Abstract
Patients with advanced epithelial ovarian cancer often experience disease recurrence after standard therapies, a critical factor in determining their five-year survival rate. Recent reports indicated that long-term or short-term survival is associated with varied gene expression of cancer cells. Thus, identification of novel prognostic biomarkers should be considered. Since the mouse genome is similar to the human genome, we explored potential prognostic biomarkers using two groups of mouse ovarian cancer cell lines (group 1: IG-10, IG-10pw, and IG-10pw/agar; group 2: IG-10 clones 2, 3, and 11) which display highly and moderately aggressive phenotypes in vivo. Mice injected with these cell lines have different survival time and rates, capacities of tumor, and ascites formations, reflecting different prognostic potentials. Using an Affymetrix Mouse Genome 430 2.0 Array, a total of 181 genes were differentially expressed (P<0.01) by at least twofold between two groups of the cell lines. Of the 181 genes, 109 and 72 genes were overexpressed in highly and moderately aggressive cell lines, respectively. Analysis of the 109 and 72 genes using Ingenuity Pathway Analysis (IPA) tool revealed two cancer-related gene networks. One was associated with the highly aggressive cell lines and affiliated with MYC gene, and another was associated with the moderately aggressive cell lines and affiliated with the androgen receptor (AR). Finally, the gene enrichment analysis indicated that the overexpressed 89 genes (out of 109 genes) in highly aggressive cell lines had a function annotation in the David database. The cancer-relevant significant gene ontology (GO) terms included Cell cycle, DNA metabolic process, and Programmed cell death. None of the genes from a set of the 72 genes overexpressed in the moderately aggressive cell lines had a function annotation in the David database. Our results suggested that the overexpressed MYC and 109 gene set represented highly aggressive ovarian cancer potential biomarkers while overexpressed AR and 72 gene set represented moderately aggressive ovarian cancer potential biomarkers. Based on our knowledge, the current study is first time to report the potential biomarkers relevant to different aggressive ovarian cancer. These potential biomarkers provide important information for investigating human ovarian cancer prognosis.
Keywords: Ovarian cancer, Bioinformatics, Prognostic biomarkers
Introduction
Ovarian cancer is the most lethal gynecological malignancy and the fifth leading cause of cancer death in women [1]. About 90 % of the disease is classified as an epithelial subtype (American Cancer Society), of which approximately 70 % is in an advanced stage (III and IV) at the time of initial diagnosis [2]. The five-year survival rate for those patients is less than 30 % [3]. This is due to acquired resistance to the standard treatment (surgery followed by platinum/taxol chemotherapy) of advanced epithelial ovarian cancer (EOC) that eventually leads to disease recurrence [4, 5]. Recent studies showed that expression or mutation of some biomarkers in epithelial ovarian cancer were important factors in influencing disease recurrence, and patients’ long-term and short-term survival. Patients with p53-positive tumors (alone/or combined with p27 and/or C-MYC) were associated with significantly lower survival rate [6]. Patients with overexpressed gene TUBA3C which encodes the production of α-tubulin were suggested to be resistant to taxol chemotherapy and had short-term survival [7]. More recently, a report indicated that deletion at 6q24.2-26 of chromosome predicts longer survival of high-grade serous epithelial ovarian cancer patients [8], suggesting that many genes in this area play a role in either chemotherapy resistance or short-term survival. Thus, identification of novel prognostic biomarkers is highly desirable. Such prognostic biomarkers would be clinically useful to predict long-term and short-term survival and to provide gene-related therapy potentials.
In this study, we used a murine model of epithelial ovarian cancer IG-10 [9] and its derived cell clones to mimic human ovarian cancer recurrence and short-term and long-term survival. The clones displayed highly aggressive and moderately aggressive phenotypes in vivo, i.e., inducing different tumor-cell-injected mice mortalities and different survival time [10]. The mice injected with highly aggressive cell lines grew more solid tumors, formed greater volumes of the malignant ascites, and had 100 % mortality. In contrast, the mice injected with moderately aggressive cell lines grew less solid tumors, formed less volumes of the malignant ascites, and had varied mortalities. Limited microarray and protein array analysis showed that MIP-2, CCL5, CXCL10, and IL-1 are expressed differently in highly and moderately aggressive phenotypes of the cell lines [10]. Thus, we believe that, by using Affymetrix GeneChip Mouse Genome 430.2.0 microarray which can analyze over 39,000 transcripts on a single array, more biomarkers can be identified. Since the gene profiles and the metabolic pathways of parental IG-10 cell line resemble with human ovarian cancer cell lines [11], the novel potential biomarkers identified in this study have a potential for human prognostic application.
Material and methods
Ethics statement
The Xavier University of Louisiana Institutional Animal Care and Use Committee (IACUC) approved animal protocol (012711-001BI) used in this study. C57BL/6 mice (6-week-old females) were purchased from Charles River Laboratories and were maintained in pathogen-free animal facilities in Xavier University of Louisiana. Each ventilated and sealed cage contained five mice with bedding materials of aspen shavings or shreds. All mice were treated in accordance with the Institute of Laboratory Animal Research (NIH, Bethesda, MD) Guide for the Care and Use of Laboratory Animals. In in vivo experiments, when the tumor size reached to a volume of 30×102 (mm3) or the mice exhibited signs of suffering, they were euthanized with CO2.
