Abstract
Background
Ischemia-reperfusion injury (IRI) leading to delayed graft function, defined by the United Network for Organ Sharing as dialysis in the first week (UNOS-DGF), associates with poor kidney transplant outcomes. Controversies remain, however, about dialysis initiation thresholds and the utility for other criteria to denote less severe IRI, or slow graft function (SGF).
Methods
Multicenter, prospective study of deceased-donor kidney recipients to compare UNOS-DGF to a definition that combines impaired creatinine reduction in the first 48 hours or >1 dialysis session for predicting 12-month estimated glomerular filtration rate (eGFR). We also assessed 10 creatinine and urine output-based SGF definitions relative to 12-month eGFR.
Results
In 560 recipients, 215 (38%) had UNOS-DGF, 330 (59%) met the combined definition, 14 (3%) died and 23 (4%) had death-censored graft failure by 12 months. Both DGF definitions were associated with lower adjusted 12-month eGFR (95% CI)–by 7.3 (3.6–10.9) and 7.4 (3.8–11.0) ml/min/1.73m2, respectively. Adjusted relative risks for 12-month eGFR <30 ml/min/1.73m2 were 1.9 (1.2–3.1) and 2.1 (1.1–3.7), with unadjusted areas under the curve of 0.618 and 0.627, respectively. For SGF definitions, postoperative day (POD) 7 creatinine had the strongest association with12-month eGFR, and POD5 creatinine and creatinine reduction between POD1-2 demonstrated modest separations in 12-month eGFR.
Conclusions
While UNOS-DGF does not adequately predict 12-month function on its own, our findings do not support changing the definition. POD7 creatinine is correlated with 12-month eGFR, but large translational studies are needed to understand the biological link between IRI severity at transplant and longer-term outcomes.
Introduction
Delayed graft function (DGF), defined by the Organ Procurement and Transplantation Network (OPTN) and the United Network for Organ Sharing (UNOS) as dialysis in the first week of kidney transplant, is an early complication that occurs in nearly a quarter of all deceased-donor kidneys.1 Studies have linked DGF with increased costs, higher rates of acute and chronic rejection, and worse allograft and patient survival.2–4 In the setting of these as well as recent clinical trial data,5,6 the Food and Drug Administration (FDA) endorsed the concept of DGF as a clinically relevant registration endpoint for drug development trials in kidney transplantation.7 Notwithstanding, the relationship between DGF and later outcomes is not absolute, and many recipients that require temporary dialysis within the first several days of transplant eventually achieve acceptable baseline allograft function. As a clear example of this disconnect, kidneys from donors with circulatory determination of death (DCD) experience DGF at higher rates (over 40%) than standard-criteria kidneys (23%), but subsequent outcomes are comparable.1,8,9
Ischemia-reperfusion injury (IRI) resulting in acute kidney injury (AKI) of varying reversibility is the most common reason allografts fail to function immediately after implantation. In nontransplant settings, both severity and duration of AKI associate with mortality,10 and AKI severity has been linked with chronic kidney disease progression.11,12 While dialysis-defined DGF can be considered a severe form of AKI, the definition lacks granularity in terms of both severity and duration of allograft injury at transplantation. Furthermore, some degree of subjectivity is unavoidable with dialysis-defined DGF given that thresholds for dialysis decisions can differ between physicians and between centers in the early posttransplant period.13
Recognizing the subjective nature of some dialysis decisions, the FDA called for “better disease definitions in short-term appraisals” regarding DGF.7 Even before this call to action, investigators used many different DGF definitions to describe the clinical experiences and outcomes at a number of centers,14 and multiple investigators (including authors of the current work) have considered the concept of “slow graft function” (SGF) to describe varying serum creatinine (SCr) concentrations in peritransplant recipients that do not require dialysis.13,15–19 Evaluating early posttransplant SCr reductions in the absence of dialysis avoids confusion because of intentional creatinine removal but still ignores variable center thresholds for early dialysis indications. A diagnostic algorithm for peritransplant graft function that incorporates all of these concepts relative to both severity and duration of allograft injury would be of tremendous benefit for kidney transplant trials.
Members of our research group previously proposed redefining DGF by combining the need for >1 dialysis session or a creatinine reduction ratio (CRR) <25% “within the first 48 h post-transplant.”14 We therefore conducted the current multicenter study to primarily test the validity of this DGF definition and secondarily determine associations between frequently described definitions for SGF in the immediate posttransplant period with the following 12-month outcomes: estimated glomerular filtration rate (eGFR), death-censored graft failure, acute rejection and mortality. We hypothesized that 12-month eGFR would differ between recipients with versus without DGF by the UNOS definition, but that other DGF and SGF “phenotypes” would also associate with 12-month eGFR after multivariable adjustment for important donor, transplant and recipient characteristics.
Materials and Methods
Study Cohort
For this observational cohort study, we collected data from deceased kidney donors whose surrogates had consented to research at 5 organ procurement organizations (OPOs) between April 2010 and December 2013. We prospectively followed recipients of these donor kidneys at 5 transplant centers. The scientific review committees for participating OPOs and institutional review boards for the participating centers approved the protocol. We excluded recipients <16 years old and recipients of dual or en-bloc kidneys, kidneys from donors <5 years old, and multi-organ transplants other than simultaneous pancreas-kidneys. One recipient of an allograft that immediately thrombosed and was removed 12 hours after transplantation was also excluded.
Data Abstraction
This study also used data from the OPTN data system, which includes information on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN, as described elsewhere. The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN contractor (currently UNOS). Donor variables were abstracted from OPO charts. We calculated the kidney donor risk index (KDRI) as described by Rao et al.20,21 We converted the KDRI, as per convention,20 to obtain the kidney donor profile index (KDPI) relative to all US deceased donors in 2010. Trained study coordinators reviewed all available medical records for recipient characteristics, treatments and outcomes. Daily SCr, urine output (UOP) and dialysis episodes were abstracted from initial transplant hospitalization records. After discharge, outpatient records were reviewed for subsequent dialysis needs and other data. The principal investigator at each site confirmed all posttransplant dialysis episodes and indications by reviewing medical records. Coordinators and analysts at the coordinating center (Yale School of Medicine) performed extensive data quality checks and validations.
Exposure Variables
We categorized recipients as having DGF, SGF or “immediate graft function” (IGF) according to several literature-based definitions. We considered the following SCr criteria for defining SGF: SCr on postoperative day 5 (POD5Cr, cutoff >3 mg/dl),13 SCr on POD7 (POD7Cr, cutoff ≥2.5 mg/dl),22,23 CRR in the first 24 hours [CRR24h; ((POD0 SCr–POD1 SCr)/POD0 SCr), cutoff <25%],24 CRR in the first 48 hours (CRR48h, cutoff <25%),14 CRR between POD1-2 (CRR2, cutoff <30%),17 CRR over 3 consecutive days within the first week (CRR3, cutoff <10%/day),25 CRR between POD0-7 (CRR7, cutoff <70%),18 and number of days to reach creatinine clearance ≥10 ml/min (CrCl10, cutoff >6 days, estimated by Cockroft-Gault equation: [Sex*((140-Age in years)/(SCr in mg/dl))*(kg/72)], where Sex=1 for male, 0.85 for female; ignoring SCr on days after dialysis posttransplant).15 We calculated average hourly UOP from transplant to the morning of POD1 (UOP1) and from the morning of POD1 to 2 (UOP2). For both assessments, we used <42 mL/h to approximate <1000 mL/24h as the cutoff.26,27 For each definition, recipients not dialyzed in the first week (ie, no UNOS-DGF) and not found to have SGF were categorized as having IGF.
