Abstract
The frequencies of multidrug resistance-associated mutations at codons 145, 151, and 69 of the human immunodeficiency virus (HIV) reverse transcriptase (RT) gene in strains from a group of 3,595 highly active antiretroviral therapy (HAART)-experienced patients were 0.22, 2.36, and 0.86%, respectively. Several amino acid substitutions different from the recently reported Gln145Met change (S. Paolucci, F. Baldanti, M. Tinelli, G. Maga, and G. Gerna, AIDS 17:924-927, 2003) were detected at position 145. Thus, amino acid substitutions selected at position 145 were introduced into the wild-type HIV type 1 (HIV-1) RT gene by site-directed mutagenesis, and recombinant HIV strains were assayed for their drug susceptibilities. Only Met and Leu substitutions at position 145 of the HIV-1 RT conferred multidrug resistance, while other amino acid changes did not. Lower levels of replication of the Gln145Met recombinant strain compared with those of both Gln151Met and wild-type recombinant strains were observed. In in vitro inhibition assays, expression and purification of the recombinant Gln145Met HIV-1 RT revealed a strong loss of catalytic efficiency of the mutated enzyme, as well as significant resistance to both zidovudine and efavirenz. Specific amino acid substitutions in the HIV RT nucleotide-binding pocket might affect both antiretroviral drug recognition and binding and decrease the level of virus replication, possibly by interfering with the enzyme activity. This finding may explain the lower frequency of Gln145Met/Leu mutations observed compared with the frequencies of Gln151Met/Leu mutations and the insertion at position 69 in HAART-experienced patients.
Treatment failure in human immunodeficiency virus (HIV) type 1 (HIV-1)-infected individuals is often associated with the emergence of virus strains resistant to antiretroviral drugs (11, 7). Most of the single resistance mutations observed in the reverse transcriptase (RT) gene of HIV-1 are associated with resistance to specific RT inhibitors. However, selection of appropriate rescue treatment schedules is complicated by wide cross-resistance events between antiretroviral drugs of the same class or even those of different classes. In fact, it is well recognized that single amino acid changes at key positions of the RT enzyme (K103N, for example) can confer high levels of resistance to an entire class of compounds (6). Moreover, defined combinations of mutations (thymidine analog mutations) primarily selected for by a single thymidine analog, such as zidovudine (AZT), have been linked to reduced susceptibilities to other compounds with similar structures (22). Recently, interclass resistance based on a point mutation in RT has been reported (2, 3). However, multidrug resistance is commonly used to refer to a broad spectrum of resistance to antiretroviral drugs. To date, three point mutations in RT have been recognized to be associated with multidrug resistance. The single amino acid substitutions Gln151Met and Gln151Leu (Gln151Met/Leu) as well as the insertion at position 69, confer high levels of resistance to multiple nucleotide or nucleoside RT inhibitors (NRTIs), while the Gln145Met amino acid change appears to be associated with resistance to both NRTIs and nonnucleoside RT inhibitors (NNRTIs) (8, 9, 19, 24, 28).
Population studies by independent research groups have indicated that the prevalence of the Gln151Met mutation and the insertion at position 69 is 1 to 3%, which is substantially lower than those of most of the other major drug resistance-associated mutations reported in highly active antiretroviral therapy (HAART)-experienced patients (12, 26, 27, 28).
The underlying mechanisms by which these mutations confer broad cross-resistance are still unclear. Recent findings indicate that the effect of the Gln151Met mutation and the insertion at position 69 on drug resistance is due to alteration of the nucleoside triphosphate-binding site of the RT enzyme (12, 14, 25, 28). On the other hand, the close proximity of Gln145Met to the Gln151Met mutation site might suggest that a possible alteration of the nucleotide analogs’ recognition ability at the enzyme-binding pocket is associated with this newly discovered multidrug resistance-associated mutation (19).
In the present study, the prevalence of different RT multidrug resistance-associated mutations in a large cohort of HAART-experienced patients was determined. In addition, the impact on drug susceptibility of different changes at position 145 of HIV-1 RT detected in strains from patients who failed HAART was analyzed. Furthermore, the effects of a Gln145Met mutation and a Gln151Met mutation on in vitro viral replication compared with the replication of the wild-type HIV strain were determined. Finally, the effects of different mutations and antiviral drugs on RT enzymatic activity were investigated.
MATERIALS AND METHODS
Frequency of multidrug resistance-associated mutations.
From 1996 to 2003, HIV-1 strains from a group of 3,595 HIV-infected patients who had failed HAART were analyzed for the presence of drug resistance-associated mutations in the RT gene at the Dipartimento di Biologia Molecolare, Universita' di Siena, Siena, Italy, and at the Servizio di Virologia, IRCCS Policlinico San Matteo, Pavia, Italy, to estimate the incidence of mutations at positions 69, 145, and 151.
Plasma HIV RNA levels and CD4+-T-cell counts were routinely determined to monitor the efficacy of HAART. Genotypic analyses were performed as part of the routine clinical examination in the presence of treatment failure.