Cell line and cell culture
Murine epithelial ovarian cancer IG-10 cell line and its clone cell lines IG-10pw, IG-10pw/agar, and IG-10 clones 2, 3, and 11 were cultured in a modified conventional Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Rockville, MD, USA). The modified DMEM contains 2 mM l-glutamine, 100 IU/ml penicillin, 10 % FCS, 100 μg/ml streptomycin, and 20 mM HEPES.
Mouse survival
Each cell line was injected intraperitoneally (i.p.) into 10 to 15 C57BL/6 mice at a concentration of 5×105 cells/mouse. Mice survival was monitored for a period of 140 days. Survival rates, solid tumor formations, and volume of ascites formations were evaluated. The Kaplan-Meier log rank survival test was used to analyze the mouse survival, and the differences were determined with P<0.05.
RNA preparation
Total RNA was isolated from IG-10 and its clones using the RNeasy isolation kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. To remove possible genomic DNA contaminants, 2 μg total RNA from each sample was treated with 2 U RNAse-free deoxyribonuclease I and 1× DNase I reaction buffer (Invitrogen, Carlsbad, CA, USA) in a final volume of 20 μl for 15 min at 25 °C. The RNA samples were used for Affymetrix mouse genome arrays and qRT-PCR analysis. After concentration, the purified RNAwas measured by spectrophotometry, and 1 μg of RNA from each sample was reverse transcribed to cDNA in a final volume of 50 μl using a TaqMan reverse transcriptase kit with random hexamers according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). The resulting cDNA (20 ng/ml) was frozen at −20 °C until use.
Expression analyses using oligonucleotide arrays
After evaluation of the RNA integrity by electrophoresis performed on the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), the RNA samples were reverse transcribed to double-stranded cDNA using superscript cDNA synthesis Kit (Invitrogen, Carlsbad, CA) and a T7-(dT)24 primer (Proligo, Boulder, CO). Approximately 5 μg of total RNA was needed for the cDNA synthesis. Biotin-labeled cRNA was synthesized in vitro from the double-stranded cDNA using Expression 3′ Amplification reagents (Affymetrix, Santa Clara, CA). Biotinylated cRNA was then purified with the Affymetrix GeneChip Sample Cleanup Module. Purified cRNA samples (20 μg) were prepared in a fragmentation buffer containing 200 mM Tris-acetate, pH 8.1, 500 mM potassium acetate, and 150 mM magnesium acetate, heated at 94 °C for 35 min, and cooled down on ice. Fragmented cRNA (10 μg) from each sample was hybridized to the GeneChip Mouse Genome 430 2.0 Array according to the manufacturer's instruction (Affymetrix). Briefly, arrays were incubated for 16 h at 45 °C with constant rotation (60 rpm) before washed. Streptavidin-R phycoerythrin (Vector Laboratories, Burlingame, CA) was used to stain the arrays for 10 min at 25 °C with 10 μg/ml and then treated with 3 μg/ml biotinylated goat anti-streptavidin antibody (Vector Laboratories) for 10 min at 25 °C. Then, the arrays were then stained once again with streptavidin-R phycoerythrin for 10 min at 25 °C. The arrays were then scanned and processed using a GeneChip Scanner 3000. The data generated were further analyzed and processed using the GeneChip Operating Software 1.4 (Affymetrix).
Microarray data processing
Global normalization with a target intensity value of 500 was performed. The absolute call (present, marginal, absent) of each gene expression in each sample, as well as the direction of change, and fold change of gene expressions between samples were identified using the commercial software package GeneChip Operating Software 1.4 (Affymetrix). The web-based software GeneSifter v. 4.0 (www.genesifter.com, VizX Labs, Seattle, WA) was used to analyze the normalized microarray data. A two-tailed t test with a quality filter value of 1 (present) in at least one out of six tested samples was used. More than twofold changes with 99 % significance (P<0.01) were used to select corresponding genes between two group samples. In addition, gene clusters were analyzed by using Partitioning Around Medoids (PAM) clustering with Euclidean for the distance and row means as the row center. Clustering these genes into two clusters yielded the largest mean silhouette width. The global normalized microarray data was in the supplement as Table 1.
Quantitative real-time PCR validation of gene expression
Quantitative real-time PCR (qRT-PCR) was performed to confirm quantitative mRNA expression in IG-10 and its clone cell lines. Six genes selected from the Affymetrix microarray results were Fstl1, Fscn1, Cyp1b1, Gadd45g, Myc, and AR, and the primer sequences were listed in Table 1. The quantitative expression of these genes in different cell lines was normalized with self-housekeeping genes Gusb. RNA primers were selected based on Mouse Genome 430 2.0 Array from Affymetrix company as shown in Table 1. RNA primers were diluted according to the manufacturer's instructions. The following reagents were mixed and added into each well of a 96-well PCR plate: 12.5 μl 2× QuantiFast SYBR, 2.5 μl forward and reverse primer, 0.25 μl QuantiFast RT Mix, 0.25 μl extracted RNA (1.11 μg/μl), and 7 μl DDI H2O. Duplicates of each sample were prepared. The 96-well plate was sealed with caps and run on iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) for reverse PCR. Negative controls were prepared by samples without adding any extracted RNA and samples without adding QuantiFast RT Mix. Forty cycles were run on the above samples. The results were analyzed by iQ5 Optical System Software (version 2.1), and the threshold was set as 200. Two experiments were performed, and the average of two experiments was shown.
Table 1.