For recipients dialyzed at least once in the first week (ie, those with UNOS-DGF), we defined dialysis duration as the number of days from transplant to the last dialysis session. We characterized dialysis timing and number based on whether recipients with only 1 session received it by POD1 (versus after POD1). Thus, we defined dialysis number as: −1=not dialyzed, 0=dialyzed once on POD1, 1=dialyzed once after POD1, 2=dialyzed twice, and 3=dialyzed ≥3 times. To analyze the previously proposed DGF definition, we categorized recipients with “DGF48h” if they had CRR48h <25% or underwent >1 dialysis session within the first week.
Outcome Variables
We calculated eGFR from clinical SCr measurements at specified time-points via the Chronic Kidney Disease Epidemiology Collaboration equation.28 No center routinely performed protocol biopsies during the study period. Acute rejection was defined according to the treating transplant physician (ie, biopsy-confirmed or clinically treated without biopsy). We defined death-censored graft failure as resumption (or initiation for preemptive transplants) of maintenance dialysis or retransplantation. Primary nonfunction was defined as continued dialysis beyond 90 days after transplant and was counted as death-censored graft failure. We imputed eGFR as 10 ml/min/1.73m2 for recipients after death-censored graft failure. In the event of recipient death with a functioning allograft, we carried forward the last available SCr to calculate eGFR.
Statistical Analysis
Descriptive statistics were reported as mean (SD–standard deviation or SE–standard error of the mean) or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. We compared donor and recipient characteristics and 12-month outcomes (eGFR as the primary outcome along with the following secondary outcomes: acute rejection, death-censored graft failure and patient death) by UNOS-DGF status using Wilcoxon rank-sum tests and Pearson's chi-square tests. During analysis planning, we anticipated a minimal detectable difference in 12-month eGFR (at 80% power and alpha=0.05) of 5 ml/min/1.73m2. These estimates indicated our final analysis was capable of detecting a 5.4 ml/min/1.73m2 difference between UNOS-DGF groups. We also calculated Pearson correlation coefficients between POD5Cr (log2-transformed), POD7Cr (log2-transformed), CRR24h, CRR48h, CRR2, CRR7, UOP1 (log2-transformed), UOP2 (log2-transformed), and 12-month eGFR. We then compared (via Wilcoxon rank-sum) 12-month eGFR by DGF48h status (capable of detecting a difference of 5.3 ml/min/1.73m2 at 80% power and alpha=0.05). The various 3-level IGF-SGF-DGF criteria were evaluated by linear models utilizing planned contrasts for pair-wise comparisons for 12-month eGFR differences between groups (without statistical adjustment for multiple comparisons).
We fit multivariable regression models to estimate the relationship between each clinical scenario (UNOS-DGF, DGF48h and each IGF-SGF-DGF definition described above) and 12-month eGFR (continuous) and 12-month eGFR <30 ml/min/1.72m2 (versus ≥30 ml/min/1.72m2, a dichotomous outcome). To account for the possible correlation of outcomes between recipients of kidneys from the same donor, we fit linear mixed regression models with a donor-specific random intercept for the continuous outcome and modified Poisson regression models to estimate relative risk for the dichotomous outcome. For multivariable adjustments, we added the following donor variables: age (years), black race, height (cm), weight (kg), hypertension, diabetes, stroke as cause of death, DCD status and terminal SCr (mg/dl); and we then added the following transplant/recipient variables: cold ischemia time (hours), number of human leukocyte antigen mismatches, age (years), black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, body mass index (kg/m2), dialysis vintage (months) before transplant, and panel reactive antibody (percent).
We used the Akaike information criterion (AIC) to compare between models to predict 12-month eGFR. The AIC is useful for comparing different types of models because it does not require the models to be nested (the smaller the AIC, the better fit that model provides relative to the others). Given the nested relationship between each of the IGF-SGF-DGF models and the UNOS-DGF model, we also compared each against UNOS-DGF for predicting 12-month eGFR with multivariable adjustment using likelihood-ratio tests. We generated penalized basis spline curves fit with 10 knots and automatically selected smoothing parameters to visualize the relationship between each continuous predictor and 12-month eGFR. We calculated the R2 value for each of these curves. For the dichotomous outcome, we performed receiver-operating characteristic curve analyses to determine the area under the curve (AUC) for each model.
Sensitivity analyses were performed after excluding graft failures or deaths (as opposed to imputing eGFR). With additional follow-up, we also analyzed 24-month eGFR as a secondary outcome. We performed additional linear regression analyses to test for possible interactions on 12-month eGFR for UNOS-DGF and DGF48h based on kidney quality (KDPI >85 and DCD status) and transplant center. We used SAS 9.3 statistical software for Windows (SAS Institute, Cary, NC) and applied a 2-sided significance level of 0.05 for all tests and confidence intervals.
Results
Cohort Description
A flow diagram for study exclusions is shown in Figure 1. Out of 560 transplants from 485 donors, 215 (38%) had UNOS-DGF. 330 (59%) recipients had DGF48h (192 with CRR48h <25% only, 33 with >1 dialysis session only, 105 with both CRR48h <25% and >1 dialysis session). By 12-month, 66 (12%) recipients experienced acute rejection, 23 (4%) had death-censored graft failure -[18 (78%) of which classified as primary non-function], 14 (3%) had died and 11 (2%) were lost to follow-up. Median 12-month eGFR was 54 [42–69] ml/min/1.73m2, and 65 (11%) recipients had 12-month eGFR <30 ml/min/1.73m2.
Figure 1.
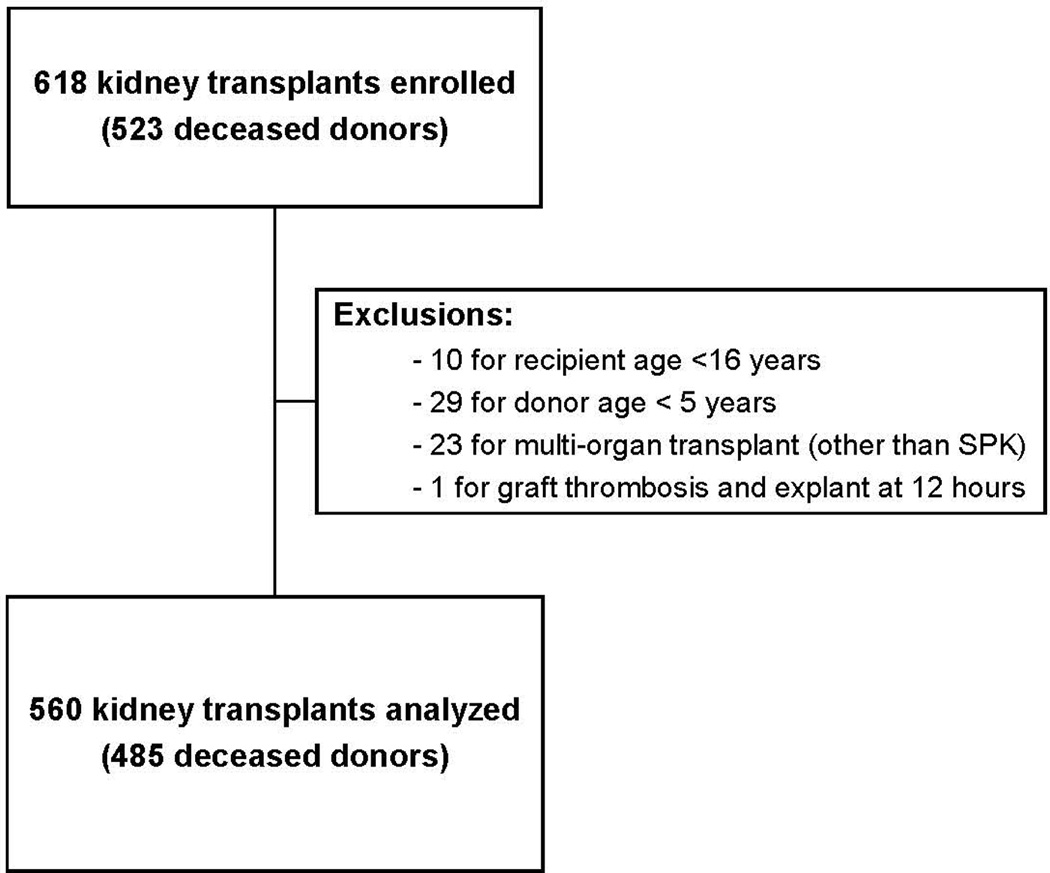
Study flow diagram. SPK, simultaneous pancreas-kidney transplant.
