging), as well as cancer care delivery research (CCDR), to individuals in their own communities, thus generating a broadly applicable evidence base that contributes to improved outcomes and a reduction in cancer disparities.
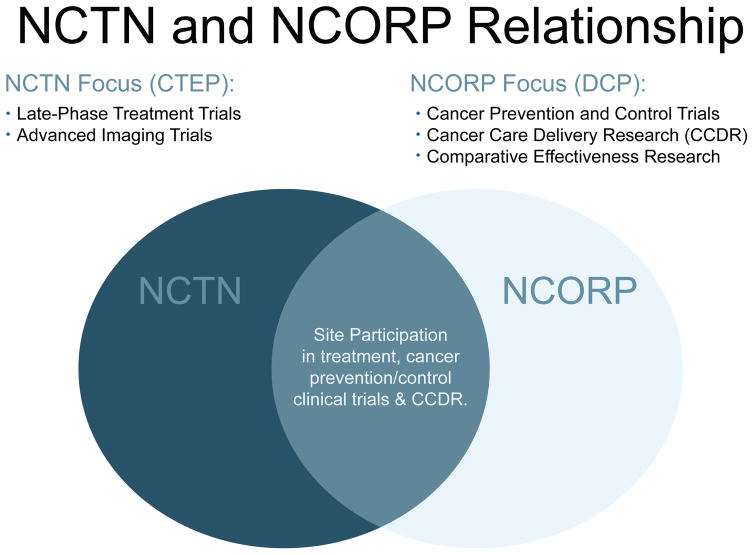
NCORP investigators both create and lead the cancer control, prevention, and screening trials in which NCTN members take part; and NCORP sites also take part in NCTN treatment and imaging trials. (Figure 1)
Figure 1.
NCI’s Cancer Therapy Evaluation Program (CTEP) and Division of Cancer Prevention (DCP) work closely to carry out NCI clinical trials and research studies.
The NCI Division of Cancer Prevention also supports an Early Phase Clinical Trials Program (Consortia) to carry out safety and preliminary efficacy phase 0/I/II clinical trials on new cancer prevention interventions (http://prevention.cancer.gov/major-programs/phase-0iii-cancerprevention). In this capacity, promising interventions can be refined before being studied in large-scale trials in NCORP. The Consortia program fills the void between preclinical studies and phase III clinical trials, with an emphasis on determining the effects of interventions on at-risk tissue, through intensive tissue collections (biopsies) and invasive biomarker monitoring.
Cancer Prevention Research Opportunities
Two recent reviews of the state of cancer prevention strongly support the need to approach prevention at the molecular level (2, 3). Cancer is a group of many diverse diseases with a great level of genetic complexity and heterogeneity. Therefore, implementing interventions akin to the level of fluoridation of the water supply to prevent dental caries will not work for the prevention of most cancers. At the same time, it has been shown that for many cancers, the progression from healthy tissue to invasive cancer can take years, allowing time for detection of these molecular changes and for intervention to stop or reverse the path to cancer.
In developing new interventions for cancer prevention, target populations are seemingly healthy and the chances of doing harm can outweigh the benefits. Therefore, rigorous phase I and II trials are necessary before starting large, population-based trials.
The biology of premalignancy is poorly studied and understood, severely limiting the development of effective interventions. For example, some of the well-described genetic drivers that occur in premalignant/malignant tissues can also occur in histologically normal tissue that is not at risk for cancer development. In fact, the same mutations can happen more often in normal people than in metastatic cancer (4).
To further develop potential prevention opportunities, the Think Tank was structured around the following topic areas: basic science, immunoprevention, precision prevention and early detection, and surveillance. The following is a summary of the meeting discussion and possible future directions.
Basic Science of Prevention
One of the critical bottlenecks in moving prevention research forward is the lack of knowledge about the earliest molecular events in progression to cancer. The specific cell of origin for many cancers remains unknown.
