Abstract
Background
To explore possible markers of developmental immunotoxicity, we prospectively examined 56 children to determine associations between exposures to methylmercury and persistent organic pollutants since birth and the comprehensive differential counts of white blood cells (WBC) at age 5 years.
Materials and methods
Extended differential count included: neutrophils, eosinophils, basophils, lymphocytes (including T cells, NK cells, and B cells), and monocytes. Organochlorine compounds (OCs) including polychlorinated biphenyls (PCBs) and pesticides, five perfluoroalkyl substances (PFASs), and total mercury (Hg) were measured in maternal (n=56) and children’s blood at 18 months (n=42) and 5 years (n=56). We constructed latent functions for exposures at three different ages using factor analyses and applied structural equation models adjusted for covariates.
Results
Prenatal mercury exposure was associated with depleted total WBC, especially for lymphocytes, where a one standard deviation (SD) increase in the exposure was associated with a decrease by 23% SD (95% CI: −43, −4) in the cell count. Prenatal exposure to OCs was marginally associated with decreases in neutrophil counts. In contrast, the 5-year PFASs concentrations were associated with higher basophil counts (B= 46% SD, 95% CI: 13, 79). Significantly reduced subpopulations of lymphocytes such as B cells, CD4-positive T helper cells and CD4 positive recent thymic emigrants may suggest cellular immunity effects and dysregulation of T-cell mediated immunity.
Conclusion
Developmental exposure to environmental immunotoxicants appears to have different impacts on WBC counts in childhood.
Keywords: Developmental exposure delayed effects, Immunotoxicity, Mercury, Perfluoroalkyl substances, Polychlorinated biphenyls, White blood cell count
1. Introduction
Exposure to environmental pollutants may significantly affect the immune system and cause immunologic suppression in children [1]. The developing immune system appears to be highly sensitive to toxic effects, and experimental animal studies and in vitro assays have shown immunomodulatory effects of persistent organic pollutants (POPs), especially polychlorinated biphenyls (PCBs) [2-5], organochlorine pesticides (OCPs) [5-7], and perfluoroalkyl substances (PFASs) [8-10], as well as mercury and other metal compounds [11, 12]. These immunotoxicants are ubiquitous, most are persistent, and they are widely detected in children and adults from the general population [13].
In humans, recent epidemiologic studies have reported immunomodulatory effects of mercury [14-16] and POPs [13, 17, 18] in children and adults. For example, higher exposures to PFASs were associated with a reduction of the humoral immune response to booster vaccination [17-19], whereas mercury exposures were associated with increased pro-inflammatory cytokines, antinuclear and antinucleolar autoantibodies and decreased anti-inflammatory cytokines [20]. Developmental exposure to PCBs and OCPs has also been shown to modulate the immune system in infants [21, 22], children [23], and adults [13]. Higher 6-month infant concentrations of PCB-153 and DDE (dichlorodiphenyldichloroethylene) were strongly associated with lower 6-month BCG-specific antibody levels [24], and higher maternal and infant PCB concentrations have been associated with a reduced volume of the infant thymus, the site of T-cell maturation [25]. Moreover, early-life environmental PCB exposure was associated with fluctuations in major lymphocyte subsets [26], and affected the dynamics of cell surface lymphocyte receptor expression [27]. Overall, previous studies provide compelling evidence of the detrimental effects of exposure to environmental toxicants on the immune system, including immune cell counts, cytokine responses, and levels of specific antibodies [28]. However, most of the human studies on immunotoxicity of POPs and mercury lack a prospective design with age-related exposure profiles, and no study has so far attempted to examine the potential joint effect of different POPs on the immune system development. These complexities also indicate a need for new statistical tools to disentangle the role of multiple exposures on multiple immune system markers [29].
The white blood cell (WBC) count has been proposed as an immunologic end point to detect immunotoxic effects of environmental contaminants in prospective epidemiologic studies [30]. In humans, WBC counts have proven useful in signaling clinically relevant hematologic changes that may result in clinically identifiable immune disorders [31], with absolute numbers of WBC providing biologically more reliable information than percentages [32].
In the present study, we explored the associations between exposures to POPs and methylmercury measured at birth and at ages 18 months and 5 years in regard to extended WBC differential counts at age 5.
2. Materials and methods
2.1. Study population
The present study focuses on fifty-six children from a cohort of consecutive births formed in the Faroe Islands to include 490 children during an 18-month period between late 2007 and early 2009. Whole blood was taken from the cord and from the mother two weeks after parturition. In addition, maternal hair at parturition was also sampled. Blood and hair were obtained from the child at successive clinical examinations at ages 18 months and 5 years. At age 5 years, children underwent detailed examinations including immune system biomarkers. On dates when fresh blood samples could be transported to the immunological laboratory in Denmark, we obtained maternal consent for a subgroup of 56 children to draw additional blood for the purposes of the present study. Standard questionnaires were used to record past medical history, current health, social factors, and nutritional habits during and before pregnancy. Relevant obstetric information, including birth weight, parity and maternal age were abstracted from hospital’s medical records.
The study protocol was approved by the ethical review committee serving the Faroe Islands and by the institutional review board at the Harvard T.H. Chan School of Public Health, and written informed consent was obtained from all mothers.
2.2. WBC counts and lymphocyte subsets
The total number of WBCs, neutrophils, eosinophils, lymphocytes, and monocytes were recorded by standard procedures using the ABX Pentra DX 120 (Horiba, United Kingdom/Germany). Furthermore, T-cell (CD3), T-helper cells (CD4), T-cytotoxic cells (CD8), B-lymphocytes (CD19), NK (CD16/56) cells and CD4+ recent thymic emigrants (CD4-RTE) absolute counts were performed by a single-platform no-lyse-no-wash procedure. Fifty μl EDTA anti-coagulated peripheral blood were incubated in TRUCount tubes (BD Biosciences, Denmark) with a panel of conjugated monoclonal antibodies. The following combinations of antibodies were used to characterize T cells as CD4 T cells (CD3-PerCP (clone SK7), CD4-FITC (clone SK3), CD8 T cells (CD3-PerCP (clone SK7), CD8-PE (clone SK1)) and CD4-RTE (recent thymic immigrant) cells as (CD3-ECD (clone UCHT1), CD4-PC7 (clone SFCT12T4D11), CD31-PE (clone WM59), CD45RA-FITC (clone L48), and CD45RO-PC5 (clone UCHL1), NK cells as (CD45-PerCP (clone 2D1), CD16/56-PE (clone B73.1 + NCAM16.2), and CD3-FITC (clone SK7)), and B cells as (CD19-PE (clone 4G7), CD45-PerCP (clone 2D1)) (BD Biosciences, Beckman Coulter and AbD Serotec, Denmark). The samples were measured on FC500 flow cytometer (Beckman Coulter, Denmark). The laboratory participates in the quality assurance program by National External Quality Assessment Site (NEQAS).