RT-PCR primer sequences for selected genes
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Fstl1 | 5′GCAGGTGACTGGGTATGAGG3′ | 5′CCCCAGTGCTGATTCTCAAT3′ |
| Fscn1 | 5′TCAACACCATGCATCTCACTC3′ | 5′CCAGTTCAAAGCTTACTATCATGG3′ |
| Cyp1b1 | 5′TTTTGCTGCTCACCATGTTC3′ | 5′GCTAAGTTCTGGGGCATGAA3′ |
| Gadd45g | 5′GAGGCTGCTAGCACAGGAAG3′ | 5′AGCAGTGCAGTCGGCTAAGT3′ |
| Myc | 5′CAACGTCTTGGAACGTCAGA3′ | 5′TGTTCTCGTCGTTTCCTCAA3′ |
| Ar | 5′CAAGTGCCCAAGATCCTTTC3′ | 5′TGAGGAACCTGTTCACGACA3′ |
| Gusb | 5′CTTTTCTTCCGTGGGGATAA3′ | 5′ATTGTGAGCCAGCCTTCACT3′ |
Pathway and gene ontology analysis
Genes with twofold differences and P<0.01 were selected and imported into Ingenuity Pathway Analysis (IPA) software (provided by Dr. Erik K. Flemington, Tulane University) for the ontology analysis, biological pathways, and potential networks. Networks were built based on the connectivity of these genes. The network score equals the negative Log of the P value calculated based on the hypergeometric distribution with the right-tailed Fisher's exact test. Thus, a score of greater than 2 indicates that the network was more than 99 % confidence of built from non-random chance selection. Functional enrichment analysis based on gene ontology (GO) database was performed by using David 6.7 program (http://david.abcc.ncifcrf.gov/).
Results
Differential aggressiveness of IG-10 and its clone cell lines
Six murine epithelial ovarian cancer cell lines were investigated. Five of them were reported previously for their tumor aggressiveness such as IG-10, IG-10pw, and IG-10 clones 2, 3, and 11 [10]. IG-10 and IG-10pw cell lines displayed highly aggressive tumorigenic capacities (i.e., 100 % mortality and short-term survival with formation of more solid tumors and more volume of ascites), while IG-10 clone 2, 3, and 11 cell lines displayed moderately aggressive tumorigenic capacities (i.e., varied mortalities and long-term survival with formation of less solid tumors and less volume of ascites) [10]. Another clone, IG-10pw/agar cell line, was derived from the IG-10pw cells that grew in the soft agar and were selected as a clone (soft agar conditions were described in [10]). This cell line displayed higher aggressive tumorigenicity similar to the IG-10 and the IG-10pw in vivo. Table 2 displays the combined results of the experiments performed at least two times, including previously published data [10]. All mice inoculated intraperitoneally (i.p) with highly aggressive tumor cell lines (5×106 cells per mouse) had no survivors during a relatively shorter period of time, and generated more ‘solid’ tumors (tumor masses) and greater volumes of malignant ascites in abdominal cavities, while mice injected with moderately aggressive tumor cell lines displayed the survivors exhibiting a range of mortality rates over a longer period time, and generated less ‘solid’ tumors and less volumes of malignant ascites in abdominal cavities (see Table 2).
Table 2.
Survival of mice injected with different cell lines
| Cell lines | Aggressiveness | Solid tumors | Ascites volume (ml) | Mice survival rate and time (days) |
|---|---|---|---|---|
| IG-10 | Highly | >30 | 0 % (62 days) | |
| IG-10pw | Highly | >30 | 10–25 ml | 0 % (44 days) |
| IG-10pw/agar | Highly | >30 | 0 % (85 days) | |
| IG-10 clone 2 | Moderately | <4 | 25 % (>140 days) | |
| IG-10 clone 3 | Moderately | <4 | <10 ml | 55 % (>140 days) |
| IG-10 clone 11 | Moderately | <4 | 75 % (>140 days) |
Each group contains 10–15 mice, and the numbers of solid tumors and ascites volume were detected at the time of death. In vivo data was collected from two to three experiments including previous published experiment [10]. The solid tumors stand for tumor masses that grew on the membrane and the organs of the abdominal cavity
Differential expression profiles of mRNAs in highly and moderately aggressive ovarian cancer cell lines
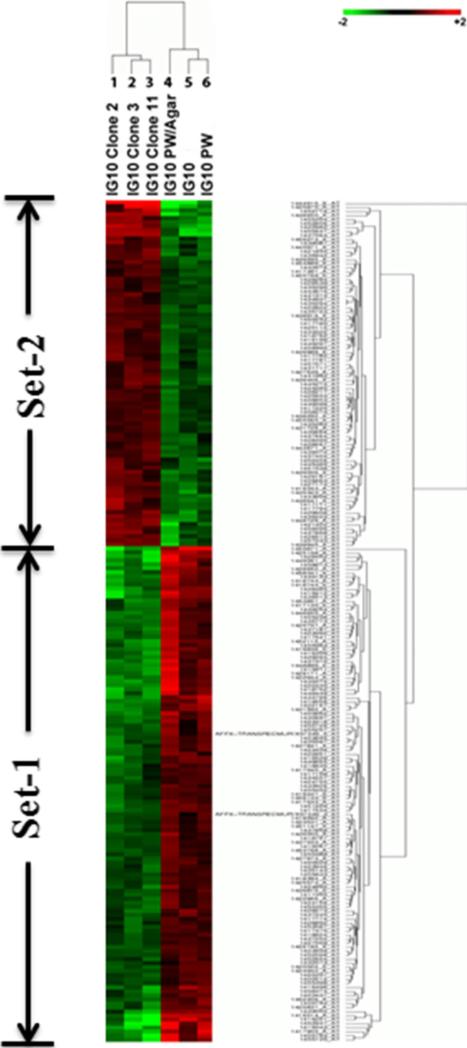
The difference in aggressiveness of murine ovarian cancer cell lines implied a possibility of different gene expression profiles. To analyze gene expression profiles, we performed a microarray assay. The mRNA samples from six ovarian cancer cell lines were analyzed using a Mouse Genome 430 2.0 Array which could analyze over 39,000 transcripts in each of our cell lines. Three highly aggressive cell lines, IG-10, IG-10pw, and IG-10pw/agar, were compared with three moderately aggressive cell lines, IG-10 clone 2, IG-10 clone 3, and IG-10 clone 11. Based on 99 % significance (P<0.01) and a two-fold change cutoff, a total of 181 gene transcripts were displayed differentially between the highly and moderately aggressive cancer cell lines. Among the 181 transcripts, 109 gene transcripts (Fig. 1, Set 1) were transcriptionally overexpressed significantly in three highly aggressive cell lines while 72 gene transcripts (Fig. 1, Set 2) were overexpressed significantly in moderately aggressive IG-10 clone 2, 3, and 11 cells. The identified genes that were overexpressed in either highly aggressive or moderately aggressive cancer cell lines are listed in Tables 3 and 4.