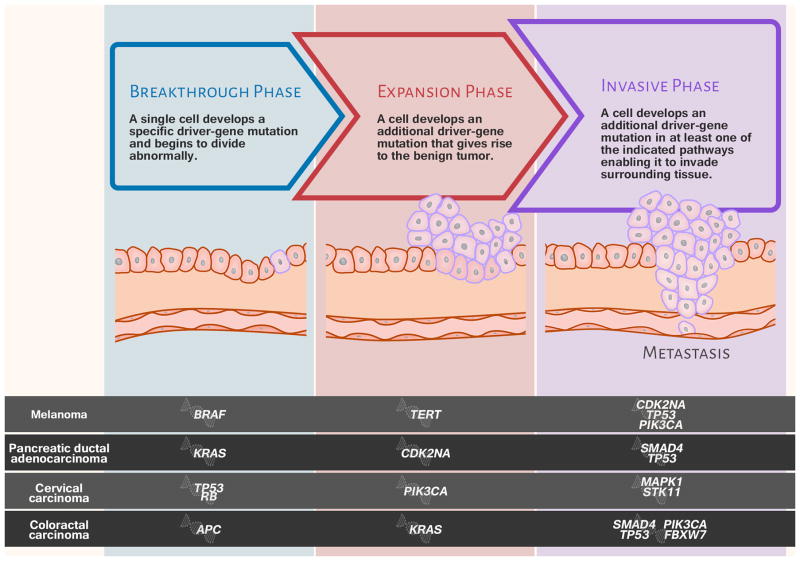
A strong case was made to begin a PremalignanT Cancer Genome Atlas (PreTCGA), modeled on The Cancer Genome Atlas (http://cancergenome.nih.gov), which has generated comprehensive, multi-dimensional maps of the key genomic changes in 33 types of cancer. For the PreTCGA, tissues would need to be collected longitudinally, over time, from the same patients. Vogelstein and his colleague conclude there are three genomic phases (breakthrough, expansion, and invasive phase), and key events happen at each stage (5). Some of the genetic changes are necessary but not sufficient for cancer to develop. There is a window of time to identify these changes before they lead to overt cancer. (Figure 2)
Figure 2.
Using four represented cancer types, this illustration depicts three broad phases of tumor development along with driver-gene mutations and their pathways (adapted from Vogelstein and Kinzler, 2015 (5)).
Targeting genetic drivers at the stage of premalignancy to stop development of cancer is one method for prevention. Research on molecular and cellular mechanisms (premalignant cancer genome) and targeted prevention strategies (precision cancer prevention) hold a vast, but unrealized, potential.
As an initial step towards the concept of a PreTCGA, Ooi et al. presented a cross-sectional approach to the transcriptomics of squamous cell carcinoma of the lung (6). Normal epithelium and premalignant dysplastic areas adjacent to resected squamous cell lung cancer within the same individual were all profiled by RNA sequencing in order to characterize the molecular changes that occur with stepwise progression to invasive carcinoma. One of the genes, CEACAM5, a cell surface glycoprotein, active in cell adhesion and intracellular signaling, is expressed more actively as tissues progressed from normal to premalignant to cancer. Immunostaining validated this change at the protein level.
A true longitudinal model of premalignant disease initiation and progress has been employed by Beane and colleagues (7) in which premalignant lesions for squamous cell lung cancer were collected from the bronchial airway of the same individuals over time. A broad range of “-omics” profiling is being performed on these samples. So far, 26 individuals with multiple specimens have been studied. Early data have shown imperfect separation of high vs. low histologic grades of tumor, suggesting that molecular profiling might contain additional information about the biological behavior of the premalignant lesion that is not captured by histology. Leveraging the longitudinal study design, these investigators have also identified molecular alterations that associate with progression and regression of these premalignant lesion over time.
Immunoprevention
Cancer is under immune surveillance from the beginning of its development, as a cancer diagnosis is considered an “escape” from immune control (8). Better animal models that incorporate immune surveillance exist now than did previously (9, 10). Immune surveillance and its interaction with the microenvironment of premalignant lesions are known as cancer “immunoediting” and have three sequential phases: elimination, equilibrium, and escape (11). Recent studies have targeted the tumor microenvironment during the escape period of the tumor-immune system interaction. The tumor escapes by modulating the immune system while it is itself the subject of modulation by the immune system. Even after primary tumor removal, the immune system continues to play a determining role in risk of recurrence and survival, as suggested in a study of MUC1 antibody-positive early breast cancer (12).