2.3. Exposure assessment
PCBs, OCPs (p,p’ and o,p isomers of dichlorodiphenyltrichloroethane [DDT] and DDE, and hexachlorobenzene [HCB]) and PFASs (perfluorohexane sulfonic acid [PFHxS], perfluorooctanoic acid [PFOA], perfluorooctane sulfonate [PFOS], perfluorononanoic acid [PFNA], and perfluorodecanoic acid [PFDA]) were measured in cord (n=56), maternal (n=56) and children’s serum at ages 18 months (n=42) and 5 years (n=56). In addition, as measures of methylmercury exposure, mercury (Hg) was measured in cord blood, maternal blood and hair, and in child blood and hair at age 5 years.
Serum analyses of DDE, DDT, HCB, and PCBs were carried out by the same procedure for all samples using a gas chromatograph with electron capture detection at Department of Environmental Health, University of Southern Denmark [23]. To avoid problems with PCB congeners not assessed or below the detection limit, ΣPCB was calculated as the sum of major congeners 138, 153, and 180 multiplied by 2 [33]. DDE at 18 months and 5 years, and DDT at 18 months were detected in less than 50% of samples and were therefore not further considered. The PFASs were measured using online solid-phase extraction and analyzed using high-pressure liquid chromatography with tandem mass spectrometry [34]. The accuracy and reliability of the data was ensured by including, in each analytical series, quality control serum samples, calibration standards, and reagent and serum blanks. Within-batch and between-batch coefficient of variations were better than 3.0% and 5.2% for all analytes. Total mercury analyses in hair and blood were performed on a Direct Mercury Analyzer (DMA-80, Milestone Inc, Sorrisole, Italy), with imprecision below 4%. All POP results below the limit of detection (LOD) were replaced by the LOD divided by √2.
2.4. Statistical analyses
WBC, Hg, and POP concentrations were all log-transformed (base 2) before they entered the models to approximate a normal distribution. One child had very high WBC count (Total=16.4 ×109 cells/L) due to a fever and was consequently removed from the analyses. Due to the large number of exposure variables, we used structural equation models (SEM) to assess the covariate-adjusted associations between prenatal, 18-month, and 5-year exposures and the extended WBC counts at 5 years.
We first conducted an exploratory factor analysis (EFA) without a priori information on the structure and correlations in the data, followed by a confirmatory factor analysis (CFA) to categorize exposures into a small number of factors, thereby reducing the extent of multiple comparisons. For prenatal exposures, this method resulted in three factors explaining 65% of the variance. Factor 1 included the 5 PFASs, factor 2 included mercury indicators (i.e., cord blood, maternal blood and hair concentrations), and factor 3 included HCB, PCB, DDE, o,p’-DDT, and p,p’-DDT. For 18-month exposures, the method yielded 2 factors explaining 65% of the variance. Factor 1 included the 5 PFASs, whereas factor 2 included HCB, PCB, o,p’-DDT, and p,p’-DDE. At 5 years, three factors explained 56% of the variance. Factor 1 included the 5 PFASs; factor 2 included child hair-Hg and blood-Hg concentrations, whereas factor 3 included HCB, PCB, p,p’-DDE, and p,p’-DDT. For the sake of simplicity, factors 1, 2, and 3 will be referred to as PFAS, mercury (Hg), and organochlorine (OC) functions, respectively. Correlations between the latent functions ranged between 0.07 (for 5-year Hg and OC) and 0.79 (for 18-month PFAS and OC). Although these correlations show good discriminant validity for the constructed factors and are considered reasonable to avoid multicollinearity issues in SEM analyses, the small sample size precluded the simultaneous analyses of all the factors in a single model. We therefore completed separate SEM analyses linking each latent exposure function with each WBC count. For each age, the exposure factors were regressed on the WBC counts, while adjusting for covariates. Models for prenatal exposures were adjusted for age at age-5 examination (months), sex, parity (no older sibling / 1 or more older siblings), and maternal smoking during pregnancy; whereas models for 18-month and 5-year exposures were further adjusted for birth weight (g) and duration of breastfeeding (< or ≥ 6 months). Missing data for breastfeeding (n=2) were compensated by Full Information Maximum Likelihood estimation.
3. Results
3.1. Descriptive results
The characteristics of the study population are presented in Table 1. The mean age of children was 60 months, with fewer boys than girls (39% vs 61%). Most of the children had an older sibling (71%), and 41% were breastfed for 6 months or longer (Table 1). Apart from one child with a total WBC count below 4 × 109 cells/L, results from all children were within the normal range (4-11 × 109 cells/L) [35]. The total WBC count did not differ in regard to age, sex, parity, maternal smoking during pregnancy, birth weight, or breastfeeding duration.
Table 1.