Generation of HIV-1 recombinant plasmids.
The effects of different amino acid changes at position 145 on antiviral drug resistance were analyzed by introducing selected amino acid changes into the RT gene of an HIV-1 strain from a treatment-naive patient (wild-type virus) by site-directed mutagenesis (10).
The Gln-to-Leu substitution at position 145 was selected to verify whether this change, in addition to the Gln-to-Met change, might confer resistance, similar to what occurs for Gln-to-Met and Gln-to-Leu changes at position 151 (8, 9). In addition, the Gln-to-Cys and Gln-to-Glu changes at position 145 were selected because they represent the most divergent structures compared with the wild-type residue among mutations found in patients receiving HAART. In parallel, the well-known multidrug resistance-associated Gln151Met mutation was inserted into the HIV-1 RT. The correct insertion of the mutations was verified by sequencing the PCR products of the region spanning RT codons 2 to 261. Then, mutagenized RT sequences as well as the wild-type RT gene were inserted into plasmid pHXB2Δ2-261RT (kindly provided by C. Boucher, Utrecht, The Netherlands), which carries the HIV-1 strain HXB2 genetic backbone. Upon transfection of CD4+ HeLa cells with each of the pHXB2Δ2-261RT constructs (Gln145Met/Leu/Cys/Glu, Gln151Met, and wild-type RT), infectious recombinant viruses were obtained.
In detail, 0.5 μg of each plasmid construct was transfected into CD4+ HeLa cells by using the lipofectin reagent, according to the recommendations of the manufacturer (Invitrogen, Groningen, The Netherlands). After 3 days of incubation at 37C°C, the cell supernatants, which contained reconstituted viable recombinant viruses, were collected (20). Quantification of the newly produced recombinant strains was obtained by determination of the HIV RNA copy number (1) in the cell culture supernatants.
HIV-1 drug susceptibility assay.
The susceptibilities of Gln145Leu, Gln145Cys, and Gln145Glu HIV-1 recombinant strains to representative NRTIs, i.e., AZT, stavudine (d4T), lamivudine (3TC), didanosine (ddI), tenofovir (TDF), and abacavir (ABC), as well as two NNRTIs, i.e., efavirenz (EFV) and nevirapine (NVP), were tested as reported previously (20). Briefly, 0.5 μg of each plasmid construct was transfected into 30% confluent HeLa CD4+ cells, and the evaluation of drug susceptibility was coincident with virus reconstitution. In fact, after 6 h of incubation at 37°C following transfection, the cell culture supernatant was removed and replaced with fourfold dilutions of antiretroviral drugs. No-drug controls for each drug dilution were included in each assay. After 72 h of incubation (the time required to perform a single replication cycle in the newly infected HeLa CD4+ cells), the HIV-1 p24 antigen in the cell culture supernatant was quantified (20). Recombinant HIV-1 strains carrying wild-type RT from treatment-naive patients and multidrug resistance-associated Gln145Met and Gln151Met changes were assayed in parallel. The degree of inhibition of viral replication was measured by determining the HIV-1 p24 antigen level (NEN Research Product, Boston, Mass.) in the supernatants of cell cultures and was expressed as the fold increase in the 50% inhibitory concentrations (IC50s) for mutagenized recombinant HIV-1 variants compared with the IC50s for the wild-type recombinant variant. Each test was performed in triplicate.
Replication of multidrug-resistant HIV-1 recombinant strains.
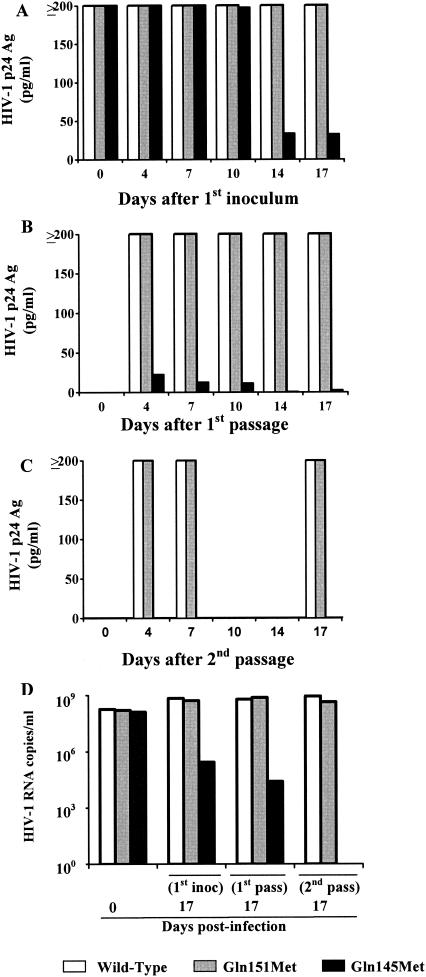
The replication rate of multidrug-resistant HIV-1 Gln145Met and Gln151Met recombinant strains in cell cultures was compared with that of the wild-type recombinant HIV-1 strain. In detail, 10 ml of transfected HeLa CD4+-cell culture supernatants containing 1.8 × 108, 1.5 × 108, and 1.2 × 108 copies of wild-type, Gln151Met, and Gln145Met recombinant HIV strain RNA per ml, respectively, was used to infect aliquots of 5 × 106 phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs) from HIV-seronegative blood donors. After 4 h of incubation, supernatants were removed and infected PBMCs were incubated at 37°C in 10 ml of RPMI 1640 medium (Eurobio, Les Ulis Cedex B, France) supplemented with 20% fetal calf serum (Life Technologies, Ltd., Paisley, Scotland), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10% interleukin-2 (ZeptoMetrix Co., Buffalo, N.Y.), and 5 μg of hydrocortisone (Sigma Chemical Co.) per ml. Then, at day 7 postinfection, the culture supernatant (10 ml) was used to infect fresh PBMCs, as reported above (first passage). A second passage was performed 7 days later. The kinetics of viral replication was determined by measuring two viral replication markers in cell culture supernatants, HIV-1 p24 antigen levels and the RNA copy number. In detail, p24 antigen levels were determined in supernatants collected at 0, 4, 7, 10, 14, and 17 days postinfection; at 4, 7, 10, 14, and 17 days following the first passage; and at 4, 7, and 17 days following the second passage. In addition, the HIV RNA level was determined in cell culture supernatants (1) at days 0 and 17 postinfection and at day 17 of the first and second passages (Fig. 1).