Both tumors and premalignant lesions establish immunosuppressive microenvironments but to different degrees. Studies that looked for infiltrating primary colon cancer have shown that, survival after cancer removal was better in patients who had T cells in their tumors compared to those who did not (13). A major independent predictive factor was the presence of immune response in the tumor, and this provides the theoretical underpinning for vaccination against tumor-associated antigens.
Few such vaccines have reached phase III trials. A novel approach is to test the vaccine at the point at which normal cells have just become slightly abnormal. The tumor microenvironment is established in very early phases of cancer development (14). Recent successes of blocking checkpoint inhibitors with antibodies, especially in advanced melanoma or lung cancer, raise the question of whether the same approach can be applied to treat premalignant disease at lower doses or longer intervals, in order to mitigate toxicity. Preliminary animal data support this possibility (15, 16).
The human papilloma virus (HPV) vaccine was a major medical advance in terms of disease prevention; however, vaccine rates remain less than optimal. Studying the efficacy of fewer than the originally recommended three doses of the regimen is an important research direction.
Much of the knowledge about HPV vaccines and immunity comes from trials conducted in Costa Rica. In a randomized trial that proved the efficacy of the bivalent vaccine against HPV 16 and HPV 18, girls achieved the same level of protection against infection whether they received one, two, or the planned three doses of vaccine (17). However, the numbers were small, especially for those receiving one dose; and the three dosing schedules were not randomly assigned. Internationally, a two dose regimen is likely to become the standard. Plans for a randomized non-inferiority study in Costa Rica comparing one versus two vaccine doses are underway, with an endpoint of protection from persistent infection of targeted HPV types. A randomized “immune-bridging” study in the US to evaluate whether one and two doses achieve stable antibody levels associated with protection from persistent infection in the Costa Rica study may be an important research project.
Immunoprevention trials in the NCI Consortia can provide the justification for larger scale trials, if successful, and include both pathogen-associated cancers and tumor-associated antigens. Studies for an alternate dosing schedule for the HPV vaccine and also for a new hepatitis C vaccine are currently underway. For tumor-associated antigens (non-infectious), there is a MUC-1 vaccine in people with history of colorectal adenomas (NCT02134925), a HER2 and multipeptide (WOKVAC) vaccines in breast cancer (NCT02780401), and a PSA vaccine (PROSTVAC) in a prostate cancer active surveillance cohort (NCT02326805).
Precision Prevention and Early Detection
A strategy to improve the efficiency of prevention interventions and, in some cases, to improve the balance of benefits and harms, is to focus on people at increased risk of cancer who are most likely to benefit.
Aspirin recently became the first medicine for cancer prevention to be included in a large scale public health guideline. In April 2016, the US Preventive Services Task Force recommended initiating low-dose aspirin for the primary prevention of cardiovascular disease (CVD) and colorectal cancer in adults aged 50 to 59 years who have a 10% or greater 10-year CVD risk, are not at increased risk for bleeding, have a life expectancy of at least 10 years, and are willing to take low-dose aspirin daily for at least 10 years (18). Future research efforts should be directed to personalizing the use of aspirin by further identifying those most likely to benefit (or identifying those most likely to be harmed so that aspirin can be avoided).
Among potential mechanisms, based on experimental models, the effect of aspirin on prostaglandins plays an important role. Aspirin is an inhibitor of arachidonic acid metabolism, cyclooxygenases 1 and 2 (COX-1, COX-2) and other pathways. Looking at the COX-2 mediator, 15-PGDH, in two key studies, there is experimental evidence that it is ubiquitously downregulated in colorectal cancer (19, 20). Molecular and genetic markers in prostaglandin and inflammatory pathways hold particular promise for colorectal cancer prevention. For other organ sites, the molecular pathways are less well characterized.
Another potential approach to “precision medicine” relies on the idea that the field of tissue injury/exposure could be measured as an individual risk biomarker. Smoking alters epithelial cell gene expression throughout the respiratory tract, but it is variable (21). Some, but not all of these changes may resolve with cessation (22). Imaging for lung cancer screening cannot distinguish between benign and malignant findings. Peripheral lung nodules present a diagnostic dilemma regarding whether the person needs surgery or watchful waiting. Assessment of gene expression patterns may help to guide clinical decisions.