Characteristics of the study population (n=55)
| Population characteristic | Mean (SD) or n (%) |
|---|---|
| Age, months | 60.1 (0.8) |
| Birth weight, grams | 3684 (537) |
| Maternal age at delivery, years | 30.6 (5.7) |
| Sex | |
| Boys | 22 (40) |
| Girls | 33 (60) |
| Parity | |
| No siblings | 16 (29) |
| ≥ 1 siblings | 39 (71) |
| Maternal smoking during pregnancy | |
| No | 44 (80) |
| Yes | 11 (20) |
| Breastfeeding duration | |
| < 6 months | 32 (60) |
| ≥6 months | 21 (40) |
| Missing | 2 |
| Differential White blood cells count (109 Cells/L) | |
| Neutrophils | 3.13 (1.75) |
| Basophils | 0.10 (0.13) |
| Lymphocytes | 2.57 (0.84) |
| CD3 | 2.00 (0.62) |
| CD4 | 1.11 (0.40) |
| CD8 | 0.75 (0.26) |
| CD4-RTE | 0.55 (0.24) |
| B Cells | 0.56 (0.24) |
| NK Cells | 0.16 (0.07) |
| Monocytes | 0.43 (0.15) |
| Eosinophils | 0.31 (0.25) |
| Total WBC | 6.54 (2.12) |
3.2. Exposures
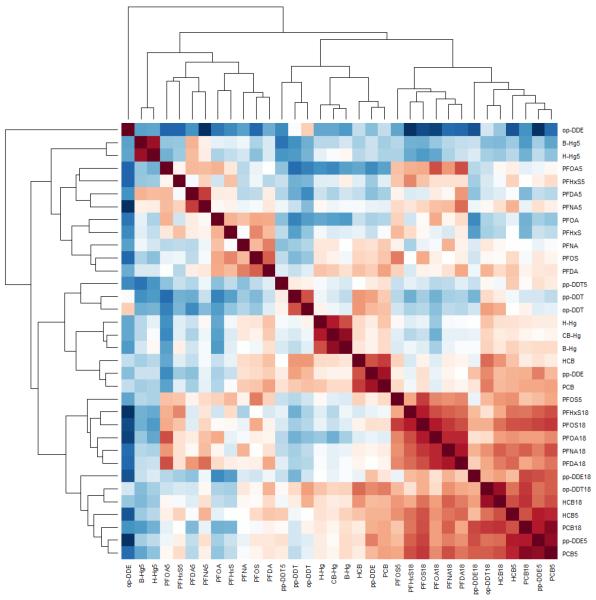
Among the PFASs, PFOS showed the highest serum concentrations at all ages, followed by PFOA and PFHxS, while PFNA and PFDA occurred in the lowest concentrations (Table 2). Between-PFAS correlation coefficients for maternal serum or child at age 18-month and 5-year ranged from 0.29 to 0.87 and were higher at 18 months and lower at 5 years. Within-PFAS correlation coefficients ranged between 0.28 and 0.79, and were generally higher for the child’s 18-month and 5-year serum samples and lower between maternal and the child’s postnatal serum samples (Figure 1). For methylmercury exposures, the higher cord blood concentrations were closely correlated with those in maternal blood (0.92). Blood- and hair-Hg concentrations were also well correlated on each occasion (0.73 for maternal concentrations and 0.89 for child concentrations at age 5). Regarding POPs, PCBs showed the highest within correlation coefficient between serum concentrations at 18 months and 5 years. PCBs also showed high correlations with the other POPs and PFASs at each age (Figure 1). PFAS concentrations at all ages were generally lower in mothers with a previous childbirth, whereas POPs at 18 months were lower in children breastfed for 6 months or more.
Table 2.
Descriptive statistics of maternal and child’s exposures (μg/L)
| Maternal exposures (n=55) | 18-month exposures (n=41) | 5-year exposures (n=53) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # < LOD |
GM | GSE | Range | # < LOD |
GM | GSE | Range | # < LOD |
GM | GSE | Range | |
| PFOA | 0 | 1.470 | 0.100 | 0.46 - 4.13 | 0 | 3.580 | 0.391 | 0.85 - 22.52 |
0 | 2.596 | 0.128 | 1.44 - 13.34 |
| PFNA | 0 | 0.789 | 0.056 | 0.30 - 4.35 | 0 | 1.244 | 0.135 | 0.23 - 7.10 |
0 | 1.358 | 0.095 | 0.38 - 5.75 |
| PFDA | 0 | 0.298 | 0.020 | 0.12 - 0.91 | 0 | 0.347 | 0.033 | 0.1 - 3.29 | 0 | 0.357 | 0.024 | 0.11 - 1.72 |
| PFHxS | 1 | 0.240 | 0.022 | <LOD - 0.86 |
3 | 0.247 | 0.045 | <LOD - 3.77 |
0 | 0.372 | 0.032 | 0.14 - 3.25 |
| PFOS | 0 | 9.075 | 0.495 | 3.67 - 19.31 |
0 | 8.252 | 0.761 | 1.95 - 28.30 |
0 | 5.058 | 0.261 | 2.17 - 11.72 |
| ΣPCB | 0 | 0.470 | 0.051 | 0.07 - 1.90 | 0 | 0.734 | 0.108 | 0.07 - 3.07 |
0 | 0.465 | 0.067 | 0.02 - 2.60 |
| HCB | 0 | 0.018 | 0.001 | 0.005 - 0.08 |
6 | 0.032 | 0.003 | <LOD - 0.11 |
0 | 0.025 | 0.002 | 0.008 - 0.08 |
| Blood-Hg | 0 | 3.066 | 0.255 | 0.53 - 19.96 |
- | - | - | - | 0 | 2.328 | 0.242 | 0.21 - 9.97 |
| Hair-Hg | 0 | 0.748 | 0.073 | 0.14 - 3.88 | - | - | - | - | 0 | 0.611 | 0.084 | 0.02 - 5.76 |
| Cord blood- Hg |
0 | 4.649 | 0.371 | 0.77 - 21.06 |
- | - | - | - | - | - | - | - |
| o,p’-DDE | 15 | n/a | n/a | <LOD - 0.02 |
38 | n/a | n/a | <LOD - 0.11 |
47 | n/a | n/a | <LOD - 0.001 |
| p,p’-DDE | 0 | 0.142 | 0.020 | 0.02 - 1.38 | 3 | 0.271 | 0.053 | <LOD - 1.95 |
0 | 0.229 | 0.032 | 0.02 - 1.78 |
| o,p’-DDT | 2 | 0.009 | 0.001 | <LOD - 0.14 |
11 | 0.033 | 0.004 | <LOD - 0.23 |
29 | n/a | n/a | <LOD - 0.003 |
| p,p’-DDT | 2 | 0.007 | 0.001 | <LOD - 0.09 |
31 | n/a | n/a | <LOD - 0.09 |
3 | 0.001 | 0.000 | <LOD - 0.008 |
Figure 1.
Correlation heat map and hierarchical clustering of maternal, 18-month, and 5-year POPs and Hg exposures. Red represents positive correlations whereas blue represents negative ones. The intensity of the color is a function of the correlation.
3.3. WBC counts in relation to exposure levels
The CFA models exhibited a good to very good fit to the data. All measured exposures showed significant and high correlations to their latent functions (Table 3) and therefore appeared as appropriate indicators. The variance in observed exposures explained by the latent exposure functions ranged between about 50 and 100%.
Table 3.
Results of the confirmatory factor analysis for prenatal, 18-month, and 5-year exposures.