FIG. 1.
Procedures used to evaluate the replication capacity of recombinant HIV strains carrying wild-type and mutated (Q145M and Q151M) RT sequences.
Cloning and expression of recombinant Gln145Met HIV-1 RT for enzyme activity testing.
The region of the HIV-1 pol gene spanning codons 2 to 261 was amplified by PCR from the pHXB2Δ2-261RT (Q145M) construct bearing the Gln145Met mutation. Then, the AccI-PvuII fragment of the RT gene, which encompasses codon 145, was isolated from the amplified fragment and ligated in place of the corresponding wild-type fragment into expression plasmid pUC12/Hisp66(ΔAccI/PvuII) containing the wild-type RT gene (4). The resulting pUC12/Hisp66 (Q145M) expression vector was used for the production of the recombinant His-tagged Gln145Met RT in Escherichia coli. The enzyme was expressed and purified as described previously (16).
Steady-state kinetic measurements.
Reactions were performed under the conditions described previously for the HIV-1 RT RNA-dependent DNA polymerase activity assay (16). Time-dependent incorporation of radioactive dTTP into poly(rA)-oligo(dT) at different substrate concentrations was monitored by removing 25-μl aliquots at the following time points: 0, 15, 30, 60, 120, 240, 480, and 900 s. The initial velocities of the reaction, determined by linear regression analysis of the data, were then plotted against the corresponding substrate concentrations. For determination of the Km and Vmax values, an interval of substrate concentrations from 0.2 to 10 Km was used.
RT inhibition assays.
Reactions were performed under the conditions described for the HIV-1 RT RNA-dependent DNA polymerase activity assay (16). Incorporation of radioactive dTTP into poly(rA)-oligo(dT) was monitored in the presence of increasing fixed amounts of AZT triphosphate (AZTTP; 0.004, 0.01, 0.1, 0.2, 1, and 5 μM), d4T triphosphate (d4TTP; 0.05, 0.1, 1, 5, 10, and 20 μM), NVP (0.1, 1, 5, 25, and 50 μM), and EFV (0.001, 0.01, 0.025, 0.1, 0.2, 0.5, 1, 4, 10, and 20 μM).
RESULTS
Frequency of multidrug resistance-associated mutations.
Among the 3,595 patients in our cohort, the HIV strains from as many as 31 patients (0.86%) showed the insertion at codon 69, and the strains from 85 patients (2.36%) carried the Gln151Met/Leu changes. In addition, the strains from 28 patients (0.78%) showed different mutations at codon 145 (Table 1). However, it was unclear whether all mutations at codon 145 could be associated with drug resistance or not. In fact, all the reported changes were detected together with several other drug resistance-associated mutations (median, 8; range, 1 to 23).
TABLE 1.
Frequency of RT mutations at position 145 compared with those of multidrug resistance-associated mutations at positions 69 and 151 in strains from a cohort of 3,595 patients failing HAART
| RT mutation | No. (%) of patient strains with mutated RT |
|---|---|
| Codon 69 insertion | 31 (0.86) |
| Gln151Met/Leu | 85 (2.36) |
| Gln145Met | 5 (0.14) |
| Gln145Leu | 3 (0.08) |
| Gln145Ile | 1 (0.02) |
| Gln145Glu | 3 (0.08) |
| Gln145Val | 4 (0.11) |
| Gln145Cys | 2 (0.05) |
| Gln145His | 4 (0.11) |
| Gln145Arg | 2 (0.05) |
| Gln145Lys | 3 (0.08) |
| Gln145Asn | 1 (0.02) |
All patients had received HAART over a period of several years. Individual drug exposures lasted from several months to several years. Treatment schedules were different, as were rates of adherence and the reported numbers of toxicity events. HAART was mostly patient tailored; and so no stratification of the patients by treatment schedule, CD4+-T-cell counts or HIV RNA loads at the time of study entry, the duration of HAART, or the number of treatment failures could be achieved in this retrospective analysis. The overall prevalences of any mutation conferring resistance to NRTIs, NNRTIs, and protease inhibitors were 70, 58, and 40% among strains from patients treated with the corresponding drug class, respectively. One-third of the patients had been exposed to all three drug classes, and resistance to two and three classes of drugs was detected in 62 and 35% of the strains from individuals in this subset, respectively.