To determine whether bronchial airway gene-expression profiles could improve the diagnostic performance of bronchoscopy, 28 medical centers participated in two prospective studies of smokers undergoing bronchoscopy for suspected lung cancer. A 23 gene biomarker panel was validated as a highly sensitive biomarker to detect lung cancer among those whose bronchoscopy was nondiagnostic, potentially allowing physicians to avoid unnecessary invasive procedures (i.e., surgical lung biopsy or transthoracic needle aspiration) among those with benign lung disease (23). Ongoing work is attempting to extend these observations to the nasal epithelium in view of its greater accessibility. Early studies have found that genes that are altered in the nasal epithelium of lung cancer patients are enriched for genes that change in the bronchial epithelium, lending credence to the nose as a surrogate for changes in the bronchus (24).
Overall, the opportunities and challenges include: Molecular profiling of relatively accessible tissue within the “field” of exposure may provide a measure of an individual’s physiological response and risk of cancer; development of highly sensitive biomarkers could serve as “rule out” tests to avoid unnecessary invasive procedures in those with non-malignant nodules, especially in the lung cancer screening setting; opportunities exist for companion diagnostics for precision chemoprevention; and the challenges in collecting surrogate tissue from screening and chemoprevention trials to develop/validate molecular biomarkers must be addressed.
Useful elements for cancer prevention trials include a population with defined, quantifiable risk; a reasonable target; an acceptable intervention from a safety perspective; a measurable endpoint such as reduction in cancer incidence; and whether efficacy is specific to tumor subtypes.
Surveillance and Screening
Screening and early detection can result in surveillance challenges, as evidenced in screening for colon cancer. Cancer surveillance includes the assessment of screening practices among patients who have undergone curative treatment for cancer (e.g., intervals between screening and screening modalities). Current US colorectal cancer screening use data shows 65% of eligible people in 2010 getting screening, and a stated goal of 80% for 2018. More people are getting screened, colonoscopy is the primary modality, and more people are being identified with adenomas (25). Surveillance colonoscopy is designed to detect lesions after a screening exam has found adenomatous polyps. Up to 25% of all colonoscopy is for surveillance, representing a huge demand on colonoscopy capacity. The optimal frequency of post-polypectomy colonoscopy surveillance is ill-defined and an important area for research. A proposed National Trial of Surveillance Colonoscopy (NToSC) aim is to evaluate 5-year and 10-year vs. 10-year only surveillance on colorectal cancer incidence in subjects with one to two non-advanced adenomas.
Overdiagnosis refers to a diagnosis that does not benefit patients because the diagnosed condition, often by screening or early diagnosis efforts, is not harmful or would not otherwise lead to symptoms or death in those individuals. This phenomenon is an important research focus within cancer prevention in association with cancer screening and the management of premalignant lesions. There is ongoing research within the NCI to discover and develop biomarkers or molecular signatures that distinguish indolent cancers from progressive cancer, and overdiagnosis must be considered as we chart the future of cancer prevention research.
Potential Research Concepts by Study Population
During the Think Tank, four break-out groups were convened to generate potential population-focused research concepts that could be carried out in the NCORP setting. The following concepts were determined by the Think Tank to be possibilities, but are not intended to be exclusive or convey an order of priority.
1. Healthy Populations
Possible target groups for consideration in future prevention studies:
Populations that may believe themselves to be “healthy” or at least at average risk of cancer who may not be, such as people with varying degrees of being overweight or obese.
Healthy cancer survivors and their families.
People who have had a positive screening test.
Potential opportunities by population include the following:
Overweight/obese
If early metformin trials produce encouraging results, NCORP could consider a definitive study of metformin vs. placebo. A combination/hybrid pragmatic study for NCORP sites could be designed to evaluate aspects of immunoprevention markers and inflammatory markers, e.g., insulin resistance at baseline and 12 months that correlates with metabolism, with a link to the electronic health records (EHR). It could be a short- or long-term study. Other agents to consider: rapamycin analogues for inflammation and nutrient-based interventions.
Healthy survivors
PremalignanT Cancer Genome Atlas (PreTCGA)–type studies could be done with colorectal cancer survivors or with those in follow-up for polyps (anyone who regularly has colonoscopies). Promising chemopreventive agents could be evaluated in sub-studies.