| Factors | Indicators | Loading | SE | p-value | Standardized estimate |
|
|---|---|---|---|---|---|---|
| Prenatal | PFAS | PFOA | 1.00 | 0.00 | NA | 0.65 |
| PFNA | 1.22 | 0.25 | <0.001 | 0.78 | ||
| PFDA | 1.43 | 0.30 | <0.001 | 0.94 | ||
| PFOS | 1.04 | 0.20 | <0.001 | 0.83 | ||
| PFHxS | 1.29 | 0.38 | 0.001 | 0.62 | ||
| OC | HCB | 1.00 | 0.00 | NA | 0.92 | |
| PCB | 1.34 | 0.17 | <0.001 | 0.84 | ||
| p,p'-DDE | 1.80 | 0.19 | <0.001 | 0.87 | ||
| o,p'-DDT | 1.44 | 0.25 | <0.001 | 0.65 | ||
| p,p'-DDT | 1.33 | 0.20 | <0.001 | 0.7 | ||
| Hg | hair Hg | 1.00 | 0.00 | NA | 0.87 | |
| blood Hg | 0.91 | 0.13 | <0.001 | 0.91 | ||
| CB-Hg | 0.95 | 0.11 | <0.001 | 0.99 | ||
|
18
months |
PFAS | PFOA | 1.00 | 0.00 | NA | 0.89 |
| PFNA | 1.03 | 0.10 | <0.001 | 0.92 | ||
| PFDA | 0.88 | 0.11 | <0.001 | 0.9 | ||
| PFOS | 0.86 | 0.12 | <0.001 | 0.9 | ||
| PFHxS | 1.56 | 0.23 | <0.001 | 0.82 | ||
| OC | HCB | 1.00 | 0.00 | NA | 0.94 | |
| PCB | 1.83 | 0.28 | <0.001 | 0.99 | ||
| p,p'-DDE | 1.62 | 0.61 | 0.008 | 0.65 | ||
| o,p'-DDT | 1.27 | 0.11 | <0.001 | 0.65 | ||
| 5 years | PFAS | PFOA | 1.00 | 0.00 | NA | 0.54 |
| PFNA | 1.74 | 0.48 | <0.001 | 0.67 | ||
| PFDA | 1.32 | 0.39 | 0.001 | 0.53 | ||
| PFOS | 1.59 | 0.52 | 0.002 | 0.85 | ||
| PFHxS | 1.95 | 0.52 | <0.001 | 0.62 | ||
| OC | HCB | 1.00 | 0.00 | NA | 0.97 | |
| PCB | 1.85 | 0.33 | <0.001 | 0.84 | ||
| p,p'-DDE | 1.80 | 0.27 | <0.001 | 0.83 | ||
| p,p'-DDT | 1.05 | 0.22 | <0.001 | 0.5 | ||
| Hg | hair Hg | 1.00 | 0.00 | NA | 0.89 | |
| blood Hg | 0.84 | 0.35 | 0.02 | 1 |
SEMs showed an acceptable (age-5 models) to a very good fit to the data. Table 4 shows the resulting associations between the latent exposure functions for PFAS, Hg, and OC at each age in regard to the WBC counts at 5 years. Higher prenatal methylmercury exposure was consistently associated with lower WBC counts. For instance, a one standard deviation (SD) increase in maternal concentrations was associated with a 23% SD decrease in lymphocytes (95% confidence intervals [CI]: −43, −4%) and a 30% SD decrease in total WBC count (95% CI: −56, −5%). Maternal OC concentrations were marginally associated with decreases in neutrophil and total WBC counts, with a 1-SD increase in maternal OC concentrations associated with a 27% SD decrease in total WBC count (95% CI: −57, 4%). Further, a 1-SD increase in 5-years OC concentrations was also marginally associated with a 23% SD decrease in total WBC count (95% CI: −47, 1%). Finally, higher PFAS concentrations at all ages were associated with higher basophil counts (B=28 % SD; 95% CI: −2, 57%, B=34 % SD; 95% CI: 0, 68%, and B=46 % SD; 95% CI: 13, 79%, respectively for maternal, 18-month, and 5-year PFAS).
Table 4.
Associations between PFAS, Hg, and POPs exposure and WBC concentrations at 5 years of age. Estimate is expressed as the change in leukocytes SD associated with a one SD change in the exposure.
| Maternal exposures | 18-month exposures | 5-year exposures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocytes | Exposures | Std Estimate |
Lower CI |
Upper CI |
p- value |
Std Estimate |
Lower CI |
Upper CI |
p- value |
Std Estimate |
Lower CI |
Upper CI |
p- value |
| Neutrophils | PFAS | −0.14 | −0.44 | 0.17 | 0.37 | 0.09 | −0.19 | 0.37 | 0.53 | −0.06 | −0.44 | 0.31 | 0.73 |
| Hg | −0.26 | −0.54 | 0.02 | 0.07 | - | - | - | - | 0.13 | −0.05 | 0.30 | 0.16 | |
| OC | −0.30 | −0.64 | 0.03 | 0.07 | −0.14 | −0.48 | 0.20 | 0.41 | −0.21 | −0.43 | 0.01 | 0.07 | |
| Basophils | PFAS | 0.28 | −0.02 | 0.57 | 0.07 | 0.34 | 0.00 | 0.68 | 0.05 | 0.46 | 0.13 | 0.79 | 0.01 |
| Hg | −0.09 | −0.32 | 0.14 | 0.46 | - | - | - | - | −0.17 | −0.59 | 0.24 | 0.41 | |
| OC | 0.21 | −0.08 | 0.50 | 0.15 | 0.25 | −0.13 | 0.62 | 0.19 | 0.18 | −0.24 | 0.59 | 0.40 | |
| Eosinophils | PFAS | −0.07 | −0.32 | 0.17 | 0.56 | 0.14 | −0.20 | 0.48 | 0.43 | −0.06 | −0.38 | 0.27 | 0.73 |
| Hg | −0.14 | −0.48 | 0.19 | 0.41 | - | - | - | - | −0.11 | −0.35 | 0.13 | 0.38 | |
| OC | −0.11 | −0.39 | 0.17 | 0.45 | 0.16 | −0.17 | 0.48 | 0.35 | −0.08 | −0.31 | 0.15 | 0.48 | |
| Lymphocytes | PFAS | −0.04 | −0.30 | 0.22 | 0.78 | 0.16 | −0.13 | 0.45 | 0.27 | −0.11 | −0.36 | 0.14 | 0.39 |
| Hg | −0.23 | −0.43 | −0.04 | 0.02 | - | - | - | - | −0.01 | −0.20 | 0.18 | 0.90 | |
| OC | −0.10 | −0.35 | 0.14 | 0.41 | 0.20 | −0.11 | 0.51 | 0.20 | −0.18 | −0.46 | 0.10 | 0.22 | |
| Monocytes | PFAS | −0.10 | −0.38 | 0.19 | 0.51 | 0.16 | −0.12 | 0.43 | 0.26 | 0.05 | −0.34 | 0.44 | 0.80 |
| Hg | −0.23 | −0.46 | 0.00 | 0.05 | - | - | - | - | −0.07 | −0.30 | 0.16 | 0.56 | |
| OC | −0.17 | −0.46 | 0.11 | 0.23 | −0.08 | −0.37 | 0.21 | 0.59 | −0.06 | −0.28 | 0.15 | 0.56 | |
| Total WBC | PFAS | −0.11 | −0.40 | 0.19 | 0.47 | 0.15 | −0.13 | 0.44 | 0.28 | −0.06 | −0.38 | 0.25 | 0.70 |
| Hg | −0.30 | −0.56 | −0.05 | 0.02 | - | - | - | - | 0.06 | −0.11 | 0.23 | 0.46 | |
| OC | −0.27 | −0.57 | 0.04 | 0.08 | 0.00 | −0.34 | 0.34 | 1.00 | −0.23 | −0.47 | 0.01 | 0.06 | |
In regard to the specific lymphocyte subpopulations, maternal Hg concentrations were significantly associated with decreased CD3+ T cells, CD4+ cells, CD4-RTE and CD19+ B Cells concentrations. For instance, a 1-SD increase in maternal Hg concentrations was associated with a 23% SD (95% CI: −40, −6%) and a 24% SD (95% CI: −47, −1%) decrease in CD4+ T cell and 19+ B cell concentrations, respectively. No association was observed with 18-month and 5-year exposures (Table 5). Attempts to incorporate all exposure information in a single model failed, as the fit indices were poor, and signs of multicollinearity issues were observed in the resulting estimates, with unstable parameter estimates and negative variances.