As shown in Table 1, only 2 out of 38 pre-emptive recipients experienced UNOS-DGF. Otherwise, those with UNOS-DGF tended to have longer dialysis vintage and were more often black race. Kidneys with UNOS-DGF tended to come from donors that were older, more frequently DCD, and had higher terminal SCr and KDPI values. For the 45 (21%) recipients with UNOS-DGF dialyzed only once by POD1, hyperkalemia was the most common indication (37 cases). For the 32 (15%) recipients with UNOS-DGF dialyzed once but after POD1, uremia was the most common indication (28 cases). Uremia was also the most common indication for recipients dialyzed more than once (>80% for uremia).
Table 1.
| a. Recipient Characteristics | |||||
|---|---|---|---|---|---|
| Characteristic | ALL (N=560) |
No UNOS-DGF (N=345) |
UNOS-DGF (N=215) |
P- value * |
|
| Age, years | 55 [46, 63] | 54 [45, 63] | 55 [46, 64] | 0.287 | |
| Sex, male | 353 (63%) | 207 (60%) | 146 (68%) | 0.059 | |
| Black race | 262 (47%) | 138 (40%) | 124 (58%) | <0.001 | |
| Body mass index, kg/m2 | 27 [24, 31.1] | 27 [24, 30] | 29 [25, 33] | <0.001 | |
| Cause of ESRD | Diabetes | 143 (26%) | 88 (26%) | 55 (26%) | 0.338 |
| Hypertension | 143 (26%) | 84 (24%) | 59 (27%) | ||
| Glomerulonephritis | 77 (14%) | 52 (15%) | 25 (12%) | ||
| CAKUT | 42 (8%) | 21 (6%) | 21 (10%) | ||
| Failed transplant | 85 (15%) | 58 (17%) | 27 (13%) | ||
| Other | 70 (13%) | 42 (12%) | 28 (13%) | ||
| Dialysis type before transplant |
Hemodialysis | 446 (80%) | 248 (73%) | 198 (92%) | <0.001 |
| Peritoneal | 72 (13%) | 57 (17%) | 15 (7%) | ||
| Pre-emptive | 38 (7%) | 36 (11%) | 2 (1%) | ||
| Dialysis vintage, months | 49 [19.5, 71] | 40.5 [10, 65.5] | 59 [36, 76.5] | <0.001 | |
| Hepatitis C seropositive | 38 (7%) | 25 (7%) | 13 (6%) | 0.451 | |
| PRA, % | 0% | 367 (66%) | 224 (65%) | 143 (67%) | 0.278 |
| 1–20% | 37 (7%) | 20 (6%) | 17 (8%) | ||
| 21–80% | 75 (13%) | 44 (13%) | 31 (14%) | ||
| >80% | 81 (14%) | 57 (17%) | 24 (11%) | ||
| b. Allograft (Donor) Characteristics | |||||
|---|---|---|---|---|---|
| Characteristic | ALL (N=560) |
No UNOS-DGF (N=345) |
UNOS-DGF (N=215) |
P- value* |
|
| Age, years | 43 [27, 53] | 38 [25, 51] | 47 [31, 56] | <0.001 | |
| Sex, male | 347 (62%) | 217 (63%) | 130 (60%) | 0.564 | |
| Black race | 96 (17%) | 62 (18%) | 34 (16%) | 0.510 | |
| Kidney Donor Risk Index | 1.22 [0.97, 1.52] | 1.11 [0.92, 1.47] | 1.36 [1.12, 1.62] | <0.001 | |
| Kidney Donor Profile Index, % | 49 [26, 70] | 40 [21, 67] | 60 [40, 76] | <0.001 | |
| Cause of death | Head trauma | 156 (28%) | 113 (33%) | 43 (20%) | 0.003 |
| Anoxia | 188 (34%) | 113 (33%) | 75 (35%) | ||
| Stroke | 208 (37%) | 116 (34%) | 92 (43%) | ||
| Other | 7 (1%) | 2 (1%) | 5 (2%) | ||
| History of diabetes | 58 (10%) | 31 (9%) | 27 (13%) | 0.177 | |
| History of hypertension | 166 (30%) | 88 (26%) | 78 (36%) | 0.007 | |
| Hepatitis C seropositive | 16 (3%) | 11 (3%) | 5 (2%) | 0.551 | |
| DCD | 100 (18%) | 46 (13%) | 54 (25%) | <0.001 | |
| Terminal SCr, mg/dl | 1 [0.7, 1.4] | 0.9 [0.7, 1.2] | 1.1 [0.7, 1.6] | 0.001 | |
| Cold ischemia time, hours | 16.2 [12.5, 22] | 16 [12, 21.5] | 17 [13, 23.4] | .064 | |
| Number of HLA mismatches |
0 | 20 (4%) | 15 (4%) | 5 (2%) | 0.844 |
| 1 | 11 (2%) | 7 (2%) | 4 (2%) | ||
| 2 | 13 (2%) | 8 (2%) | 5 (2%) | ||
| 3 | 75 (13%) | 48 (14%) | 27 (13%) | ||
| 4 | 140 (25%) | 89 (26%) | 51 (24%) | ||
| 5 | 186 (33%) | 109 (32%) | 77 (36%) | ||
| 6 | 111 (20%) | 67 (20%) | 44 (21%) | ||
Values are median [interquartile range] or n (%). DGF, delayed graft function; ESRD, end-stage renal disease; CAKUT, congenital anomalies of the kidney and urinary tract; PRA, panel reactive antibody.
Wilcoxon rank sum test for continuous variables and Pearson’s chi-square test for categorical variables.
Values are median [interquartile range] or n (%). DGF, delayed graft function; DCD, donation after circulatory determination of death. HLA, human leukocyte antigen.
Associations with 12-month Graft Function
As shown in Table 2, recipients with UNOS-DGF had significantly lower 12-month eGFR than those without UNOS-DGF with a difference (95% CI) of −11.9 (−15.6, −8.2) ml/min/1.73m2. Results were similar for DGF48h with a difference of −13.2 (−16.9, −9.5) ml/min/1.73m2 compared to recipients without DGF48h. For eGFR <30 ml/min/1.73m2 at 12 months, sensitivity and specificity were 58% and 64%, respectively for UNOS-DGF–81% and 44%, respectively for DGF48h. Adjusted relative risk (95% CI) for 12-month eGFR <30 ml/min/1.73m2 with UNOS-DGF and DGF48h was 1.9 (1.2–3.1) and 2.1 (1.1–3.7), respectively. The AUC (95% CI) for predicting 12-month eGFR <30 ml/min/1.73m2 was 0.62 (0.55–0.68) and 0.63 (0.57–0.68), respectively. The AUC with the clinical model alone was 0.78 (0.72–0.84), increasing slightly to 0.80 (0.74–0.86) with the addition of either UNOS-DGF or DGF48h (Supplemental Figure S1).
Table 2.