Another potential concept involves microbiome mutagenicity and how exposures or preventive agents, e.g., metformin, affect the microbiome (26). NCORP has the capacity to enroll many colon cancer survivors to assess the microbiome, colonic tissue, germline alterations, and immune function.
Screening
The radiology community is interested in a randomized trial comparing standard digital mammography with and without tomosynthesis (3-D mammography) to assess the occurrence of advanced cancers during a specific screening period.
Because there is no chemopreventive agent ready for a definitive trial in healthy populations, a possibility might be to collect baseline data as a way to engage people, thereby creating a healthy cohort to effectively and efficiently launch an intervention study in the future when appropriate.
2. Persons with Preneoplastic Lesions
Molecular atlas of cancer initiation
TCGA did not provide information about cancer initiation or premalignancy, and the study population was heavily dominated by Caucasians. However, NCORPs could look at tumor initiation/premalignancy at the molecular level, and in racial/ethnic minority populations, possibly using breast as a prototype. High-risk populations undergoing screening may provide a setting for longitudinal studies of molecular indicators of early disease.
There are emerging technologies to look at proteins in small quantities; every single protein is archived. Protein signatures within the stroma and immune cells may provide indicators of risk or progression of premalignant lesions. An unanswered question is whether a normal biopsy truly is normal. Biochemical changes may belie a morphologically normal biopsy.
Erlotinib prevention of oral cancer (EPOC II)
The primary hypothesis for the EPOC II clinical trial is that the EGFR tyrosine kinase inhibitor, erlotinib, will improve oral cancer-free survival in high-risk patients with oral pre-malignant lesions, as determined by loss of heterozygosity (LOH) profiles.
Primary findings from the EPOC study showed that erlotinib did not prevent oral cancer in those with LOH+ cancers, and frequent dose reductions were required, primarily due to skin rash. However, when efficacy was analyzed according to development of rash, erlotinib-treated patients who exhibited grade 2 or 3 rash at month 1 had statistically significant superior oral cancer-free survival, compared to erlotinib-treated patients with grade 1 or no rash (27).
Rash associated with EGFR inhibitors is mediated at least in part by macrophage and mast cell induced T cell infiltration in skin, and may be a favorable prognostic and predictive marker of benefit from EGFR inhibitors (28, 29). Intermittent dosing might lessen the severity of skin rash, as is being tested in an ongoing NCI-funded Phase 0/I/II Consortia study (NCT02169284).
Nicotinamide for prevention of atypical nevi
Chen and colleagues reported that oral nicotinamide was safe and effective in reducing the rates of new nonmelanoma skin cancer and actinic keratosis in high-risk patients (30). Ultraviolet (UV) radiation is an important risk factor for these conditions. UV radiation increases the risk of both melanoma and non-melanoma skin cancer by damaging DNA, suppressing cutaneous anti-tumor immunity, and inhibiting DNA repair by depleting cellular adenosine triphosphate (ATP). Nicotinamide is an amide form of vitamin B3 and the precursor of nicotinamide adenine dinucleotide (NAD+), an essential cofactor for ATP production. The compound prevents ATP depletion and glycolytic blockade induced by UV radiation thereby boosting cellular energy and enhancing DNA repair. Nicotinamide also reduces the level of immunosuppression induced by UV radiation, which is triggered by DNA damage, without altering baseline immunity. Nicotinamide has been reported to enhance repair of UV-induced DNA damage in primary melanocytes (31).
A proposed early stage trial would be in people with atypical nevi who are at increased risk for skin cancers. They would be randomized to take nicotinamide or placebo for 12 months with a measured endpoint of the numbers of atypical nevi.
3. High-Risk Populations
There are FDA-approved prevention options for high-risk populations, but their uptake in the community is limited.
Tamoxifen/Raloxifene use in women at elevated risk for breast cancer
In general, in women with atypical ductal hyperplasia or atypical lobular hyperplasia, less than 10% take selective estrogen receptor modulators (SERMs), such as tamoxifen or raloxifene. However, some institutes have had greater success, particularly when physicians participate in a comprehensive education program, with quality metric assessments of frequency of prescribing SERMs as incentives. For example, The University of Texas MD Anderson’s high risk clinic reports their experience of a 40% uptake in women with biopsy-proven atypia breast lesions.