Table 5.
Associations between PFAS, Hg, and POP exposures and specific lymphocyte counts at 5 years of age. Estimates are expressed as the relative change in regard to the leukocyte SD associated with a one SD increase in the exposure.
| Maternal exposures | 18-month exposures | 5-year exposures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocytes | Exposures | Std Estimate |
Lower CI |
Upper CI |
p- value |
Std Estimate |
Lower CI |
Upper CI |
p- value |
Std Estimate |
Lower CI |
Upper CI |
p- value |
| CD3 T cells | PFAS | −0.12 | −0.36 | 0.13 | 0.36 | 0.09 | −0.31 | 0.49 | 0.66 | −0.15 | −0.40 | 0.10 | 0.24 |
| Hg | −0.22 | −0.41 | −0.04 | 0.02 | - | - | - | - | 0.00 | −0.08 | 0.09 | 0.95 | |
| OC | −0.11 | −0.34 | 0.12 | 0.37 | 0.13 | −0.20 | 0.47 | 0.42 | −0.19 | −0.51 | 0.14 | 0.26 | |
| CD4 T cells | PFAS | −0.12 | −0.37 | 0.13 | 0.35 | 0.07 | −0.29 | 0.43 | 0.71 | −0.18 | −0.44 | 0.07 | 0.16 |
| Hg | −0.23 | −0.40 | −0.06 | 0.01 | - | - | - | - | 0.04 | −0.16 | 0.24 | 0.72 | |
| OC | −0.10 | −0.33 | 0.13 | 0.41 | 0.12 | −0.20 | 0.44 | 0.46 | −0.22 | −0.56 | 0.11 | 0.19 | |
| CD8 T cells | PFAS | −0.12 | −0.36 | 0.12 | 0.32 | 0.16 | −0.27 | 0.59 | 0.46 | −0.08 | −0.33 | 0.17 | 0.53 |
| Hg | −0.16 | −0.38 | 0.06 | 0.15 | - | - | - | - | −0.05 | −0.26 | 0.17 | 0.68 | |
| OC | −0.13 | −0.37 | 0.10 | 0.27 | 0.17 | −0.20 | 0.53 | 0.37 | −0.09 | −0.40 | 0.21 | 0.55 | |
| CD4-RTE | PFAS | −0.05 | −0.32 | 0.22 | 0.70 | 0.23 | −0.08 | 0.53 | 0.14 | −0.12 | −0.35 | 0.11 | 0.31 |
| Hg | −0.26 | −0.45 | −0.08 | 0.01 | - | - | - | - | 0.00 | −0.07 | 0.08 | 0.91 | |
| OC | −0.10 | −0.34 | 0.14 | 0.41 | 0.20 | −0.28 | 0.69 | 0.42 | −0.05 | −0.40 | 0.29 | 0.76 | |
| NK cells | PFAS | −0.12 | −0.41 | 0.18 | 0.43 | −0.20 | −0.50 | 0.09 | 0.18 | 0.16 | −0.16 | 0.48 | 0.33 |
| Hg | −0.11 | −0.33 | 0.11 | 0.32 | - | - | - | - | −0.02 | −0.10 | 0.06 | 0.63 | |
| OC | 0.11 | −0.13 | 0.35 | 0.38 | −0.22 | −0.66 | 0.22 | 0.33 | −0.13 | −0.40 | 0.15 | 0.36 | |
| B cells | PFAS | −0.06 | −0.33 | 0.21 | 0.65 | 0.19 | −0.08 | 0.47 | 0.17 | −0.12 | −0.42 | 0.17 | 0.41 |
| Hg | −0.24 | −0.47 | −0.01 | 0.04 | - | - | - | - | 0.05 | −0.16 | 0.26 | 0.62 | |
| OC | −0.10 | −0.38 | 0.17 | 0.46 | 0.09 | −0.22 | 0.40 | 0.59 | −0.14 | −0.42 | 0.14 | 0.33 | |
4. Discussion
In this prospective study with age-related methylmercury and POPs exposure profiles, we found an association between prenatal methylmercury exposures and depleted total white blood cell counts, especially for monocytes, basophils and CD3+ T lymphocytes, CD4+ T lymphocytes, CD4+ recent thymic emigrants (CD4-RTE), and CD19+ B lymphocytes. Further, prenatal and concurrent concentrations of organochlorine compounds were marginally associated with decreased neutrophil and total WBC counts. Finally, higher 18-month and 5-year PFAS concentrations were associated with increased basophil counts in the children at age 5 years.
Very few studies investigated WBC count in relation to developmental exposure to methylmercury and POPs. Of greatest interest, our study suggests a negative impact on recent thymic immigrant cells, as well as on mature lymphocyte subsets. The results are in line with other human studies showing decreased lymphocyte [16, 36], and monocyte [37] counts in relation to mercury exposures, but contradict a recent cross-sectional study showing a positive correlation of the serum-mercury concentration – likely reflecting inorganic mercury exposure – with lymphocyte count [15]. In the Faroes, total mercury concentrations in blood and hair primarily indicate exposures to methylmercury [38], thus suggesting that different chemical speciations may affect the mercury-associated toxicity. Mercury-induced cytotoxicity has been demonstrated in cells of the immune system, including B cells, T cells, and monocytes [39, 40], and two in vitro studies demonstrated that Hg exposure causes mitochondrial dysfunction and enhances the apoptosis of neutrophils and mononuclear cells including T cells [41, 42]. However, mercury has also been shown to exert immunomodulatory and anti-apoptotic effects in animals [43] and to be associated with autoimmune diseases in both animals and humans [44]. Although no clear picture has emerged so far, the differences between studies may mainly be attributable to differences in concentrations and mercury species, i.e., whether inorganic mercury or methylmercury [45].