Drug susceptibilities of HIV-1 mutagenized recombinant strains.
The IC50s of RT inhibitors for recombinant HIV-1 strains carrying the Gln145Met and the Gln145Leu RT mutations showed a marked increase (>12-fold) compared with those for the wild-type recombinant virus. These data confirm the association of Q145M with resistance to both NRTIs and NNRTIs (6) and extend the range of multidrug resistance to TDF and ABC. Interestingly, strains with a different amino acid change (Gln145Leu) showed overlapping levels of resistance to both NRTIs and NNRTIs (Table 2). In contrast, Gln145Cys and Gln145Glu recombinant strains did not show reduced susceptibilities to NRTIs or NNRTIs (Table 2). As expected, the Q151M recombinant strain showed resistance to NRTIs, while it retained susceptibility to NVP. The striking difference in TDF resistance levels associated with the Q145M, Q145L, and Q151M mutations (>2,560-, 375-, and 3.75-fold, respectively) is worth mentioning.
TABLE 2.
Susceptibilities to HIV-1 RT inhibitors of wild-type, Gln145Met/Leu/Cys/Glu, and Gln151Met recombinant virus RTs, as determined by the phenotypic assaya
| Recombinant HIV strain RT mutation | Susceptibility to antiretroviral drugs (mean IC50 [μM] [fold increase])
|
|||||||
|---|---|---|---|---|---|---|---|---|
| AZT | 3TC | d4T | ddI | TDF | ABC | EFV | NVP | |
| Wild typeb | 0.04 ± 0.03 | 0.7 ± 0.36 | 0.9 ± 0.26 | 2.06 ± 1.5 | 0.02 ± 0.01 | 8 ± 5.2 | 0.002 ± 0.002 | 0.21 ± 0.12 |
| Mutagenized | ||||||||
| Gln145Met | >2 (>50) | >16 (>22.9) | >16 (>17.7) | >40 (>19.4) | >51.2 (>2,560) | >96 (>12) | >0.3 (>150) | >24 (>114.2) |
| Gln145Leu | >2 (>50) | >16 (>22.9) | >16 (>17.7) | >40 (>19.4) | 7.5 ± 3.75 (375) | >96 (>12) | >0.3 (>150) | >24 (>114.2) |
| Gln145Cys | 0.03 ± 0.02 (0.8) | 0.8 ± 0.4 (1.1) | 1 ± 0.28 (1.1) | 2.2 ± 1.58 (1.06) | <0.02 (<1) | 6 (0.75) | 0.003 ± 0.001 (1.5) | 0.14 ± 0.08 (0.7) |
| Gln145Glu | 0.06 ± 0.04 (1.5) | 1.3 ± 0.66 (1.9) | 1.3 ± 0.36 (1.4) | 2.1 ± 1.51 (1.01) | <0.02 (<1) | 5.5 (0.68) | 0.0007 ± 0.0004 (0.4) | 0.15 ± 0.08 (0.7) |
| Gln151Met | 3.5 ± 2.62 (87.5) | NDc | >24 (26.6) | ND | 0.075 ± 0.03 (3.75) | ND | ND | 0.35 ± 0.19 (1.66) |
Data are from triplicate experiments.
Polymorphism in the RT gene of the wild-type recombinant virus (Met16Thr, Arg83Lys, Lys101Arg, Lys166Arg, Arg211Lys).
ND, not done.
In vitro replication of mutant HIV-1 recombinant viruses and in vivo estimate of replicative capacity of Q145M/L variants.
Following infection with Gln145Met, Gln151Met, and wild-type recombinant strains, the p24 antigen levels in the cell culture supernatant at the baseline (time zero) were comparable for all viruses, thus confirming the comparable viral inocula (Fig. 2). However, while the p24 antigen level was consistently at a plateau in supernatants from cultures infected with Gln151Met and wild-type recombinant strains, it rapidly declined in supernatants from cultures infected with the Gln145Met recombinant strain, until it became negative at day 4 of the second passage.
FIG. 2.
(A to C) p24 antigen (Ag) levels in the supernatants of cell cultures infected with recombinant HIV-1 strains carrying wild-type and mutagenized RT genes. (A) First virus inoculum; (B) first passage; (C) second passage. (D) HIV-1 RNA levels in the supernatants of cell cultures infected with recombinant HIV-1 strains carrying wild-type and mutagenized RT genes (inoc, inoculum; pass, passage).