Women, identified via pathology report who are eligible for SERMs as chemoprevention, could be studied to better understand the determinants of uptake and use of these proven interventions in a cancer-care delivery study. The intervention could be targeted towards physician education or patient decision aids with a goal of achieving informed decision-making. Possible study endpoints include frequency of discussion and offering drug, frequency of the decision to start a preventive agent, and frequency of continuing medication after 1, 2 and 5 years.
Create a cohort of high-risk individuals that could be followed over time and a source of individuals for phase 2 prevention trials
Crowd sourcing enrollment/recruitment tools could be used to create a cohort of high-risk individuals. People could self-identify as being at high-risk (e.g., certain genetic mutations, tobacco use, obesity) using defined criteria. Personal, clinical data would be collected in a database via electronic health record or self-report. Blood, buccal or nasal swabs could be obtained for comprehensive genomic analysis. Similar to the NCI MATCH trial, which uses genomic testing of patients’ tumors in order to match them to a targeted therapy, a MATCH trial for prevention, could be developed. In a prevention MATCH trial, each participant would have a model calculated based on information they enter into a database. The data could be used to better understand risk. Such a database could also be used as a potential referral source for smaller phase II trials. Blood banking could be used for circulating tumor DNA studies.
4. Children/Adolescents and Young Adults
In pediatric cancer patients or survivors, there may be genetic markers in patients at risk of developing second malignancies (e.g., breast cancer in girls irradiated for lymphoma). Researchers could also study the effect of associated conditions such as obesity in the rate of relapse. Many children who start chemotherapy treatments at a normal weight end up obese due to change of diet, lack of activity or steroids. Additional studies are needed on the effects of alcohol, tobacco and obesity on the development of second malignancies. Another question is whether cancer survivors get vaccinated against HPV at the same rate as children without a history of cancer. For this population, the utilization of HPV vaccines could also be studied, especially how uptake varies among girls of different racial groups and economic backgrounds.
Another potential research area in children and adolescents without cancer pertains to the diversity of use of tobacco products. The increased availability of e-cigarettes (vaping) has an unknown influence on use of tobacco, marijuana or other drugs. A substantial barrier to conducting research in this area is access to the population of interest: healthy children, adolescents, and young adults. NCORP will collaborate with other NCI Divisions that conduct tobacco research to identify potential opportunities in this population.
Summary
Based on the presentations and discussion from this Think Tank, six priority areas were identified for prevention studies. Two of them are concepts that are already under active consideration or in development: 1) defining the appropriate interval of surveillance colonoscopy in people who have low-risk adenomas found during cancer screening, and 2) a study to compare standard digital mammography with and without tomosynthesis for breast cancer screening (TMIST). Although focused on detection, these studies provide potential opportunities to collect tissues that could be analyzed as part of 3) a pre-cancer genome atlas (PreTCGA). The remaining priority areas for consideration are: 4) pursuing the question of one versus two HPV vaccine doses in establishing immunity (would depend upon outcomes from other ongoing studies before it could reach large-scale testing); 5) immunoprevention of noninfectious origins; and 6) overdiagnosis.
Next Steps
Following the Think Tank, a summary highlighting the six priority areas was shared with the NCORP Research Base Prevention Committee Chairs. Overall, the priorities and suggestions were met with enthusiasm, particularly with regards to PreTCGA, immune function/vaccines, uptake of available strategies, lifestyle factors/obesity, and surveillance studies.
Challenges exist and were discussed. The priority list is an ambitious and potentially expensive one. Studies need to be carefully designed and feasible to maximize accrual and collection of prospective tissue. Exploring existing co-funding mechanisms will be necessary to secure resources in order to develop and carry out these new initiatives in NCORP. Additionally, NCI will need to cultivate relationships with other physician specialists (gastrointestinal, primary care, etc.) in order to reach the target populations. Moving forward, NCI will internally review and further prioritize the ideas that came forth from the Think Tank based upon readiness of those ideas and preliminary data. To keep the momentum of the Think Tank, and to build a stronger relationship between the NCORP Research Bases and the DCP Phase 0/I/II Clinical Trials Consortia, NCI plans to convene an in-person meeting of the NCORP Prevention Committee Chairs, as well as working groups to cultivate and sustain partnerships with primary care and non-oncology specialists that will include representation from stakeholders, including NCTN investigators.