Our study also found that OCs tended to have similar effects as methylmercury, though not significantly so. Previous studies have reported similar findings in various settings. Higher PCB concentrations were associated with lower neutrophil and lymphocyte percentages, and a lower total WBC count [13, 46]. Further, Inuit infants whose mothers had elevated levels of PCBs and dioxins in their breast milk had decreased CD4+/CD8+ T cell ratio at 6 and 12 months of age [47]. A joint effect of higher exposures of DDT and PCBs on CD16+ NK cells was also observed in Japanese infants [22]. Finally, neutrophil counts were inversely related to OC pesticides concentrations in the general U.S. population form NHANES 1999-2002 [48]. Similar findings have also been reported in animal and in vitro studies [49-52]. The strong correlations between PCBs and other OCs exposures observed in this population make it difficult to distinguish between effects of specific OC substances. It is also worth mentioning that although we were able to collect serum samples and examine OC concentrations at different time points, with apparent different associations with neutrophils and total WBC count, it is still challenging to state about the exact timing of greatest susceptibility. Given the long half-lives of OCs, the serum concentrations at 18 months and 5 years are likely still affected by prenatal and lactational transfer from the mother.
We also observed an association between PFAS exposure and increased basophil count. Although the role that basophils play in the immune system has not been thoroughly investigated in the past, recent studies revealed their crucial role in various immune responses such as allergy and anti-parasitic protective immunity [53]. Importantly, elevated IgE concentrations correlate with increased numbers of circulating basophils both in mice and in humans [54], and recent evidence from epidemiologic and animal studies suggests that PFASs may be associated with allergy and asthma-related outcomes, perhaps as a result of a shift in the host’s immune state toward a more TH2-like state [55-59].
Based on the cell counts obtained in the present study, current evidence does not allow any conclusions regarding any increased risk of adverse outcomes. As WBC counts could be easily generated in population studies, further investigation is warranted, even though the clinical significance of these changes is unclear at this point. However, the results are indicative of immunotoxic and/or immunomodulatory effects of methylmercury and POPs, and the demonstrated effects on the cellular and humoral immune responses might lead to increased susceptibility to various infectious diseases or to immune dysfunction with an impaired immune tolerance. Interestingly, our findings point to developmental toxicity on the thymus, as demonstrated by the lower CD4-RTE concentration, associated with perinatal methylmercury exposure. One may speculate that a compromised thymic function could lead to insufficient T cell helper activity for developing B-cell based immunity and in the longer term perhaps also an increased risk of autoimmune disease, as may be seen in immune deficiency conditions.
A major limitation of this study is the small sample size. Although the use of structural equation models allowed a reduction of multiple comparisons and a gain in statistical power due to the by reduced measurement error, the small sample size did not allow for mutual adjustment of the three families of exposures, nor mutual adjustment for pre- and postnatal exposures.
To our knowledge, this is the first study to investigate associations of both prenatal and postnatal exposure to multiple POPs and methylmercury and children’s WBC counts. Despite the small sample size, we were able to adjust for several potentially relevant confounders, and their effect on the results was marginal. The potential influence of any unmeasured determinant of WBC would appear minimal, especially when taking into regard the homogeneity of the Faroese population in terms of socio-demographic, lifestyle, and genetic characteristics. Thus, these findings should inspire further study of the possible effects of developmental exposure to environmental chemicals on children’s immune functions.
5. Conclusions
In this prospective study of Faroese children, we report that prenatal methylmercury and OC exposures are inversely associated with the total WBC counts at age 5 years. The finding of significantly reduced subpopulations of lymphocytes such as B-lymphocytes, CD4 positive T helper cells and CD4 positive recent thymic emigrants suggest an effect of prenatal methylmercury exposure on cellular immunity and a dysregulation of T-cell mediated immunity. Prenatal OC exposure was marginally associated with decreases in neutrophil counts, while postnatal PFAS concentrations appeared to be associated with increased basophil counts. These observations suggest different effects of developmental exposures to immunotoxicants on circulating leukocyte populations in childhood. This study provides new evidence of the potential immunomodulatory effects of persistent organic pollutants and methylmercury and suggests that extended differential counts may be useful in future research.
HIGHLIGHTS.
White blood cell counts may be affected by developmental exposure to immunotoxicants,
We assessed associations of latent pollutant exposures with cell counts at age 5 years,
Prenatal methylmercury exposure was associated with depleted white cells, especially lymphocytes,
Perfluorinated compound exposures at age 5 were associated with higher basophil counts,
White cell counts appear to be affected in different ways by immunotoxicant exposures.
Acknowledgments
Funding
This study was funded by grant number ES012199 from the National Institute of Environmental Health Sciences of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Winans B, Humble MC, Lawrence BP. Environmental toxicants and the developing immune system: a missing link in the global battle against infectious disease? Reprod toxicol. 2011;31(3):327–336. doi: 10.1016/j.reprotox.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tryphonas H. Immunotoxicity of PCBs (Aroclors) in relation to Great Lakes. Environ Health Perspect. 1995;103(Suppl 9):35–46. doi: 10.1289/ehp.95103s935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arkoosh MR, Clemons E, Myers M, Casillas E. Suppression of B-cell mediated immunity in juvenile chinook salmon (Oncorhynchus tshawytscha) after exposure to either a polycyclic aromatic hydrocarbon or to polychlorinated biphenyls. Immunopharmacol Immunotoxicol. 1994;16(2):293–314. doi: 10.3109/08923979409007096. [DOI] [PubMed] [Google Scholar]