Quantification of HIV-1 RNA in culture supernatants of the three recombinant HIV strains showed that they had similar kinetics. In fact, while HIV RNA levels were comparable at time zero (Fig. 2), those in supernatants from cell cultures infected with the Gln151Met and wild-type recombinant strains were 2 to 3 log10 higher than those found in supernatants from cell cultures infected with the Gln145Met recombinant strain at day 17 postinfection and at day 17 of the first passage. Moreover, HIV RNA levels in the supernatants of cell cultures infected with the Gln151Met and wild-type recombinant strains at day 17 of the second passage reached 4.3 × 108 and 8.7 × 108 copies/ml, respectively, whereas the supernatants of cultures infected with the Gln145Met recombinant strain were negative for HIV RNA (Fig. 2).
From the analysis of plasma HIV RNA levels of the patients in the cohort, a trend associating a reduced HIV load with the presence of Q145M/L was observed. In fact, patients harboring Q145M/L mutants (n = 8) showed plasma HIV RNA levels (mean, 19,594 ± 22,962 copies/ml; median, 9,537 copies/ml; range, 100 to 62,626 copies/ml) lower than those in patients who had failed HAART in the absence of mutated Q145 (n = 3,257; mean, 124,691 + 602,684 copies/ml; median 12,400 copies/ml; range, 30 to 19,000,000 copies/ml). However, the great disproportion between patient strains without changes at position 145 and those with the Q145M/L mutation is likely responsible for the lack of statistical significance (P = 0.5, Mann-Whitney U test).
Enzymatic characterization of recombinant Q145M RT.
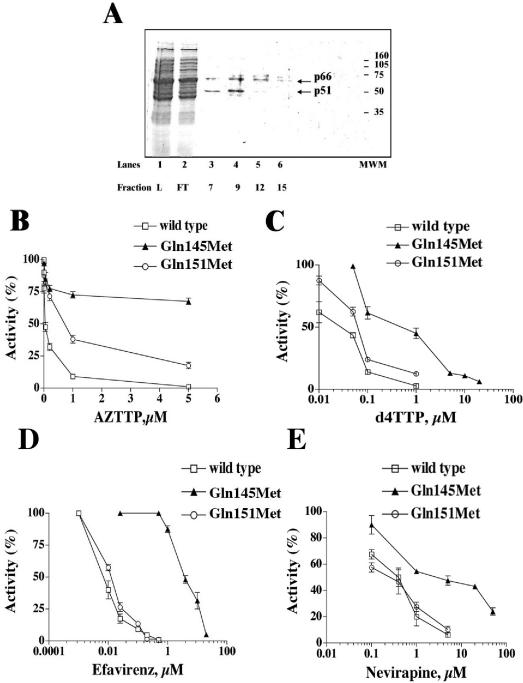
Recombinant His-tagged Gln145Met RT was produced in E. coli and was purified to near homogeneity. Figure 3A shows the results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the fractions eluted from the last purification step. As judged from Coomassie staining, the protein was >90% pure. Fraction 9 was used for further enzymatic characterization. The kinetic parameters (Km, kcat, and kcat/Km) for nucleotide utilization were determined under RNA-dependent DNA synthesis conditions and are listed in Table 3. For comparison, the same values were also determined for the wild-type and Gln151Met mutant RTs. The Gln145Met mutation resulted in a 200-fold reduction in the catalytic rate (kcat) compared with that of the wild-type enzyme. On the contrary, the Gln151Met mutant RT showed only a fourfold reduction in the kcat value compared with that of the wild type. Next, the sensitivities of the wild-type, Gln145Met, and Gln151Met RTs to AZTTP, EFV, d4TTP, and NVP were tested. As shown in Fig. 3B, both the Gln145Met and Gln151Met mutants had significant resistance to AZTTP. However, the Gln145Met mutant showed high-level resistance to EFV, d4TTP, and NVP, contrary to the Gln151Met mutant, which displayed a sensitivity similar to that of the wild-type enzyme (Fig. 3C to E). In summary, the biochemical properties of the RT carrying the Gln145Met mutation are comparable to the phenotypes of the viruses carrying the same mutation in the pol gene, providing a molecular explanation for both the multidrug resistance and the reduced fitness of the Gln145Met mutant viruses.
FIG. 3.
(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the recombinant Q145M RT eluted from a nickel-nitrilotriacetic acid-agarose column. L, loading; FT, flowthrough; MWM, molecular weight markers. The two RT subunits are indicated with arrows. (B and C) Sensitivity of wild-type RT and the Q145M and Q151M mutants to AZTTP (B) and d4TTP (C). Inhibition assays were performed as described in Materials and Methods. (D and E) Sensitivities of wild-type RT and the Q145M and Q151M mutants to EFV (D) and NVP (E).
TABLE 3.