Acknowledgments
We wish to thank the Cancer Prevention Think Tank Participants. Planning Committee members are noted with an asterisk.
-
*Anthony J. Alberg, PhD, MPH
Hollings Cancer Center
Medical University of South Carolina
Charleston, SC
-
*Howard Bailey, MD
University of Wisconsin School of Medicine and Public Health
Madison, WI
-
Isabelle Bedrosian, MD
The University of Texas MD Anderson Cancer Center
Houston, TX
-
L. Michelle Bennett, PhD
National Cancer Institute
Bethesda, MD
-
Raymond Bergan, MD
K night Cancer Center
Oregon Health & Science University
Portland, OR
-
Michele Bloch, MD, PhD
National Cancer Institute
Bethesda, MD
-
Powel Brown, MD, PhD
The University of Texas MD Anderson Cancer Center
Houston, TX
-
*Andy Chan, MD, MPH
Massachusetts General Hospital
Boston, MA
-
Joseph P. Costantino, DrPH
University of Pittsburgh
Pittsburgh, PA
-
Andy Dannenberg, MD
Weill Cornell Medical College
New York, NY
-
*Olivera (Olja) J. Finn, PhD
University of Pittsburgh School of Medicine
Pittsburgh, PA
-
*Leslie Ford, MD
National Cancer Institute
Bethesda, MD
-
Judy Garber, MD, MPH
Dana-Farber Cancer Institute
Boston, MA
-
Gary Goodman, MD, MS
Swedish Cancer Institute
Seattle, WA
-
Peter Greenwald, MD, DrPH
National Cancer Institute
Bethesda, MD
-
J. Silvio Gutkind, PhD
UC San Diego Moores Cancer Center
La Jolla, CA
-
Allan Hildesheim, PhD
National Cancer Institute
Bethesda, MD
-
Elizabeth Jaffee, MD
Johns Hopkins Sidney Kimmel Comprehensive Cancer Center
Baltimore, MD
-
Barnett Kramer, MD, MPH
National Cancer Institute
Bethesda, MD
-
J. Jack Lee, PhD, MS, DDS
The University of Texas MD Anderson Cancer Center
Houston, TX
-
*Scott Lippman, MD – Chair
UC San Diego Moores Cancer Center
La Jolla, CA
-
Ana Maria Lopez, FACP, MD, MPH
University of Utah
Salt Lake City, UT
-
Doug Lowy, MD
National Cancer Institute
Bethesda, MD
-
*Pamela Maxwell
National Cancer Institute
Bethesda, MD
-
*Worta McCaskill-Stevens, MD, MS
National Cancer Institute
Bethesda, MD
-
Frank Meyskens, MD, FACP
University of California Irvine
Orange, CA
-
Wynne Norton, PhD
National Cancer Institute
Bethesda, MD
-
Augusto Ochoa, MD
Louisiana State University Health Science Center
New Orleans, LA
-
*Olufunmilayo (Funmi) Olopade, MD, FACP
University of Chicago Medicine
Chicago, IL
-
Lee Pai-Scherf, MD
Food and Drug Administration
Rockville, MD
-
Howard Parnes, MD
National Cancer Institute
Bethesda, MD
-
*Deborah Pearson, RN, MPH
National Cancer Institute
Bethesda, MD
-
Robert Schoen, MD, MPH
University of Pittsburgh
Pittsburgh, PA
-
Vicki Seewaldt, MD
City of Hope
Duarte, CA
-
Avrum (Avi) Spira, MD, MSc
Boston University School of Medicine
Boston, MA
-
Lillian Sung, MD, PhD
Hospital for Sick Children
Ontario, Canada
-
*Eva Szabo, MD
National Cancer Institute
Bethesda, MD
-
*Catherine M. Tangen, DrPH
Fred Hutchinson Cancer Research Center
Seattle, WA
-
*Martina V. Taylor, MT, ASCP
National Cancer Institute
Bethesda, MD
-
Asad Umar, DVM, PhD
National Cancer Institute
Bethesda, MD
-
Victor G. Vogel, MD, MHS
Geisinger Cancer Institute
Danville, PA
-
Liewei Wang, MD, PhD
Mayo Clinic
Rochester, MN
-
Erica Warner, ScD
Brigham and Women’s Hospital
Boston, MA
-
*Larry Wickerham, MD
Pittsburgh Campus of the Drexel University School of Medicine
Pittsburgh, PA
-
William Nassib William, Jr, MD
The University of Texas MD Anderson Cancer Center
Houston, TX
-
*Marie Wood, MD
University of Vermont College of Medicine
Burlington, VT
We also wish to thank Kara Smigel-Croker and Gwen Moulton of the NCI Division of Cancer Prevention for their editorial assistance.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.IOM (Institute of Medicine) A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 2.Maresso KC, Tsai KY, Brown PH, Szabo E, Lippman S, Hawk ET. Molecular cancer prevention: Current status and future directions. CA Cancer J Clin. 2015;65:345–83. doi: 10.3322/caac.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenzler TW, Spira A, Garber JE, Szabo E, Lee JJ, Dong Z, et al. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev Research. 2016;9(1):2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, Lippman SM, Flaherty KT, Kurzrock R. The Conundrum of Genetic “Drivers” in Benign Conditions. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw036. Print 2016 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. The Path to Cancer – Three Strikes and You’re Out. N Engl J Med. 2015;373:1895–8. doi: 10.1056/NEJMp1508811. [DOI] [PubMed] [Google Scholar]