Fig. 1.
Differentially expressed genes between the highly and moderately aggressive ovarian cancer cells. Six samples from left to right are IG-10 clone 2, IG-10 clone 3, IG-10 clone 11, IG-10pw/agar, IG-10, and IG-10pw, respectively. The genes were selected with a twofold difference and P<0.01 between the two groups. Genes in set 1 were overexpressed in highly aggressive ovarian cancer cell lines; genes in set 2 were overexpressed in moderately aggressive ovarian cancer cell lines
Table 3.
Genes overexpressed in highly aggressive ovarian cancer cell lines
| Gene ID | Ratioa | Gene ID | Ratioa | Gene ID | Ratio |
|---|---|---|---|---|---|
| Bcat1 | 12.6 | Nusap1 | 3.45 | Bag2 | 2.33 |
| Gprc5c | 9.02 | Mum1l1 | 3.39 | Fam110a | 2.32 |
| Fscn1 | 7.64 | Trip13 | 3.38 | Phlda1 | 2.32 |
| 2810025M15Rik | 7.00 | E2f7 | 3.35 | Speg | 2.32 |
| Fstl1 | 6.65 | Ammecr1 | 3.29 | Skp2 | 2.28 |
| Nasp | 6.63 | 4930547N16Rik | 3.26 | Bok | 2.27 |
| Fam149a | 6.40 | Gadd45g | 3.25 | Flt1 | 2.25 |
| BC004728 | 6.24 | Sec16b | 3.11 | Tinagl1 | 2.25 |
| Cyp1b1 | 5.82 | Incenp | 3.10 | Trim16 | 2.24 |
| Aldh3a1 | 5.43 | Sh3kbp1 | 3.04 | Ntn4 | 2.23 |
| Prim1 | 5.43 | Hells | 3.03 | Pdlim2 | 2.23 |
| Dlg7 (Dlgap5) | 5.31 | Emp1 | 2.98 | Naaa | 2.19 |
| Vldlr | 5.29 | Rad18 | 2.98 | Elk3 | 2.18 |
| Ppp1r12b | 5.28 | C79407 | 2.97 | Tbc1d1 | 2.17 |
| Tanc2 | 4.93 | Lbh | 2.93 | Cd97 | 2.16 |
| Kntc1 | 4.66 | Traip | 2.91 | Ggt6 | 2.15 |
| Scn5a | 4.54 | Ccrn4l | 2.86 | Ifrd1 | 2.15 |
| Ripk3 | 4.47 | Sema3a | 2.83 | Slk | 2.15 |
| Car12 | 4.42 | Angptl4 | 2.79 | Afap1l2 | 2.15 |
| Fignl1 | 4.41 | Itgb3 | 2.76 | Apobec3 | 2.14 |
| Hopx | 4.41 | Erdr1 | 2.70 | Pgm2 | 2.13 |
| Sgol2 | 4.23 | Spp1 | 2.70 | Dpy19l3 | 2.12 |
| Rad51ap1 | 4.22 | Hmga2 | 2.66 | Nup155 | 2.12 |
| Uhrf1 | 4.19 | Atad5 | 2.65 | 6230416J20Rik | 2.11 |
| Sprr1a | 4.14 | Bmper | 2.64 | Nans | 2.11 |
| Rtn2 | 4.01 | Csdc2 | 2.63 | Asap1 | 2.10 |
| Slc16a3 | 4.01 | Gpsm2 | 2.63 | Hip1 | 2.08 |
| Pde5a | 3.96 | Gsta3 | 2.62 | Arf6 | 2.06 |
| Plk4 | 3.96 | Rbl1 | 2.58 | Lrrc8c | 2.06 |
| Sema5a | 3.90 | Gm71 | 2.57 | Ppil1 | 2.05 |
| Ncapg | 3.88 | Itgb5 | 2.53 | Zdhhc2 | 2.03 |
| Diap3 | 3.81 | G2e3 | 2.49 | 1700113i22rik | 2.02 |
| Ppic | 3.66 | Suz12 | 2.48 | Gats | 2.01 |
| Pole | 3.63 | Tfrc | 2.39 | Hnrnpa1 | 2.01 |
| Tesc | 3.63 | Med1 | 2.35 | Tmem184b | 2.01 |
| Cdca2 | 3.62 | Myc | 2.35 | ||
| Rad54l | 3.62 | Sema3e | 2.35 |
The ratio was calculated with the genes’ intensity of highly aggressive cell lines over moderately aggressive cell lines. Genes’ intensity of different cell lines obtained from Affymetrix was analyzed by GeneSifter. After global normalization, the ratio was generated with the average normalized intensity of highly aggressive cells lines and moderately aggressive cell lines. A minimum of twofold change (P<0.05) was used as the standards for selecting genes of interest. In some ratio, we added parentheses which contained the digits. These digits are the mean of intensity in the relevant genes obtained from microarray data. These relevant genes were involved in cell cycle, DNA metabolic process, and/or apoptosis and cell death and displayed in Table 8
Table 4.