Kinetic parameters for nucleotide incorporation during RNA-dependent DNA synthesis catalyzed by wild-type HIV-1 RT as well as Q151M and Q145M mutant RTsa
| Kinetic parameter | Parameter value (mean [SD])
|
||
|---|---|---|---|
| Wild type | Q145M | Q151M | |
| Km (μM) | 3.9 (±0.5) | 5.3 (±0.6) | 8.9 (±1) |
| kcat (s−1) | 0.2 (±0.02) | 0.001 (±0.0002) | 0.05 (±0.001) |
| kcat/Km (μM−1s−1) | 0.05 (±0.01) | 1.8 (±0.2) × 10−4 | 0.006 (±0.001) |
Data are from triplicate experiments.
DISCUSSION
One of the major problems in AIDS treatment is the ability of HIV-1 to develop resistance to antiretroviral compounds. Recently, to increase virus suppression and delay or avoid the development of drug-resistant HIV-1 variants, aggressive combination chemotherapy with multiple antiviral drugs has been used (17, 23). Specifically, RT, a key enzyme in the replication cycle of HIV-1, has become a major target for antiretroviral therapy (5, 18). However, antivirals for which the RT gene is the target also induce the appearance of mutations that confer resistance to single or multiple drugs. Mutations that confer multidrug resistance are less frequent than mutations that confer resistance to single drugs or a few related drugs. The lower frequency of multidrug resistance mutations at positions 151 and 69 does not appear to be completely explained by the reduced viral fitness of the HIV-1 strains carrying these mutations, since the replicative capacity of mutant strains appears to be comparable to or marginally increased (13, 15) as well as reduced (21) compared with the replicative capacity of wild-type virus. Recently, a new Gln145Met mutation conferring resistance to all RT inhibitors was reported in a patient who had failed HAART (19).
In the present study, the type and frequency of RT mutations at codon 145 were analyzed in strains from a large cohort of patients who had failed HAART and were compared to those of other mutations that confer multidrug resistance (Gln151Met/Leu and an insertion at codon 69). The results showed a wide variety of amino acid changes at codon 145 with a frequency (0.77%) lower than that of the Glu151Met/Leu mutation (2.36%) but comparable to that of the insertion at codon 69 (0.86%). However, a definite association between the presence of mutations at codon 145 and multidrug resistance could not be envisioned, since all mutants simultaneously carried multiple resistance-associated mutations. Thus, the impact of different amino acid changes at codon 145 in determining antiretroviral drug resistance was investigated. The results showed that only a subset of mutations at this position determine drug resistance. In fact, in addition to the reported Gln145Met change, the Gln145Leu substitution was also found to sharply reduce the susceptibilities to both NRTIs and NNRTIs, while other changes (Gln145Cys/Glu) had no impact on drug susceptibility. Interestingly, multidrug resistance appears to be related to the replacement of a Gln residue with a Met or a Leu residue at either position 145 or 151.
Initially, a possible explanation for the lower frequency of resistance mutations at codon 145 compared with those at codons 151 (10.7-fold) and 69 (3.9-fold) was that Gln145Met/Leu mutants could have reduced fitness. This hypothesis was confirmed by the finding that Gln145Met recombinant virus showed a reduced replicative capacity compared to those of Gln151Met and wild-type recombinant viruses. In contrast, the replicative capacities of Gln151Met and wild-type recombinant virus were comparable, as previously reported by others (15). Similarly, recombinant RT carrying the Gln145Met mutation was found to be 200-fold less efficient than the wild-type enzyme in catalyzing RNA-dependent DNA synthesis, whereas the Gln151Met mutation had a much weaker impact on the catalytic efficiency (fourfold reduction). A defect in the viral replication of Q145M/L mutants can also be hypothesized on the basis of the lower mean plasma HIV RNA levels associated with the presence of such viral variants in vivo compared with the levels associated with the presence of other variants conferring drug resistance and inducing HAART failure. However, this observation is not conclusive for two main reasons: (i) the great disproportion of patient strains with and without these amino acid changes and (ii) the impacts of other mutations in RT that potentially compensate for the effect of Q145M/L on viral replication. Indeed, Q145M/L provides a great selective advantage together with a dramatic impairment of the replicative capacity. Thus, it appears to be conceivable that Q145M/L variants are frequently selected in vivo during treatment but are systematically outgrown by fitter variants. In this scenario, Q145M/L variants could emerge only after they acquire additional mutations that compensate for the replicative defect. Indeed, all Q145M/L variants showed high numbers of additional RT mutations.
Mutations at codons 145, 151, and 69 confer multidrug resistance and alter key residues in the nucleotide-binding pocket of the RT enzyme active site. However, the effects of single amino acid substitutions at codons 145 and 151 on resistance to antiretroviral drugs and viral fitness appear to differ. In fact, only some mutations at position 145 appear to be associated with a wider spectrum of resistance to both NRTIs and NNRTIs and with a marked impairment of crucial RT functions which affect the replicative capacity of the virus.
In conclusion, the selection of virus strains with mutations in the nucleotide-binding pocket is a relatively rare event. However, it appears to entail a number of virologic, biochemical, and possibly, clinical implications.