- 6.Ooi AT, Gower AC, Zhang KX, Vick JL, Hong L, Nagao B, et al. Molecular profiling of premalignant lesions in lung squamous cell carcinomas identifies mechanisms involved in stepwise carcinogenesis. Cancer Prev Res. 2014 May;7(5):487–95. doi: 10.1158/1940-6207.CAPR-13-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beane JE, Campbell J, Moy C, Perdomo C, Schaffer M, Mazzilli S, et al. Abstract 2878: Development of the pre-cancer genome atlas (PCGA) for squamous cell lung carcinoma. Cancer Research. 2015;75(15 Supplement) [Google Scholar]
- 8.Dunn GP, Bruce AT, Ikeda H, Old LJ, Scheiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3(11):991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 9.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 10.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009 Jun 28;279(1):1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Mensdorff-Pouilly S, von Verstraeten AA, Kenemans P, Snijdewint FGM, Kok A, Van Kamp GI, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18(3):574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Keenan B, Jaffee EM. Immunotherapy in preneoplastic disease: targeting early procarcinogenic inflammatory changes that lead to immune suppression and tumor tolerance. Ann NY Acad Sci. 2013 May;1284:12–6. doi: 10.1111/nyas.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ai M, Curran M. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother. 2015;64(7):885–92. doi: 10.1007/s00262-014-1650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proofof-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011 Oct 5;103(19):1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement [published online April 12, 2016] Ann Intern Med. 2016;164(12):836–45. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 19.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci. 2004;101:10143–48. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8:R201. doi: 10.1186/gb-2007-8-9-r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373(3):243–51. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Rogers J, Gerrein J, Anderlind C, Liu G, Zhang S, Alekseyev Y, et al. Nasal epithelial gene expression is altered in patients with lung cancer and reflects the cancerassociated gene expression changes observed in the bronchial epithelium. Am J Respir Crit Care Med. 2016;193:A7535. [Google Scholar]
- 25.CDC. Vital Signs: Colorectal Cancer Screening Test Use — United States, 2012. MMWR. 2013;62(44):881–88. [PMC free article] [PubMed] [Google Scholar]
- 26.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.William WN, Jr, Papadimitrakopoulou V, Lee JJ, Mao L, Cohen EE, Lin HY, et al. Erlotinib and the risk of oral cancer: the erlotinib prevention of oral cancer (EPOC) randomized clinical trial. JAMA Oncol. 2016;2(2):209–16. doi: 10.1001/jamaoncol.2015.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013;5(199):199ra11. doi: 10.1126/scitranslmed.3005886. [DOI] [PubMed] [Google Scholar]
- 29.Mascia F, Lam G, Keith C, Garber C, Steinberg SM, Kohn E, et al. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Transl Med. 2013;5(199):199ra110. doi: 10.1126/scitranslmed.3005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618–26. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 31.Thompson BC, Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Exp Dermatol. 2014;23(7):509–1. doi: 10.1111/exd.12430. [DOI] [PubMed] [Google Scholar]