Genes overexpressed in moderately aggressive ovarian cancer cell lines
| Gene ID | Fold changea | Gene ID | Fold change | Gene ID | Fold change |
|---|---|---|---|---|---|
| Runx1t1 | 28.74 | Gm9776 | 2.71 | Zbtb33 | 2.18 |
| Kcnip2 | 7.18 | Fars2 | 2.7 | Zfp68 | 2.17 |
| AR | 5.73 | Nrip2 | 2.66 | Nek4 | 2.15 |
| Gja1 | 4.80 | Apln | 2.61 | Odf3b | 2.15 |
| 5730469M10Rik | 4.36 | Lamp2 | 2.59 | 1600012H06Rik | 2.14 |
| 5033411D12Rik | 4.00 | Cd55 | 2.57 | Kifc3 | 2.13 |
| Sept4 | 3.82 | Rbmx | 2.43 | Zfp69 | 2.13 |
| Unc13c | 3.64 | Rbbp6 | 2.38 | Gnpda1 | 2.12 |
| Itgb8 | 3.49 | Mast4 | 2.35 | Ptch1 | 2.12 |
| Fam46a | 3.41 | Lass4 | 2.34 | Casp12 | 2.11 |
| Dnaja2 | 3.18 | Slc12a6 | 2.34 | Tmem176a | 2.09 |
| Rbm28 | 3.18 | Tmcc3 | 2.33 | Fah | 2.07 |
| Fam57b | 3.16 | Rab14 | 2.32 | 6720401G13Rik | 2.06 |
| Mboat1 | 2.96 | Ccdc112 | 2.31 | Tmem80 | 2.05 |
| A930035D04Rik | 2.91 | Stard5 | 2.31 | Adk | 2.04 |
| Snrnp48 | 2.87 | 5830428H23Rik | 2.29 | Hoxd3 | 2.04 |
| Fam70a | 2.86 | A230072C01Rik | 2.28 | Mospd1 | 2.02 |
| OTTMUSG00000016321 | 2.85 | Gm19569 | 2.27 | Usp11 | 2.02 |
| EG434179 | 2.84 | Zfp771 | 2.26 | Cdk7 | 2.01 |
| Zmym3 | 2.84 | C1galt1 | 2.23 | Il16 | 2.01 |
| Abca1 | 2.75 | Pde4d | 2.21 | Ssbp1 | 2.01 |
| Man1a | 2.75 | Maoa | 2.20 | Lrba | 2.00 |
| Tpmt | 2.75 | Ptar1 | 2.20 | Neo1 | 2.00 |
| OTTMUSG00000004461 | 2.74 | Akap5 | 2.19 | Zfp259 | 2.00 |
The fold change was calculated with the genes’ intensity of moderately over highly aggressive cell lines. A minimum of twofold change (P<0.05) was used as the standards for selecting genes of interest
In Table 3, the transcripts of the 109 genes were shown to be overexpressed in IG-10, IG-10pw, and IG-10pw/agar cells and were underexpressed in IG-10 clone 2, 3, and 11 cells. Differences in the normalized microarray data for these genes between highly and moderately aggressive cell lines were shown by ratios between a minimum of 2.01 (for Tmem184b gene) and a maximum of 12.6 (for Bcat1 gene). Table 4 displayed the transcripts of the 72 genes that were overexpressed in IG-10 clone 2, 3, and 11 cell lines and were underexpressed in IG-10, IG-10pw, and IG-10pw/agar cell lines. The ratio range of the normalized microarray data was shown between 2.00 (for Lrba gene) and 28.74 (for Runx1t1 gene).
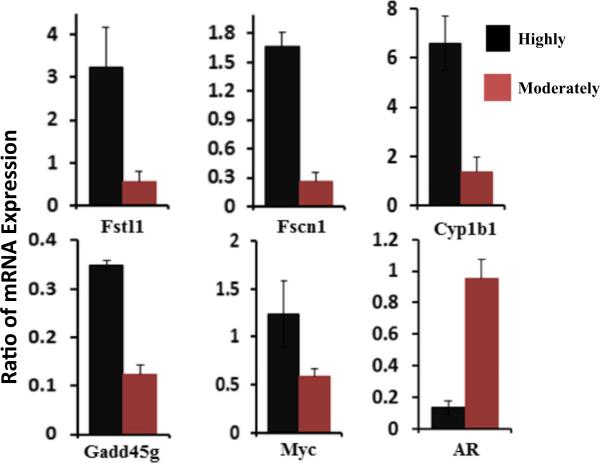
To validate the microarray results, we performed qRT-PCR to determine the expression of six randomly selected genes Fstl1, Fscn1, Cyp1b1, Gadd45g, Myc, and AR from Tables 3 and 4. Expression of the housekeeping gene Gusb in each cell lines was used as an internal control to adjust the level of gene expression. The used RNA samples were the aliquots of those samples used for the microarray analysis. Figure 2 showed that five genes Fstl1, Fscn1, Cyp1b1, Gadd45g, and Myc were overexpressed by more than two-fold in the highly aggressive cell group, and another AR gene was overexpressed by more than two-fold in the moderately aggressive cell group. This data was concordant to microarray data displayed in Tables 3 and 4. Thus, the qRT-PCR results validated the data from microarray analysis.