Comparison of UNOS-DGF and DGF48h as predictors of 12-month eGFR
| 12-month Outcome | UNOS-DGF | DGF48h | ||||||
|
No n=345 |
Yes n=215 |
Unadjusted Association (95% CI) * |
Adjusted Association (95% CI) ** |
No n=230 |
Yes n=330 |
Unadjusted Association (95% CI) * |
Adjusted Association (95% CI) ** |
|
| eGFR, ml/min/1.73m2 | 60±22 | 48±22 | −11.9 (−15.6, −8.2) | −7.3 (−10.9, −3.6) | 64±22 | 50±21 | −13.2 (−16.9, −9.5) | −7.4 (−11.0, −3.8) |
| eGFR <30 ml/min/1.73m2 | 27 (8) | 38 (18) | 2.3 (1.4, 3.6) | 1.9 (1.2, 3.1) | 12 (5) | 53 (16) | 3.1 (1.7, 5.6) | 2.1 (1.1, 3.7) |
| Acute Rejection | 41 (12) | 25 (12) | 1.0 (0.6, 1.6) | 1.0 (0.6, 1.7) | 23 (10) | 43 (13) | 1.3 (0.8, 2.1) | 1.4 (0.9, 2.4) |
| Death-Censored Graft Failure |
6 (2) | 17 (8) | 4.5 (1.8, 11.3) | 4.7 (1.6, 13.9) | 4 (2) | 19 (6) | 3.3 (1.1, 9.5) | 2.2 (0.7, 6.3) |
| Recipient Death | 7 (2) | 7 (3) | 1.6 (0.6, 4.5) | 1.4 (0.4, 5.7) | 3 (1) | 11 (3) | 2.5 (0.7, 9.0) | 2.1 (0.5, 8.0) |
|
Sensitivity Analyses (after removing 37 recipients with graft failure or death) | ||||||||
| 12-month Outcome | UNOS-DGF | DGF48h | ||||||
|
No n=332 |
Yes n=191 |
Unadjusted Association (95% CI) * |
Adjusted Association (95% CI) ** |
No n=223 |
Yes n=300 |
Unadjusted Association (95% CI) * |
Adjusted Association (95% CI) ** |
|
| eGFR, ml/min/1.73m2 | 60±21 | 52±19 | −8.2 (−11.7, −4.7) | −3.7 (−7.1, −0.3) | 65±20 | 52±19 | −12.2 (−15.6, −8.7) | −7.1 (−10.5, −3.8) |
| eGFR <30 ml/min/1.73m2 | 21 (6) | 18 (9) | 1.5 (0.8, 2.7) | 1.2 (0.7, 2.2) | 7 (3) | 32 (11) | 3.4 (1.5, 7.6) | 2.5 (1.1, 5.4) |
| Acute Rejection | 38 (11) | 22 12) | 1.0 (0.6, 1.7) | 1.0 (0.6, 1.7) | 21 (9) | 39 (13) | 1.4 (0.8, 2.3) | 1.5 (0.9, 2.6) |
United Network for Organ Sharing-defined delayed graft function (UNOS-DGF) is any dialysis in the first posttransplant week. DGF48h is delayed graft function defined as a serum creatinine reduction of <25% in the first 48 h or >1 dialysis session in the first posttransplant week. Values are mean ± standard deviation, n (%), or measure of association (95% confidence interval) as indicated.
Unadjusted associations with continuous eGFR tested via mixed effects linear regression and with dichotomous eGFR <30 ml/min/1.73m2 via modified Poisson regression
Same regressions as above adjusted for the following donor variables: age, circulatory death (rather than brain death), black race, hypertension, diabetes, height, weight, stroke as cause of death, and terminal serum creatinine; and the following transplant/recipient variables: cold ischemia time, age, black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, number of human leukocyte antigen mismatches, body mass index, duration (vintage) of dialysis before transplant, and percent panel reactive antibody.
Continuous values for POD5Cr, POD7Cr, CRR24h, CRR48h, CRR2, CRR3, CRR7, UOP1 and UOP2 were significantly correlated with 12-month eGFR with Pearson coefficients of −0.37, −0.43, 0.25, 0.32, 0.28, −0.10, 0.38, 0.31 and 0.28 respectively (all p-values <0.0001). Using the typical literature-based cutoff and as shown in Table 3, mean 12-month eGFR ranged between 60–67 ml/min/1.73m2 for IGF, compared with values between 50–58 ml/min/1.73m2 for SGF. Mean (SD) 12-month eGFR with UNOS-DGF was 48 (22) ml/min/1.73m2. Table 4 shows adjusted mean (SE) 12-month eGFR by the different IGF-SGF-DGF cutoffs for each of these variables and UNOS-DGF. Only POD5Cr and CRR2 demonstrated significant separation in adjusted mean 12-month eGFR between IGF-SGF-DGF groups (i.e., each group was statistically different from the others via pair-wise comparisons). However, the AIC was smallest for POD7Cr, indicating the fully adjusted model that incorporates a SCr cutoff of 2.5 mg/dL on POD7 for recipients that do not require dialysis may be the best overall predictor of 12-month eGFR relative to all other models studied.
Table 3.
Mean 6, 12 and 24-month eGFR by literature-based definitions of IGF and SGF in recipients that did not require posttransplant dialysis
| Mean 6-month eGFR | Mean 12-month eGFR | Mean 24-month eGFR | |||||
|---|---|---|---|---|---|---|---|
| IGF | SGF | IGF | SGF | IGF | SGF | ||
| Serum creatinine |
Postoperative day 5 | 64 (20) | 51 (20) | 66 (25) | 58 (25) | 66 (25) | 60 (24) |
| Postoperative day 7 | 63 (20) | 48 (20) | 66 (24) | 54 (25) | 67 (24) | 56 (25) | |
| Creatinine reduction ratio |
Over 1st 24 h | 68 (19) | 55 (20) | 70 (26) | 59 (24) | 70 (25) | 61 (24) |
| Over 1st 48 h | 63 (20) | 52 (21) | 65 (25) | 59 (24) | 66 (25) | 60 (24) | |
| Postoperative day 1 to 2 | 65 (20) | 54 (21) | 67 (26) | 59 (24) | 69 (26) | 60 (24) | |
| Over 3 consecutive days in 1st week | 61 (21) | 52 (19) | 63 (25) | 59 (25) | 65 (25) | 60 (24) | |
| Postoperative day 0 to 7 | 64 (20) | 53 (20) | 66 (25) | 58 (24) | 66 (26) | 60 (24) | |
| Days to reach creatinine clearance >10 ml/min | 59 (21) | 44 (17) | 62 (25) | 63 (32) | 64 (25) | 63 (32) | |
| Urine output |
Postoperative day 1 | 60 (21) | 45 (17) | 63 (25) | 52 (22) | 64 (25) | 55 (22) |
| Postoperative day 1 to 2 | 59 (20) | 55 (26) | 63 (25) | 57 (24) | 64 (25) | 62 (23) | |
Values are mean (standard deviation) estimated glomerular filtration rate (eGFR, calculated from serum creatinine via the Chronic Kidney Disease Epidemiology Collaboration equation) in mL/min/1.73m2, that result from categorizing all 345 recipients that did not require dialysis in the first 7 days after transplant as having either immediate graft function (IGF) or slow graft function (SGF) according to each literature-based cutoff as described below. Mean (standard deviation) eGFR at 6, 12 and 24 months for all 345 recipients was 59 (21), 60 (22) and 62 (25) ml/min/1.73m2, respectively. Postoperative day 5 serum creatinine (POD5Cr: IGF <3 mg/dL, n=197; SGF ≥3 mg/dl, n=148), postoperative day 7 serum creatinine (POD7Cr: IGF <2.5 mg/dl, n=240; SGF ≥2.5 mg/dl, n=10), creatinine reduction ratio over the first 24 h (CRR24h: IGF ≥0.25, n=93; SGF <0.25, n=252), creatinine reduction ratio over the first 48 h (CRR48h: IGF ≥0.25, n=204; SGF <0.25, n=141), creatinine reduction ratio from postoperative day 1 to 2 (CRR2: IGF ≥0.3, n=141; SGF <0.3, n=204), creatinine reduction ratio over 3 consecutive days in the first week (CRR3: IGF ≥0.1, n=262; SGF <0.1, n=83), creatinine reduction ratio from postoperative day 0 to 7 (CRR7: IGF ≥0.7, n=184; SGF <0.7, n=161), days to reach a creatinine clearance by Cockroft-Gault equation ≥10 ml/min (CrCl10: IGF ≤6 days, n=331; SGF >6 days, n=14), average hourly urine output from transplant to the morning of postoperative day 1 (UOP1: IGF ≥42 ml/h, n=307; SGF <42 ml/h, n=30), average hourly urine output from the morning of postoperative day 1 to 2 (UOP2: IGF ≥42 ml/h, n=302; SGF <42 ml/h, n=42).