- [4].Harper N, Connor K, Safe S. Immunotoxic potencies of polychlorinated biphenyl (PCB), dibenzofuran (PCDF) and dibenzo-p-dioxin (PCDD) congeners in C57BL/6 and DBA/2 mice. Toxicology. 1993;80(2-3):217–27. doi: 10.1016/0300-483x(93)90183-s. [DOI] [PubMed] [Google Scholar]
- [5].Mori C, Morsey B, Levin M, Nambiar PR, De Guise S. Immunomodulatory effects of in vitro exposure to organochlorines on T-cell proliferation in marine mammals and mice. J Toxicol Environ Health A. 2006;69(3-4):283–302. doi: 10.1080/15287390500227472. [DOI] [PubMed] [Google Scholar]
- [6].Misumi I, Vella AT, Leong JA, Nakanishi T, Schreck CB. p,p'-DDE depresses the immune competence of chinook salmon (Oncorhynchus tshawytscha) leukocytes. Fish Shellfish Immunol. 2005;19(2):97–114. doi: 10.1016/j.fsi.2004.11.005. [DOI] [PubMed] [Google Scholar]
- [7].Mangum LC, Borazjani A, Stokes JV, Matthews AT, Lee JH, Chambers JE, Ross MK. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem Res Toxicol. 2015;28(4):570–84. doi: 10.1021/tx500323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40(2):300–11. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- [9].Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol Sci. 2008;104(1):144–54. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- [10].Fair PA, Driscoll E, Mollenhauer MAM, Bradshaw SG, Yun SH, Kannan K, Bossart GD, Keil DE, Peden-Adams MM. Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J Immunotoxicol. 2011;8(1):17–29. doi: 10.3109/1547691X.2010.527868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Begam M, Sengupta M. Immunomodulation of intestinal macrophages by mercury involves oxidative damage and rise of pro-inflammatory cytokine release in the fresh water fish Channa punctatus Bloch. Fish Shellfish Immunol. 2015;45(2):378–85. doi: 10.1016/j.fsi.2015.04.017. [DOI] [PubMed] [Google Scholar]
- [12].Jorissen A, Plum LM, Rink L, Haase H. Impact of lead and mercuric ions on the interleukin-2-dependent proliferation and survival of T cells. Arch Toxicol. 2013;87(2):249–58. doi: 10.1007/s00204-012-0926-z. [DOI] [PubMed] [Google Scholar]
- [13].Serdar B, LeBlanc WG, Norris JM, Dickinson LM. Potential effects of polychlorinated biphenyls (PCBs) and selected organochlorine pesticides (OCPs) on immune cells and blood biochemistry measures: a cross-sectional assessment of the NHANES 2003-2004 data. Environ Health. 2014;13:114. doi: 10.1186/1476-069X-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nyland JF, Fillion M, Barbosa F, Jr., Shirley DL, Chine C, Lemire M, Mergler D, Silbergeld EK. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ Health Perspect. 2011;119(12):1733–8. doi: 10.1289/ehp.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim JH, Lee KH, Hong SC, Lee HS, Lee J, Kang JW. Association between serum mercury concentration and leukocyte differential count in children. Pediatr Hematol Oncol. 2015;32(2):109–14. doi: 10.3109/08880018.2013.853222. [DOI] [PubMed] [Google Scholar]
- [16].Kim KN, Bae S, Park HY, Kwon HJ, Hong YC. Low-level Mercury Exposure and Risk of Asthma in School-age Children. Epidemiology. 2015;26(5):733–9. doi: 10.1097/EDE.0000000000000351. [DOI] [PubMed] [Google Scholar]
- [17].Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jorgensen E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environ Health. 2015;14:47. doi: 10.1186/s12940-015-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama. 2012;307(4):391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jorgensen E, Heilmann C. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol. 2016;13(2):270–3. doi: 10.3109/1547691X.2015.1067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hui LL, Chan MH, Lam HS, Chan PH, Kwok KM, Chan IH, Li AM, Fok TF. Impact of fetal and childhood mercury exposure on immune status in children. Environ Res. 2016;144(Pt A):66–72. doi: 10.1016/j.envres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- [21].Glynn A, Thuvander A, Aune M, Johannisson A, Darnerud PO, Ronquist G, Cnattingius S. Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ Health. 2008;7:62. doi: 10.1186/1476-069X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nagayama J, Tsuji H, Iida T, Nakagawa R, Matsueda T, Hirakawa H, Yanagawa T, Fukushige J.i., Watanabe T. Immunologic effects of perinatal exposure to dioxins. PCBs and organochlorine pesticides in Japanese infants, Chemosphere. 2007;67(9):S393–S398. doi: 10.1016/j.chemosphere.2006.05.134. [DOI] [PubMed] [Google Scholar]
- [23].Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jorgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3(8):e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jusko TA, Roos A.J. De, Lee SY, Thevenet-Morrison K, Schwartz SM, Verner M-A, Murinova LP, Drobná B, Kočan A, Fabišiková A, Čonka K, Trnovec T, Hertz-Picciotto I, Lawrence BP. A Birth Cohort Study of Maternal and Infant Serum PCB-153 and DDE Concentrations and Responses to Infant Tuberculosis Vaccination. Environ Health Perspect. 2016;124(6):813–821. doi: 10.1289/ehp.1510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jusko TA, Sonneborn D, Palkovicova L, Kocan A, Drobna B, Trnovec T, Hertz-Picciotto I. Pre- and Postnatal Polychlorinated Biphenyl Concentrations and Longitudinal Measures of Thymus Volume in Infants. Environ Health Perspect. 2012;120(4):595–600. doi: 10.1289/ehp.1104229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horváthová M, Jahnová E, Palkovičová Ľ, Trnovec T, Hertz-Picciotto I. The kinetics of cell surface receptor expression in children perinatally exposed to polychlorinated biphenyls. J Immunotoxicol. 2011;8(4):367–380. doi: 10.3109/1547691X.2011.620037. [DOI] [PubMed] [Google Scholar]
- [27].Horváthová M, Jahnová E, Palkovičová Ľ, Trnovec T, Hertz-Picciotto I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J Immunotoxicol. 2011;8(4):333–345. doi: 10.3109/1547691X.2011.615767. [DOI] [PubMed] [Google Scholar]
- [28].Cao J, Xu X, Hylkema MN, Zeng EY, Sly PD, Suk WA, Bergman Å, Huo X. Early-life Exposure to Widespread Environmental Toxicants and Health Risk: A Focus on the Immune and Respiratory Systems. Ann Glob Health. 2016;82(1):119–131. doi: 10.1016/j.aogh.2016.01.023. [DOI] [PubMed] [Google Scholar]
- [29].Gascon M, Morales E, Sunyer J, Vrijheid M. Effects of persistent organic pollutants on the developing respiratory and immune systems: a systematic review. Environ Int. 2013;52:51–65. doi: 10.1016/j.envint.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [30].Tryphonas H. Approaches to detecting immunotoxic effects of environmental contaminants in humans. Environ Health Perspect. 2001;109(Suppl 6):877–84. doi: 10.1289/ehp.01109s6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luster MI, Portier C, Pait DG, White KL, Jr., Gennings C, Munson AE, Rosenthal GJ. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam Appl Toxicol. 1992;18(2):200–10. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- [32].Perkins S. Examination of the blood and bone marrow, Wintrobe’s Clinical Hematology. 10th ed Lippincott Williams & Wilkins; Baltimore, MD: 1999. pp. 9–35. [Google Scholar]