Fig. 2.
RT-PCR verification of selected microarray data. RT-PCR results were shown. The quantitative expression of these genes in different cell lines was normalized with self-housekeeping gene Gusb. The average of two experiments was shown
Pathways associated with genes differentially expressed between highly and moderately aggressive ovarian cancer cells
To analyze the function of the 181 genes displayed in Tables 3 and 4, we used GeneSifter software. In the 109 overexpressed genes in highly aggressive ovarian cancer cell lines, most genes were summarized in seven functional categories listed in Table 5. Category I was cell cycle [12, 13], cell division [14], cell growth and proliferation [15–17]; category II was axon guidance, adhesion, migration and invasion [17]; category III was apoptosis and necrosis [18]; category IV was DNA repair, regulation, replication and recombination [19]; category V was metabolism; and category VI was angiogenesis. The genes involved in these categories were mainly summed up in two groups in which genes are reported or unreported in ovarian cancer. In the reported group, our results were mostly consistent with reports in which the genes were overexpressed in ovarian cancer (Table 5). In the unreported group, the genes identified in our study as overexpressed in highly aggressive ovarian cancer cells for the first time had been implicated in other types of cancer (Table 5). In addition to overexpression, some genes have been reported in gene methylation, mutation and deletion elsewhere (Table 5).
Table 5.
Genes overexpressed in highly aggressive ovarian cancer cell lines involved in different cellular functions
| Cellular functions | Genes reported in ovarian cancer by other investigators |
Genes not reported in ovarian cancerg | Genes related with ovary tissue | |
|---|---|---|---|---|
| High expressiond, e | Low expressionf | |||
| (I) Cell cycle, cell division, cell growth, and proliferation | Myc [1]; [2], Suz12 [3], Bcat1 [4], Rad51ap1 [5], Spp1 [6], Apobec3 (deletion) [7], [8], Hmga2 [9], Zdhhc2 [10], Ca12 [11], Afap1l2a [12], Med1b [13]; Nasp [4], Vldlrd, e [14], Emp1d, e [15]; Skp2 [16]; Nusap1 [17], [5]; Ncapg [18], [19]; Sema5a [20] | hnRNPA1 [21]; Dlgap5 [22], [23]; Trim16 [24]; E2f7 | Gm4975/Sgol2, Incenp [25]; Csdc2 [26]; Elk3 [27]; Aldh3a1 [28]; Cdca2 [29], [19]; Rbl1 [30]; Plk4 [31]; Tfrc [32]; Tis7 [33]; Prim1 [34]; Tinagl1 (↓) [35]; Traip (↑) [36]; Trip13 [37]; Fignl1 [38]; Gpsm2 [39]; Hopx (↓) [40]; Angptl4 [41]; Kntc1 [42]; Sprr1a [43]; Tesc [44]; Gadd45g (cell cycle arrest) [45]; Sema3a (↓) [46]; Bmper [47]; Tbc1d1 [48]; Erdr1 [49]; Gprc5c [50]; Gm71 [51]; G2e3 [52]; Tanc2 [53]; Fam110a [54]; Speg [55]; Pde5a [56]; 4930547N16Rik [57]; Nans [58]; Hells [59]; Ppil1 [60] [61]; C79407 [62]; Nup155 [63]; 6230416J20Rik [64]; Slk [65]; Lrrc8c [66]; Uhrf1 [67] | Bmper [68]; Tbc1d1 [69]Sema3a [46]; Nasp [4] |
| (II) Axon guidance, adhesion, migration, and invasion | Ca12 [11]; Bcat1 [70]; Spp1 [6]; Itgb3 [71]; Fscn1 [72]; Asap1 [17]; Zdhhc2 [73]; Afap1l2a {Bai, 2014 #5999}; Sema5a [20] | Diaph3 [74]; Trim16 [75]; Fstl1 [76]; Sh3kbp1 [77], [78]; Ntn4 [79], [80] | Rtn2 [81], Pdlim2 [82]; Aldh3a1 [83]; Arf6 [84]; Itgb5 [85]; Tinagl1 [35]; Angptl4 [41]; Gprc5c [50]; Erdr1 [86]; Gm71 [51]; Slk [65] | Bmper [68], Sema3a [46] |
| (III) Apoptosis and necrosis | Rad51ap1 [5]; Spp1 [6]; Bokc [87], Hip1 [88]; Myc | E2f7 [89], Trim16 [75], Fstl1 (↑, ovarian cancer ↓) [76]; Sh3kbp1 | Aldh3a1 [83]; Ripk3 [90]; Phlda1 [91]; Emp1 [92], [93]; Prim1 [34]; Traip (↑) [36]; Bag2 [94]; Gadd45g [45]; Sema3a (↓) [46]; Slk [65] | Sema3a (↓ [46] |