Acknowledgments
We thank C. Boucher for providing plasmid pHXB2Δ2-261RT, U. Hübscher and S. H. Hughes for the coexpression vectors pUC12/Hisp66, and Linda D'Arrigo for revision of the English.
This work was partly supported by the Ministero della Sanitá, Istituto Superiore di Sanitá, Programma Nazionale di Ricerca sull'AIDS (grants 30D.36 and 30D.82), IRCCS Policlinico San Matteo Ricerca Finalizzata (grant 126), and Ricerca Corrente (grant 80207) and by EC Project LSHG-CT-2003-503480 TRIoH. R.C. was supported by an ICGEB fellowship.
REFERENCES
- 1.Bagnarelli, P., A. Valenza, S. Menzo, A. Manzin, G. Scalise, P. E. Varaldo, and M. Clementi. 1994. Dynamics of molecular parameters of human immunodeficiency virus type 1 activity in vivo. J. Virol. 68:2495-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldanti, F., S. Paolucci, G. Maga, N. Labó, U. Hübscher, A. Y. Skoblov, L. Victorova, S. Spadari, L. Minoli, and G. Gerna. 2003. Nevirapine-selected mutations Y181I/C of HIV-1 reverse transcriptase confer cross-resistance to stavudine. AIDS 17:1568-1570. [DOI] [PubMed] [Google Scholar]
- 3.Blanca, G., F. Baldanti, S. Paolucci, A. Y. Skoblov, L. Victorova, U. Hubscher, G. Gerna, S. Spadari, and G. Maga. 2003. Nevirapine resistance mutation at codon 181 of the HIV-1 reverse transcriptase confers stavudine resistance by increasing nucleotide substrate discrimination and phosphorolytic activity. J. Biol. Chem. 278:15469-15472. [DOI] [PubMed] [Google Scholar]
- 4.Boyer, P. L., J. Ding, E. Arnold, and S. H. Hughes. 1994. Drug resistance of human immunodeficiency virus type 1 reverse transcriptase: subunit specificity of mutations that confer resistance to nonnucleoside inhibitors in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 38:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Clercq, E. 1992. HIV inhibitors targeted at the reverse transcriptase. AIDS Res. Hum. Retrovir. 8:119-134. [DOI] [PubMed] [Google Scholar]
- 6.Deeks, S. G. 2001. Nonnucleoside reverse transcriptase inhibitor resistance. J. Acquir. Immune Defic. Syndr. 26:S25-S33. [DOI] [PubMed] [Google Scholar]
- 7.De Gruttola, V., L. Dix, R. D'Aquila, D. Holder, A. Phillips, M. Ait-Khaled, J. Baxter, P. Clevenbergh, S. Hammer, R. Harrigan, D. Katzenstein, R. Lanier, M. Miller, M. Para, S. Yerly, A. Zolopa, J. Murray, A. Patick, V. Miller S. Castillo, L. Pedneault, and J. Mellors. 2000. The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir. Ther. 5:41-48. [DOI] [PubMed] [Google Scholar]
- 8.Dunne, A. L., F. M. Mitchell, S. K. Coberly, N. S. Hellmann, J. Hoy, A. Mijch, C. J. Petropoulos, J. Mills, and S. M. Crowe. 2001. Comparison of genotyping and phenotyping methods for determining susceptibility of HIV-1 to antiretroviral drugs. AIDS 15:1471-1475. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales, M. J., R. N. Machekano, and R. W. Shafer. 2001. Human immunodeficiency virus type 1 reverse-transcriptase and protease subtypes: classification, amino acid mutation patterns, and prevalence in a northern California clinic-based population. J. Infect. Dis. 184:998-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi, R. 1990. Recombinant PCR, p. 176-183. In T. J. White (ed.), PCR protocols. A guide to method and applications. Academic Press, Inc., San Diego, Calif.