Table 4.
12-month adjusted eGFR by phenotypes for IGF-SGF-DGF.
| IGF-SGF Definition | Adjusted 12-month eGFR | AIC** | P-value*** | |||
|---|---|---|---|---|---|---|
| IGF | SGF | DGF | ||||
| Serum creatinine |
Postoperative day 5 | 56 (3)* | 50 (3)* | 45 (2) | 4786.5 | 0.004 |
| Postoperative day 7 | 56 (3)* | 47 (3) | 45 (2) | 4780.3 | <.001 | |
| Creatinine reduction ratio |
Over 1st 24 h | 55 (3) | 52 (2) | 45 (2)* | 4793.1 | 0.169 |
| Over 1st 48 h | 54 (3) | 51 (3) | 45 (2)* | 4792.8 | 0.144 | |
| Postoperative day 1 to 2 | 56 (3)* | 51 (2)* | 46 (2)* | 4790.6 | 0.036 | |
| Over 3 consecutive days in 1st week | 54 (3) | 50 (3) | 46 (2) | 4791.6 | 0.066 | |
| Postoperative day 0 to 7 | 55 (3) | 51 (3) | 45 (2)* | 4791.6 | 0.068 | |
| Days to reach creatinine clearance >10 ml/min | 53 (2) | 52 (5) | 45 (2) | 4794.9 | 0.899 | |
| Urine output |
Postoperative day 1 Postoperative day 1 to 2 |
53 (2) | 48 (4) | 45 (2) | 4792.8 | 0.144 |
| 53 (2) | 53 (4) | 45 (2)* | 4795.0 | 0.916 | ||
Predicted mean (standard error) 12-month estimated glomerular filtration rate (eGFR) in ml/min/1.73m2 via linear regression models adjusted for the following donor variables: age, circulatory death (rather than brain death), black race, hypertension, diabetes, height, weight, stroke as cause of death, and terminal serum creatinine; and the following transplant/recipient variables: cold ischemia time, age, black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, number of human leukocyte antigen mismatches, body mass index, duration (vintage) of dialysis before transplant, and percent panel reactive antibody.
Immediate graft function (IGF) and slow graft function (SGF) were defined according to the literature-based cutoffs described below. Delayed graft function (DGF) was defined as any dialysis in the first week after transplant according to the United Network for Organ Sharing definition. Postoperative day 5 serum creatinine (POD5Cr: IGF <3 mg/dL, n=197; SGF ≥3 mg/dl, n=148), postoperative day 7 serum creatinine (POD7Cr: IGF <2.5 mg/dl, n=240; SGF ≥2.5 mg/dl, n=10), creatinine reduction ratio over the first 24 h (CRR24h: IGF ≥0.25, n=93; SGF <0.25, n=252), creatinine reduction ratio over the first 48 h (CRR48h: IGF ≥0.25, n=204; SGF <0.25, n=141), creatinine reduction ratio from postoperative day 1 to 2 (CRR2: IGF ≥0.3, n=141; SGF <0.3, n=204), creatinine reduction ratio over 3 consecutive days in the first week (CRR3: IGF ≥0.1, n=262; SGF <0.1, n=83), creatinine reduction ratio from postoperative day 0 to 7 (CRR7: IGF ≥0.7, n=184; SGF <0.7, n=161), days to reach a creatinine clearance by Cockroft-Gault equation ≥10 ml/min (CrCl10: IGF ≤6 days, n=331; SGF >6 days, n=14), average hourly urine output from transplant to the morning of postoperative day 1 (UOP1: IGF ≥42 ml/h, n=307; SGF <42 ml/h, n=30), average hourly urine output from the morning of postoperative day 1 to 2 (UOP2: IGF ≥42 ml/h, n=302; SGF <42 ml/h, n=42).
Single asterisk indicates which (if any) IGF-SGF-DGF group is significantly different from the other 2 groups in terms of 12-month eGFR via pair-wise comparisons for that particular row.
AIC, Akaike information criterion (smaller values indicate better estimated statistical quality of the adjusted model).
Likelihood ratio test for each adjusted IGF-SGF-DGF model against the adjusted null model of UNOS-DGF versus Non-DGF.
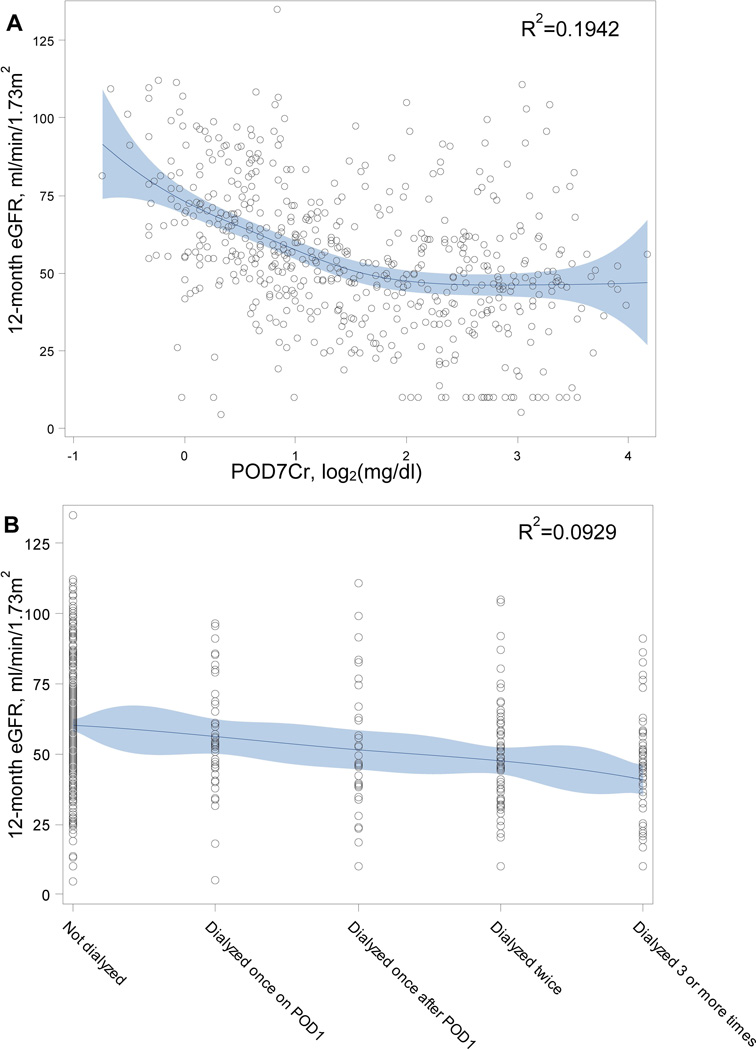
Table 5 provides additional unadjusted and adjusted linear regression results for 12-month eGFR utilizing continuous SCr and UOP-based variables (along with dialysis number and duration) rather than typical cutoffs. AIC results again point to POD7Cr as the strongest predictor. Figure 2 shows spline curves for 12-month eGFR versus POD7Cr and dialysis duration with resulting R2 values (spline curves for the other continuous predictors included as Supplemental Figures S2–S4).