| (IV) DNA regulation, replication, recombination, and repair | Rad51ap1 [5]; Apobec3 (deletion) [95]; [8]; Parpbp [96]; Afap1l2a [12]; Polea [97] | E2f7 [89]; Rad54l (methylation) [98] | Uhrf1 [67], [99]; Ncapg [18]; Prim1 [34]; Atad5/Egl1 [100]; Fignl1 [38]; Rad18 [101]; Gadd45g [45]; Gm71 (methylation) [102]; G2e3 [52]; Pdlim2 (↑↓) [82], [103]; Slk [65], Trip13 [37] | |
| (V) Metabolism | Myc [104]; Polea [97]; Nasp [4]; Rad51ap1 [5]; Cyp1b1 [105] | Rad541b [98] | Ccrn4l [106]; Tbc1d1 [48]; Naaa [107]; Nans [108]; Sec16b [109]; Lrrc8c (fat) [110]; Prim1 [34]; Uhrf1 [67]; Slk [65], Trip13 [37]; Rad18 [101]; Hells [59] | Pgm1 [111], Tbc1d1 [69] |
| (VI) Angiogenesis | Sema3e [112]; Cd97 [113]; Flt1 (VEGFR1) [114] | Fstl1 (↑↓) [76]; Ntn4 (↓) [79], [115] | Elk3 [116]; Itgb5 [117]; Tinagl1 [35]; Angptl4 [41]; Sema3a (↓) [46] | Sema3a [46] |
| (VII) DNA/chromatin/chromosome composition | Suz12 [118]; Hmga2 [119] | Gm4975/Sgol2, Incenp [25]; Prim1 [34]; Kntc1 [42]; Rad18 [101]; Hells [59]; C79407 | ||
| (VIII) Carcinogenesis and tumorigenesis | Hip1 [88]; Gsta3 [120]; Spp1 [6]; Afap1l2a [12]; Polea [97] | hnRNPA1 (↓) [21] | Emp1 [121]; Atad5/Egl1a [122]; Gadd45g [45]; [123]; Nans [124] | |
| (IX) Transcription, transcriptional modification, and mRNA stability | Med1b [13] | hnRNPA1 (↓) [21] | Lbh [125]; [126]; Ccrn4l [106] | |
| (X) Differentiation | Hmga2 [119] | Csdc2 [26]; Tis7 [33]; Speg [55] | ||
| (XI) Membrane transporters/channel | Scn5a [127]; Slc16a3 [128] | Tesc ([44], Tfrc [32], Nup155 (nuclear membrane macromolecule transporting) [129] | ||
| (XII) Immune and inflammatory responses | Asap1 [17] | Angptl4 [41]; Ripk3 [90] | ||
| Asap1 [17] | Arf6 [130] | |||
| (XIII) Autophagy and membrane trafficking | ||||
| (XIV) Ubiquination | Skp2 [131] | Bag2 (↓) [132]; Ppil1 (protein folding) [60], [61] | ||
| (XV) Signaling | Ppp1r12b [133], [134]; Gprc5c [135]; Lrrc8c [110] | |||
| (XVI) Adipogenesis | Gsta3 [120] | Ccrn4l [136]; [123]; Lrrc8c [110] | ||
| (XVII) Other | ||||
| (XVIII) Unknown | Ggt6 [137]; Gm4975/Sgol2, Ppic [138]; 2810025M15Rik, Fam149a, BC004728; Mum1l1; Pgm2 [139]; Dpy19l3 [140]; Ammecr1 [141]; Gats; Tmem184b [142]; 1700113i22rik [143] | |||
The 109 genes overexpressed in highly aggressive cell lines are listed here. . Genes in boldface are reported oncogenes [144]. Due to the high number of citations, the references for this table are shown in the supplemental materials
Down-pointing arrow genes were underexpressed or they were tumor suppressors, up-pointing arrow genes overexpressed, up-pointing and down-pointing arrow genes were sometimes underexpressed and sometimes overexpressed
Mutation is associated with ovarian cancer
Gene methylation is associated with ovarian cancer
Gene was reported to be associated with drug resistance in ovarian cancer cell lines
Genes were reported in mouse ovarian cancer IG10 cell line
Genes with high/overexpression were reported to be associated with ovarian cancers by other investigators
Genes with low/down-expression were reported to be associated with ovarian cancers by other investigators
Genes that were not reported in ovarian cancer, but these genes may have been reported in other cancers. The references were in the supplement
In the moderately aggressive ovarian cancer cell lines, most of the overexpressed genes only accumulated in category I, II, and III (Table 6). Our results suggested that the genes that were involved in category I, II, and III reflected ovarian cancer aggressiveness status, while the genes that were highly expressed in category IV, V, and VI indicated highly aggressive cancer characteristics.
Table 6.