- 11.Hirsch, M. S., B. Conway, R. T. D'Aquila, V. A. Johnson, F. Brun-Vezinet, B. Clotet, L. M. Demeter, S. M. Hammer, D. M. Jacobsen, D. R. Kuritzkes, C. Loveday, J. W. Mellors, S. Vella, and D. D. Richman. 1998. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. JAMA 279:1984-1991. [DOI] [PubMed] [Google Scholar]
- 12.Iversen, A. K. N., R. W. Shafer, K. Wehrly, M. A. Winters, J. I. Mullins, B. Chesebro, and T. C. Merigan. 1996. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J. Virol. 70:1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 73:5356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larder, B. A., S. Bloor, S. D. Kemp, K. Hertogs, R. L. Desmet, V. Miller, M. Sturmer, S. Staszewski, J. Ren, D. K. Stammers, D. I. Stuart, and R. Pauwels. 1999. A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance. Antimicrob. Agents Chemother. 43:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda, Y., D. J. Venzon, and H. Mitsuya. 1998. Altered drug sensitivity, fitness, and evolution of human immunodeficiency virus type 1 with pol gene mutations conferring multi-dideoxynucleoside resistance. J. Infect. Dis. 177:1207-1213. [DOI] [PubMed] [Google Scholar]
- 16.Maga, G., M. Amacker, N. Ruel, U. Hubscher, and S. Spadari. 1997. Resistance to nevirapine of HIV-1 reverse transcriptase mutants: loss of stabilizing interactions and thermodynamic or steric barriers are induced by different single amino-acid substitutions. J. Mol. Biol. 274:738-747. [DOI] [PubMed] [Google Scholar]
- 17.Maldarelli, F. 2003. HIV-1 fitness and replication capacity: what are they and can they help in patient management? Curr. Infect. Dis. Rep. 5:77-84. [DOI] [PubMed] [Google Scholar]
- 18.Mansky, L. M. 2003. Mutagenic outcome of combined antiviral drug treatment during human immunodeficiency virus type 1 replication. Virology 307:116-121. [DOI] [PubMed] [Google Scholar]
- 19.Paolucci, S., F. Baldanti, M. Tinelli, G. Maga, and G. Gerna. 2003. Detection of a new HIV-1 reverse transcriptase mutation (Q145M) conferring resistance to nucleoside and non-nucleoside inhibitors in a patient failing highly active antiretroviral therapy. AIDS 17:924-927. [DOI] [PubMed] [Google Scholar]
- 20.Paolucci, S., F. Baldanti, M. Zavattoni, and G. Gerna. 2004. Novel recombinant phenotypic assay for clonal analysis of reverse transcriptase mutations conferring drug resistance to HIV-1 variants. J. Antimicrob. Chemother. 53:766-771. [DOI] [PubMed] [Google Scholar]
- 21.Quinones-Mateu, M. E., M. Tadele, M. Parera, A. Mas, J. Weber, H. R. Rangel, B. Chakraborty, B. Clotet, E. Domingo, L. Menendez-Arias, and M. A. Martinez. 2002. Insertions in the reverse transcriptase increase both drug resistance and viral fitness in a human immunodeficiency virus type 1 isolate harboring the multi-nucleoside reverse transcriptase inhibitor resistance 69 insertion complex mutation. J. Virol. 76:10546-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross, L., A. Scarsella, S. Raffanti, K. Henry, S. Becker, R. Fisher, Q. Liao, A. Hirani, N. Graham, M. St Clair, J. Hernandez, and the NZT40012 Study Team. 2001. Thymidine analog and multinucleoside resistance mutations are associated with decreased phenotypic susceptibility to stavudine in HIV type 1 isolated from zidovudine-naive patients experiencing viremia on stavudine-containing regimens. AIDS Res. Hum. Retrovir. 17:1107-1115. [DOI] [PubMed] [Google Scholar]
- 23.Sankatsing, S. U., R. M. van Praag, R. P. van Rij, R. Rientsma, J. M. Lange, J. M. Prins, and H. Schuitemaker. 2003. Dynamics of the pool of infected resting CD4 HLA-DR-T lymphocytes in patients who started a triple class five-drug antiretroviral regimen during primary HIV-1 infection. Antivir. Ther. 8:137-142. [PubMed] [Google Scholar]
- 24.Shirasaka, T., M. F. Kavlick, T. Ueno, W. Y. Gao, E. Kaojima, M. L. Alcaide, S. Chokekijchai, B. M. Roy, E. Arnold, R. Yarchoan, and H. Mitsuya. 1995. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc. Natl. Acad. Sci. USA 92:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluis-Cremer, N., D. Arion, and M. A. Parniak. 2000. Molecular mechanisms of HIV-1 resistance to nucleoside reverse transcriptase inhibitors (NRTIs). Cell. Mol. Life Sci. 57:1408-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamelet, C. N., N. Yahi, C. Tourres, P. Colson, A. M. Quinson, I. Poizot-Martin, C. Dhiver, and J. Fantini. 2000. Multidrug resistance genotypes (insertion in the β3-β4 finger subdomain and MDR mutations) of HIV-1 reverse transcriptase from extensively treated patients: incidence and association with other resistance mutations. Virology 270:310-316. [DOI] [PubMed] [Google Scholar]
- 27.Van Vaerenbergh, K., K. Van Laethem, J. Albert, C. A. Boucher, B. Clotet, M. Floridia, J. Gerstoft, B. Hejdeman, C. Nielsen, C. Pannecouque, L. Perrin, M. F. Pirillo, L. Ruiz, J. C. Schmit, F. Schneider, A. Schoolmeester, R. Schuurman, H. J. Stellbrink, L. Stuyver, J. Van Lunzen, B. Van Remoortel, E. Van Wijngaerden, S. Vella, M. Witvrouw, S. Yerly, E. De Clercq, J. Destmyer, and A. M. Vandamme. 2000. Prevalence and characteristics of multinucleoside-resistant human immunodeficiency virus type 1 among European patients receiving combinations of nucleoside analogues. Antimicrob. Agents Chemother. 44:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winters, M. A., K. L. Coolley, Y. A. Girard, D. J. Levee, H. Hamdan, R. W. Shafer, D. A. Katzenstein, and T. C. Merigan. 1998. A 6-base pair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Investig. 102:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]