Table 5.
Linear regression for 12-month eGFR using continuous predictors
| Effect | Unadjusted | Adjusted* | AIC | |
|---|---|---|---|---|
| Dialysis number ** | −4.5 (−5.7, −3.3) | −2.9 (−4.1, −1.7) | 4699.3 | |
| Dialysis duration (days) | −0.02 (−0.07, 0.02) | 0.00 (−0.03, 0.04) | 4719.5 | |
| Serum creatinine | Postoperative day 5 (log2 mg/dl) | −7.9 (−9.5, −6.2) | −5.9 (−7.7, −4.1) | 4680.8 |
| Postoperative day 7 (log2 mg/dl) | −8.7 (−10.3, −7.0) | −6.5 (−8.2, −4.8) | 4666.9 | |
| Creatinine reduction ratio |
Over 1st 24 h | 17.5 (10.1, 25.0) | 4.8 (−2.2, 11.9) | 4719.7 |
| Over 1st 48 h | 18.4 (13.1, 23.7) | 9.5 (4.4, 14.6) | 4708.7 | |
| Postoperative day 1 to 2 | 22.8 (15.6, 29.9) | 14.3 (7.7, 20.8) | 4703.5 | |
| Postoperative day 0 to 7 | 20.5 (15.8, 25.3) | 12.8 (8.2, 17.5) | 4692.7 | |
| Days to reach creatinine clearance >10 ml/min | −0.7 (−1.0, −0.3) | −0.5 (−0.8, −0.2) | 4710.2 | |
| Urine Output | Postoperative day 1 (log2 ml/h) | 3.2 (2.4, 4.1) | 2.0 (1.1, 2.9) | 4700.6 |
| Postoperative day 1 to 2 (log2 ml/h) | 3.2 (2.2, 4.2) | 1.9 (0.9, 2.9) | 4707.0 | |
The unadjusted and adjusted values are linear regression coefficients (95% confidence intervals). AIC, Akaike information criterion (smaller values indicate better estimated statistical quality when comparing all of the adjusted models).
Adjusted for the following donor variables: age, circulatory death (rather than brain death), black race, hypertension, diabetes, height, weight, stroke as cause of death, and terminal serum creatinine; and the following transplant/recipient variables: cold ischemia time, age, black race, gender, previous kidney transplant, diabetes as the cause of end stage renal disease, number of human leukocyte antigen mismatches, body mass index, duration (vintage) of dialysis before transplant, and percent panel reactive antibody.
Continuous variable coded as follows: −1 = Not dialyzed, 0 = Dialyzed once on POD1, 1 = Dialyzed once after POD1, 2 = Dialyzed twice, 3 = Dialyzed 3 or more times.
Figure 2.
Basis spline curves. A. 12-month estimated glomerular filtration reate (eGFR) vs. postoperative day 7 serum creatinine concentration (POD7Cr, log2-transformed). B. 12-month eGFR vs. number of dialysis sessions in the first week. Solid line represents the penalized basis spline curve fit with 10 knots and automatically selected smoothing parameter. Shaded area represents the 95% confidence limits.
Secondary Outcomes and Sensitivity Analyses
Table 2 shows acute rejection, death-censored graft failure and recipient death at 12 months separated by UNOS-DGF and DGF48h status. While neither definition was associated with acute rejection or death, both were associated with 12-month death-censored graft failure with unadjusted relative risks of 4.5 (1.8, 11.3) for UNOS-DGF and 3.3 (1.1, 9.5) for DGF48h. However, only UNOS-DGF remained independently associated with an adjusted relative risk for 12-month death-censored graft failure of 4.7 (1.6, 13.9). Rates of acute rejection, death-censored graft failure and death at 12 months varied slightly by the different SCr, UOP and dialysis-based criteria (Supplemental Table S1).
Between 12 and 24 months, an additional 11 recipients experienced acute rejections [total of 77 (14%) by 24 months], 8 developed death-censored graft failure [31 (6%) total], and 7 died [21 (4%) total]. Unadjusted and adjusted results for UNOS-DGF and DGF48h with regard to 24-month outcomes were slightly attenuated but otherwise similar to 12-month results (Supplemental Table S2). Table 3 provides mean (SD) 6-month and 24-month eGFR values in addition to mean 12-month eGFR separated by the different SCr and UOP-based criteria. Figure 3 depicts mean 6, 12 and 24-month eGFR according to dialysis number and duration.
Figure 3.
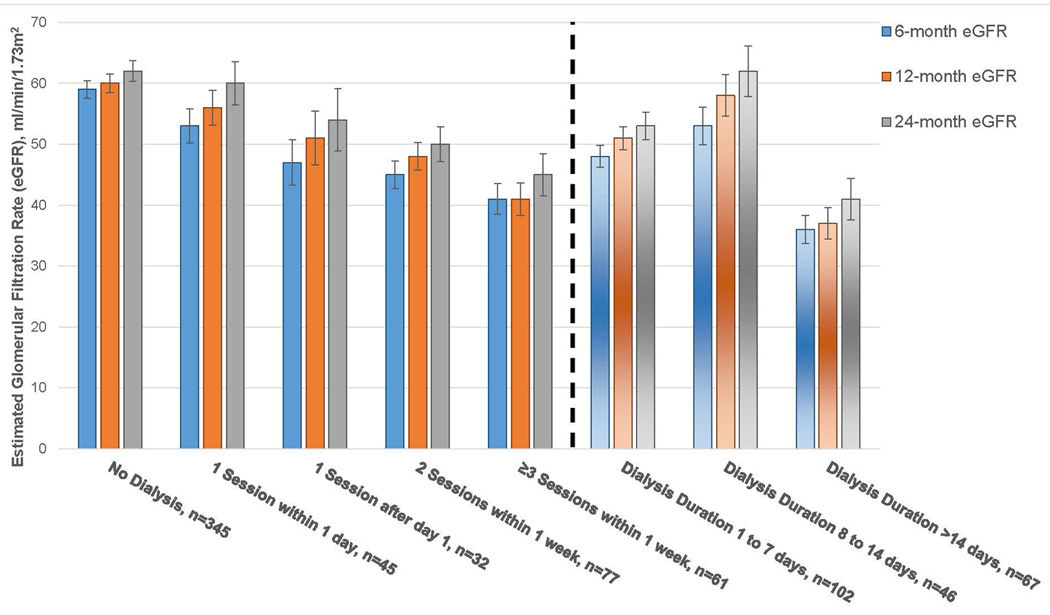
Mean 6, 12 and 24-month estimated glomerular filtration rate (eGFR) by dialysis number and duration. Standard error bars are shown above and below each mean value. A total of 215 recipients were dialyzed at least once in the first week of dialysis. A dotted vertical line separates the different dialysis-based criteria for the cohort.
As shown in Table 2, sensitivity analyses did not substantially alter the primary 12-month results. However, excluding the 37 recipients with graft failure or death within 12 months attenuated the inverse association between UNOS-DGF and 12-month eGFR slightly more than between DGF48h and 12-month eGFR.
There was no apparent effect modification between UNOS-DGF or DGF48h and kidney quality (KDPI >85 or DCD status, all interaction p-values >0.05) with 12-month eGFR. There was also no discernible effect modification between transplant center and DGF48h on 12-month eGFR (p=0.59). The deleterious effect of UNOS-DGF on 12-month eGFR was potentially more severe (interaction p-values <0.05) at 2 of the participating centers with linear regression coefficients for UNOS-DGF ranging from −2.3 to −15.3 among all 5 centers.