- [33].Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DG, Jr., Sampson EJ, Jorgensen PJ, Vahter M. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res. 1995;71(1):29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- [34].Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216(3):385–93. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- [35].Wintrobe MM, Greer JP. Wintrobe's clinical hematology. 2014 [Google Scholar]
- [36].Belles-Isles M, Ayotte P, Dewailly E, Weber JP, Roy R. Cord blood lymphocyte functions in newborns from a remote maritime population exposed to organochlorines and methylmercury. J Toxicol Environ Health A. 2002;65(2):165–82. doi: 10.1080/152873902753396794. [DOI] [PubMed] [Google Scholar]
- [37].Cardenas A, Koestler DC, Houseman EA, Jackson BP, Kile ML, Karagas MR, Marsit CJ. Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics. 2015;10(6):508–15. doi: 10.1080/15592294.2015.1046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grandjean P, Weihe P, Jorgensen PJ, Clarkson T, Cernichiari E, Videro T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47(3):185–95. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- [39].Shenker BJ, Berthold P, Decker S, Mayro J, Rooney C, Vitale L, Shapiro IM. Immunotoxic Effects of Mercuric Compounds on Human Lymphocytes and Monocytes. II. Alterations in Cell Viability. Immunopharmacol Immunotoxicol. 1992;14(3):555–577. doi: 10.3109/08923979209005411. [DOI] [PubMed] [Google Scholar]
- [40].Kim SH, Sharma RP. Mercury-induced apoptosis and necrosis in murine macrophages: role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2004;196(1):47–57. doi: 10.1016/j.taap.2003.11.020. [DOI] [PubMed] [Google Scholar]
- [41].Shenker BJ, Guo TL, Shapiro IM. Mercury-induced apoptosis in human lymphoid cells: evidence that the apoptotic pathway is mercurial species dependent. Environ Res. 2000;84(2):89–99. doi: 10.1006/enrs.2000.4078. [DOI] [PubMed] [Google Scholar]
- [42].Moisan E, Arbour S, Nguyen N, Hebert MJ, Girard D, Bernier J, Fournier M, Kouassi E. Prolongation of human neutrophil survival by low-level mercury via inhibition of spontaneous apoptosis. J Toxicol Environ Health A. 2002;65(2):183–203. doi: 10.1080/152873902753396802. [DOI] [PubMed] [Google Scholar]
- [43].Vas J, Monestier M. Immunology of Mercury. Ann N Y Acad Sci. 2008;1143(1):240–267. doi: 10.1196/annals.1443.022. [DOI] [PubMed] [Google Scholar]
- [44].Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30(10):502–9. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- [45].Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett. 2010;198(2):182–90. doi: 10.1016/j.toxlet.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Daniel V, Huber W, Bauer K, Suesal C, Conradt C, Opelz G. Associations of blood levels of PCB, HCHS, and HCB with numbers of lymphocyte subpopulations, in vitro lymphocyte response, plasma cytokine levels, and immunoglobulin autoantibodies. Environ Health Perspect. 2001;109(2):173–178. doi: 10.1289/ehp.01109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dewailly E, Nantel A, Bruneau S, Laliberté C, Ferron L, Gingras S. Breast milk contamination by PCDDs, PCDFs and PCBs in Arctic Québec: A preliminary assessment. Chemosphere. 1992;25(7):1245–1249. [Google Scholar]
- [48].Lee DH, Jacobs DR, Kocher T. Associations of serum concentrations of persistent organic pollutants with the prevalence of periodontal disease and subpopulations of white blood cells. Environ Health Perspect. 2008;116(11):1558–62. doi: 10.1289/ehp.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vos JG, Driel-Grootenhuis LV. PCB-induced suppression of the humoral and cell-mediated immunity in guinea pigs. Sci Total Environ. 1972;1(3):289–302. doi: 10.1016/0048-9697(72)90024-1. [DOI] [PubMed] [Google Scholar]
- [50].Arnold DL, Bryce F, Karpinski K, Mes J, Fernie S, Tryphonas H, Truelove J, McGuire PF, Burns D, Tanner JR, Stapley R, Zawidzka ZZ, Basford D. Toxicological consequences of aroclor 1254 ingestion by female rhesus (macaca mulatta) monkeys. Part 1B. Prebreeding phase: Clinical and analytical laboratory findings. Food Chem Toxicol. 1993;31(11):811–824. doi: 10.1016/0278-6915(93)90219-o. [DOI] [PubMed] [Google Scholar]
- [51].Schwacke LH, Zolman ES, Balmer BC, De Guise S, George RC, Hoguet J, Hohn AA, Kucklick JR, Lamb S, Levin M, Litz JA, McFee WE, Place NJ, Townsend FI, Wells RS, Rowles TK. Anaemia, hypothyroidism and immune suppression associated with polychlorinated biphenyl exposure in bottlenose dolphins (Tursiops truncatus) Proc R Soc Lond [Biol] 2012;279(1726):48–57. doi: 10.1098/rspb.2011.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Alegria-Torres JA, Diaz-Barriga F, Gandolfi AJ, Perez-Maldonado IN. Mechanisms of p,p'-DDE-induced apoptosis in human peripheral blood mononuclear cells. Toxicol In Vitro. 2009;23(6):1000–6. doi: 10.1016/j.tiv.2009.06.021. [DOI] [PubMed] [Google Scholar]
- [53].Karasuyama H, Yamanishi Y. Basophils have emerged as a key player in immunity. Curr Opin Immunol. 2014;31:1–7. doi: 10.1016/j.coi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- [54].Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14(5):559–70. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Anderson-Mahoney P, Kotlerman J, Takhar H, Gray D, Dahlgren J. Self-reported health effects among community residents exposed to perfluorooctanoate. New Solut. 2008;18(2):129–43. doi: 10.2190/NS.18.2.d. [DOI] [PubMed] [Google Scholar]
- [56].Mark L, Sujata B, Andrew JH, Anita K, Genevieve B, Allan BB. The Impact Of A Perfluorinated Compound (PFC) On Airway Function In An Allergic Murine Model. TOXICOLOGY OF OCCUPATIONAL AND ENVIRONMENTAL DISEASE. American Thoracic Society. 2011:A3249–A3249. [Google Scholar]
- [57].Humblet O, Diaz-Ramirez LG, Balmes JR, Pinney SM, Hiatt RA. Perfluoroalkyl chemicals and asthma among children 12-19 years of age: NHANES (1999-2008) Environ Health Perspect. 2014;122(10):1129–33. doi: 10.1289/ehp.1306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dong GH, Tung KY, Tsai CH, Liu MM, Wang D, Liu W, Jin YH, Hsieh WS, Lee YL, Chen PC. Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of Taiwanese children. Environ Health Perspect. 2013;121(4):507–13. 513e1–8. doi: 10.1289/ehp.1205351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dong GH, Liu MM, Wang D, Zheng L, Liang ZF, Jin YH. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch Toxicol. 2011;85(10):1235–44. doi: 10.1007/s00204-011-0661-x. [DOI] [PubMed] [Google Scholar]