Discussion
Using prospective, multicenter cohort data for deceased-donor kidney transplant recipients in the modern era, we have shown that UNOS-defined DGF is associated with a reduced eGFR at 12 months by approximately 7 ml/min/1.73m2 after adjusting for multiple donor, transplant and recipient factors. We found a previously proposed but not validated DGF definition (based on the combination of impaired creatinine reduction in the first 48 hours or >1 dialysis session in the first week)14 resulted in 12-month allograft outcomes that were generally similar to UNOS-DGF. We also found several SCr and UOP-based criteria reported in the literature to describe SGF in the absence of dialysis are associated with 12-month eGFR. Two criteria (POD5Cr and CRR2) demonstrated significant separation in adjusted 12-month eGFR between all 3 IGF-SGF-DGF groups; however, POD7Cr demonstrated the strongest overall association with 12-month eGFR.
There continues to be much interest in appraising peri-transplant function. Mallon et al recently reported comparable predictive power, in terms of 12-month eGFR and long-term graft survival, for 10 DGF definitions at a single center in the UK.29 The authors concluded by recommending universal adoption of the UNOS-DGF definition given the arguable lack of superiority for any other definition evaluated, its descriptive simplicity, and its applicability regarding both length of stay and retrospective analyses. We would offer (what we believe also reflects FDA recommendations) that the primary motivation to improve peri-transplant functional assessment should be the development of a reliable early surrogate outcome for use in clinical trials, with less emphasis on retrospective studies.
Future trials to reduce in-hospital dialysis after transplant could be worthwhile in moderating costs. However, with meaningful improvements in long-term allograft function and longevity as the primary goal, we could expect even greater and more durable savings in terms of the economics of chronic kidney disease care, need for retransplantation, and recipient work productivity. As an example of the potential impact of using another definition, data from the current study show that 59% of recipients without UNOS-DGF would be correctly reclassified as high risk (14% with UNOS-DGF correctly reclassified as low risk) for poor 12-month allograft function (eGFR <30 ml/min/1.73m2) if the early outcome were instead defined by DGF48h. This alternate definition is clearly not perfect, however, and could even be deleterious to future trial design given tradeoffs between improved sensitivity and worse specificity for 12-month eGFR <30 ml/min/1.73m2 compared with the UNOS-DGF definition. In terms of other dialysis-based definitions, we noted a significant step-wise decrease in 12-month eGFR according to dialysis number, but we were somewhat surprised by numerically (though not significantly) better eGFRs for dialysis durations between 8–14 days compared with durations between 1–7 days. Most importantly, these analyses highlight the limitations of current DGF definitions based on SCr, UOP and/or dialysis.
The current study also demonstrates an important consideration about allograft functional assessments both peri-transplant and during follow-up–ie, our continued reliance on SCr itself. SCr does not accurately reflect GFR in the nonsteady state, which is the rule peri-transplant and a common occurrence during follow-up. Numerous conditions other than GFR also affect SCr, including muscle mass and medications. In addition, because dialysis removes creatinine, pre-transplant dialysis timing can greatly affect CRRs, which we observed while investigating apparent outliers in this cohort. This issue leads some to ignore SCr on post-dialysis days (eg, CrCl10 in the current study based on work by Giral-Classe et al.).15 Furthermore, because SCr is used to estimate GFR, the fact that POD7Cr had the strongest association with 12-month eGFR is not too surprising and is consistent with the SCr data (particularly POD7 and 10) reported by Mallon et al.29 In terms of allograft survival, a recently proposed tool to predict 5-year survival on POD7 incorporated discharge eGFR with dialysis-defined DGF along with multiple other variables.30
We would anticipate that later SCr and/or eGFR values would associate with subsequent allograft function and survival even better than POD7Cr. Kaplan et al showed each 1 mg/dl increase in SCr during the first year of follow-up was associated with over 2-fold risk for allograft failure,31 but they argued that the change in SCr had poor predictive value with an AUC for allograft failure of only 0.627. While Smail and colleagues noted worse death-censored allograft survival in recipients of expanded-criteria kidneys with SGF (which they defined as CRR <20% in the first 24 hours) but no allograft survival difference based on dialysis-defined DGF, they found a >30% decline in eGFR from month 1 to 12 after transplant was associated with worse allograft survival (hazard ratio of 2.2, p=0.02).32 Most recently, Clayton et al also found that a ≥30% decline in eGFR (though from year 1 to 3 after transplant) was associated with over 5-fold risk of subsequent death-censored graft failure.33 As these prior reports infer, rather than relying on predictors that only become available years later, additional prospective studies are needed to discover effective, mechanistic peritransplant predictors of long-term allograft survival. We believe more studies are needed to determine whether measuring the degree of ischemic injury at transplant with biomarkers like neutrophil gelatinase-associated lipocalin or interleukin-18,19,27 or assessing the allograft’s early response to injury with biomarkers like YKL-40,34 better capture the biology of peri-transplant renal IRI and recovery as it relates to long-term allograft function and longevity.
Study strengths include careful, standardized and prospective data collection for a relatively large number of recipients at multiple transplant centers with very few losses to follow-up. There are some limitations to consider, however. Despite multivariable adjustment, residual confounding is possible given the observational design. Also, we did not adjust for multiple comparisons in order to identify potential relationships requiring additional study. To evaluate the effects of DGF related to severe allograft IRI at transplant on 12-month outcomes, living-donor kidney transplants were not included. Though 12-month (and subsequently 24-month) follow-up data are now complete, longer-term outcome information is not yet available for the entire cohort.
In conclusion, experimental evidence as well as clinical data from native kidney settings suggest the severity and duration of renal IRI affects subsequent function following recovery. DGF is the corollary to this concept in kidney transplantation. With unquestioning acceptance of DGF as dialysis in the first week, however, we run the risk of forgetting that DGF is a (dichotomous) clinical term to describe poor allograft recovery after IRI (which likely varies across a spectrum). Early dialysis may be indicated for reasons unrelated to severe allograft injury (eg, hyperkalemia with surgical fasting), which may not adversely affect long-term allograft function. We noted SCr on POD7 had the strongest association with 12-month eGFR while 2 other SCr-based criteria demonstrated clear separation in 12-month eGFR with regard to SGF in the absence of dialysis. However, our data indicate that none of the current definitions for DGF or SGF are ideal, stand-alone surrogate outcomes in transplantation, though some are probably sufficient in certain situations (eg, as practical endpoints for low-cost pragmatic trials to optimize peri-transplant care). Nonetheless, the question remains whether pathophysiologic biomarkers of kidney injury and recovery could improve early prognostic determinations at the individual-patient level and prove useful for therapeutic trials. The transplant community should not grow complacent with a DGF definition that is simply “good enough” for more retrospective analyses.
Supplementary Material
Acknowledgments
Dr. Parikh had full access to all of the study data and takes responsibility for its integrity and the accuracy of all analyses. Dr. Parikh also affirms that everyone who made substantial contributions to this work has been properly listed.
The authors wish to acknowledge the advice and support from the study Advisory Board members: Amit Garg, M.D., Ph.D., Peter Heeger, M.D., and Fadi Lakkis, M.D. The authors thank Isabel Butrymowicz, M.D. for assistance with data coordination for this multicenter study and for the participation of study partners at the following OPOs: Gift of Life Philadelphia (Sharon West, Vicky Reilly), the New York Organ Donor Network (Harvey Lerner, Anthony Guidice, Allison Hoffman), the Michigan Organ and Tissue Donation Program (Burton Mattice, Susan Shay), and the New Jersey Sharing Network (William Reitsma, Cindy Godfrey, Alene Steward, Joel Padilla Benitez).
This work was supported by 1) the National Institutes of Health grant R01DK-93770, grant K24DK090203, 2) a Roche Organ Transplantation Research Foundation Award to Dr. Parikh, and 3) a career development award from the American Heart Association to Dr. Hall. None of these organizations or funding agencies were involved in study design, analysis, interpretation, or manuscript creation. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
Abbreviations
- AKI
acute kidney injury
- CRR
creatinine reduction ratio
- DCD
donation after circulatory determination of death
- DGF
delayed graft function
- eGFR
estimated glomerular filtration rate
- FDA
Food and Drug Administration
- IGF
immediate graft function
- IRI
ischemia-reperfusion injury
- KDPI
kidney donor profile index
- KDRI
kidney donor risk index
- OPTN
Organ Procurement and Transplantation Network
- POD
postoperative day
- SCr
serum creatinine
- SGF
slow graft function
- UNOS
United Network for Organ Sharing
- UOP
urine output
Footnotes
-
Isaac E. Hall, MD, MSDr. Hall participated in the design and analyses for the study, interpreted the results and drafted the manuscript. This work was funded in part by a career development award that Dr. Hall received from the American Heart Association.
-
Peter P. Reese, MD, MSCEDr. Reese participated by contributing study subjects, interpreting results and helping with manuscript revisions.
-
Mona D. Doshi, MDDr. Doshi participated by contributing study subjects, interpreting results and helping with manuscript revisions.
-
Francis L. Weng, MD, MSCEDr. Weng participated by contributing study subjects, interpreting results and helping with manuscript revisions.
-
Bernd Schröppel, MDDr. Schröppel participated by contributing study subjects, interpreting results and helping with manuscript revisions.
-
William S. Asch, MD, PhDDr. Asch participated by interpreting results, providing important feedback and helping with manuscript revisions.
-
Joseph Ficek, MA, MSMr. Ficek participated by performing the statistical analyses and helping with manuscript revisions.
-
Heather Thiessen-Philbrook, MMathMs. Thiessen-Philbrook participated by providing important statistical feedback and helping with manuscript revisions.
-
Chirag R. Parikh, MD, PhDDr. Parikh conceived of the study, participated in its design, interpreted the results and helped write the manuscript.
The authors have no relevant financial conflicts of interest to report.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2014. pp. 11–44. [Google Scholar]
- 2.Hagenmeyer EG, Haussler B, Hempel E, et al. Resource use and treatment costs after kidney transplantation: impact of demographic factors, comorbidities, and complications. Transplantation. 2004;77(10):1545–1550. doi: 10.1097/01.tp.0000121763.44137.fa. [DOI] [PubMed] [Google Scholar]
- 3.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 4.Butala NM, Reese PP, Doshi MD, Parikh CR. Is Delayed Graft Function Causally Associated With Long-term Outcomes After Kidney Transplantation? Instrumental Variable Analysis. Transplantation. 2013;95 doi: 10.1097/TP.0b013e3182855544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J. Med. 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 6.Moers C, Pirenne J, Paul A, Ploeg RJ. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J. Med. 2012;366(8):770–771. doi: 10.1056/NEJMc1111038. [DOI] [PubMed] [Google Scholar]
- 7.Cavaille-Coll M, Bala S, Velidedeoglu E, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13(5):1134–1148. doi: 10.1111/ajt.12210. [DOI] [PubMed] [Google Scholar]
- 8.Singh RP, Farney AC, Rogers J, et al. Kidney transplantation from donation after cardiac death donors: lack of impact of delayed graft function on post-transplant outcomes. Clin Transplant. 2011;25(2):255–264. doi: 10.1111/j.1399-0012.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 9.Wadei HM, Heckman MG, Rawal B, et al. Comparison of kidney function between donation after cardiac death and donation after brain death kidney transplantation. Transplantation. 2013;96(3):274–281. doi: 10.1097/TP.0b013e31829807d1. [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney. Int. 2010;78(9):926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern. Med. 2011;171(3):226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 12.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney. Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akkina SK, Connaire JJ, Israni AK, Snyder JJ, Matas AJ, Kasiske BL. Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. Am J Transplant. 2009;9(6):1460–1466. doi: 10.1111/j.1600-6143.2009.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giral-Classe M, Hourmant M, Cantarovich D, et al. Delayed graft function of more than six days strongly decreases long-term survival of transplanted kidneys. Kidney. Int. 1998;54(3):972–978. doi: 10.1046/j.1523-1755.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 16.Govani MV, Kwon O, Batiuk TD, Milgrom ML, Filo RS. Creatinine reduction ratio and 24-hour creatinine excretion on posttransplant day two: simple and objective tools to define graft function. J Am Soc Nephrol. 2002;13(6):1645–1649. doi: 10.1097/01.asn.0000014253.40506.f6. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo E, Ruiz JC, Pinera C, et al. Creatinine reduction ratio on post-transplant day two as criterion in defining delayed graft function. Am J Transplant. 2004;4(7):1163–1169. doi: 10.1111/j.1600-6143.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnston O, O'Kelly P, Spencer S, et al. Reduced graft function (with or without dialysis) vs immediate graft function--a comparison of long-term renal allograft survival. Nephrol Dial Transplant. 2006;21(8):2270–2274. doi: 10.1093/ndt/gfl103. [DOI] [PubMed] [Google Scholar]
- 19.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR. Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol. 2012;7(8):1224–1233. doi: 10.2215/CJN.00310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OPTN: Organ Procurement and Transplantation Network. A Guide to Calculating and Interpreting KDPI. [Accessed January 17, 2013];2012 http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=81.
- 21.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 22.Turkowski-Duhem A, Kamar N, Cointault O, et al. Predictive factors of anemia within the first year post renal transplant. Transplantation. 2005;80(7):903–909. doi: 10.1097/01.tp.0000173791.42893.08. [DOI] [PubMed] [Google Scholar]
- 23.Zeraati AA, Naghibi M, Kianoush S, Ashraf H. Impact of slow and delayed graft function on kidney graft survival between various subgroups among renal transplant patients. Transplant Proc. 2009;41(7):2777–2780. doi: 10.1016/j.transproceed.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt R, Kupin W, Dumler F, Venkat KK, Mozes M. Influence of the pretransplant hematocrit level on early graft function in primary cadaveric renal transplantation. Transplantation. 1993;55(5):1034–1040. doi: 10.1097/00007890-199305000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Boom H, Mallat MJ, de Fijter JW, Zwinderman AH, Paul LC. Delayed graft function influences renal function, but not survival. Kidney. Int. 2000;58(2):859–866. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Halloran PF, Hunsicker LG. Delayed graft function: state of the art, November 10–11, 2000. Summit meeting, Scottsdale, Arizona, USA. Am J Transplant. 2001;1(2):115–120. [PubMed] [Google Scholar]
- 27.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. 2013;96(10):885–889. doi: 10.1097/TP.0b013e3182a19348. [DOI] [PubMed] [Google Scholar]
- 30.Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Peng Y, Weinhandl ED. A simple tool to predict outcomes after kidney transplant. Am J Kidney. Dis. 2010;56(5):947–960. doi: 10.1053/j.ajkd.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan B, Schold J, Meier-Kriesche HU. Poor predictive value of serum creatinine for renal allograft loss. Am J Transplant. 2003;3(12):1560–1565. doi: 10.1046/j.1600-6135.2003.00275.x. [DOI] [PubMed] [Google Scholar]
- 32.Smail N, Tchervenkov J, Paraskevas S, et al. Impact of early graft function on 10-year graft survival in recipients of kidneys from standard- or expanded-criteria donors. Transplantation. 2013;96(2):176–181. doi: 10.1097/TP.0b013e318297443b. [DOI] [PubMed] [Google Scholar]
- 33.Clayton PA, Lim WH, Wong G, Chadban SJ. Relationship between eGFR Decline and Hard Outcomes after Kidney Transplants. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt IM, Hall IE, Kale S, et al. Chitinase-Like Protein Brp-39/YKL-40 Modulates the Renal Response to Ischemic Injury and Predicts Delayed Allograft Function. J Am Soc Nephrol. 2013;24(2):309–319. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.