Genes overexpressed in moderately aggressive ovarian cancer cell lines involved in cellular functions
| Categories of genes involved in cellular functions | Genes reported in ovarian cancer by other investigators |
Genes not reported in ovarian cancer | Genes related with ovary tissue | |
|---|---|---|---|---|
| High expressiond | Low expressione | |||
| (I) Cell cycle, cell division, cell growth, and proliferation | Ar (↑) [145]; Slc12a6 (↑) (proliferation and invasion) [146]; Pde4d (↑) [147], [148]; Il16 (↑) [149], [150], [151]; Kifc3 (↓) [152], [153] | Ptch1 (↓) [154]; Cdk7 (↓) [155]; Abca1 (↓) [156]; Ssbp1 (↓) [157]; Gja1 (↓) [158], [159], [160]; Neo1 (↓↑) [161]; Runx1t1 [162], [163] | Maoa (↓) [164], [48], [165], Rbbp6 (↑↓ depends on isoforms) [166], Mast4 [167], [168]; Zmym3 (m and ↓) [169], [170]; Znf259 [171]; Lrba [172]; Usp11 [173], [174]; Itgb8 (↑) [175], [176], Fam46a (tooth) [177]; Zbtb33 (↓) [178] | C1galt1 [179]; [180], Kifc3 [181], [182] |
| (II) Axon guidance, adhesion, migration and invasion | Ar (↑) [145]; Neol (↑) [183], Lamp2 [184], [185], [186] | Cd55 [187]; Gjal (↓) [159], [160]; Neol (↓↑) [161] | Itgb8 (motility) [188], Rab14 [189] | C1galt1 [179], [180] |
| (III) Apoptosis and necrosis | Casp12 [190], [191]; Gja1 (↓) [159], [160]; Rbbp6 (overexpression of both isoforms is related with apoptosis) [166] | Rbmx [192], Adk [193], Sept4 [194], Usp11 [173], Maoa (inhibit apoptosis) [10], Fam46a (tooth) [177] | ||
| (IV) DNA regulation, replication, recombination, and repair | Casp12 [190], [191] | Rbmx [3], snRNP48 [195], Usp11 [174], [196] | Rbm28 | |
| (V) Metabolism | Man1a1 [197]; [198], Fah [199], Mboat1 (lipid metabolism) [200], Znf259 [9] | Gnpda1 [201] | ||
| (VI) Angiogenesis | Il16 (↑) [149], [202]; Hoxd3 (↑) [203] | Ptch1 (↓) [154] | Rbmx [192], Apln [204], [205], Adk [193] | |
| (VII) DNA/chromatin/chromosome composition | ||||
| (VIII) Carcinogenesis and tumorigenesis | Rbbp6 (isoform3) [166] | |||
| (IX) Transcription, transcriptional modification and mRNA stability | Znf259 [206] | |||
| (X) Differentiation | Mospd1 (↓) [207] | Fam213a [208], Fam46a (tooth) [177]; Zbtb33 [209] | ||
| (XI) Membrane transporters/channel | Kcnip2 [210], [211] | |||
| (XII) Immune and inflammatory responses | Casp12 (↓) [212], Il16 [149] | Adk [193], Akap5 [213], [214], Usp11 [173], Tmem176a [215]; Zbtb33 [209] | ||
| (XIII) Autophagy and membrane trafficking | Lamp2 (↑ promoting cell resistance) [216], [217] | Sept4 [194], Lrba [172], Rab14 [218], [219] | ||
| (XIV) Ubiquination | Usp11 [220] | |||
| (XV) Signaling | Slc12a6 (and ion concentration control) [221]; [146] | Maoa [10], Itgb8 [222], Dnaja2 (via G protein-mediated signal transduction) [223], Nrip2 (Toll-like signaling pathway) [224] | ||
| (XVI) Adipogenesis, | Fam57b [225] | |||
| (XVII) Other | Stard5 (↑ chlestrol) [226] | Unc13c (synapses) [227], Dnaja2 (cochaperone) [228], OTTMUSG00000004461 (cell and nuclear size) [229], Fars2 (Mg ion binding and neurotoxicity prevention) [230], 6720401G13Rik (nuclear architecture of the chromosome) [15], Lass4 (fat storage) [231] | Nek4 (cell expansion) [232] | |
| (XVIII) Unknown | Odf3b, Zfp932, TPMT, 5033411D12Rik, A930035D04Rik, Fam70a, OTTMUSG00000016321, EG434179, Gm9776, 5830428H23Rik, A230072C01Rik, 1600012H06Rik, Ccdc112, Gm19569, Zfp771, Ptar1, Zfp68, Zfp69, Tmem80, Tmcc3 | |||
The 72 genes overexpressed in highly aggressive cell lines are listed here. Gene in boldface is a reported tumor suppressor gene [144]. Due to the high number of citations, the references for this table are shown in the supplemental materials
Down-pointing arrow genes were underexpressed or they were tumor suppressors, up-pointing arrow genes overexpressed, up-pointing and down-pointing arrow genes were sometimes underexpressed and sometimes overexpressed
a Mutation is associated with ovarian cancer
b Gene methylation is associated with ovarian cancer
c Gene was reported to be associated with drug resistance in ovarian cancer cell lines.
Genes with high/overexpression were reported to be associated with ovarian cancers by other investigators
Genes with low/down-expression were reported to be associated with ovarian cancers by other investigators
f Genes that were not reported in ovarian cancer, but these genes may have been reported in other cancers. The references were in the supplement
Two of the main types of genes that play important roles in carcinogenesis are oncogenes and tumor suppressor genes. The oncogenes are relevant to uncontrolled cell growth, which can lead to cancer [20]. Tumor suppressor genes slow down cell division, repair DNA mistakes, or provide a process known as programmed cell death, which inhibit cancer development (Genes and Cancer from American Cancer Society). Our results indicated that two oncogenes, Myc and ELK3 [21–24], were overexpressed in the highly aggressive ovarian cancer cell lines (Table 5), while a tumor suppressor gene PTCH1 [25, 26] was overexpressed in moderately aggressive ovarian cancer cell lines (Table 6).
Potential networks affiliated with Myc and AR genes significantly overexpressed in highly and moderately aggressive ovarian cancer cells, respectively
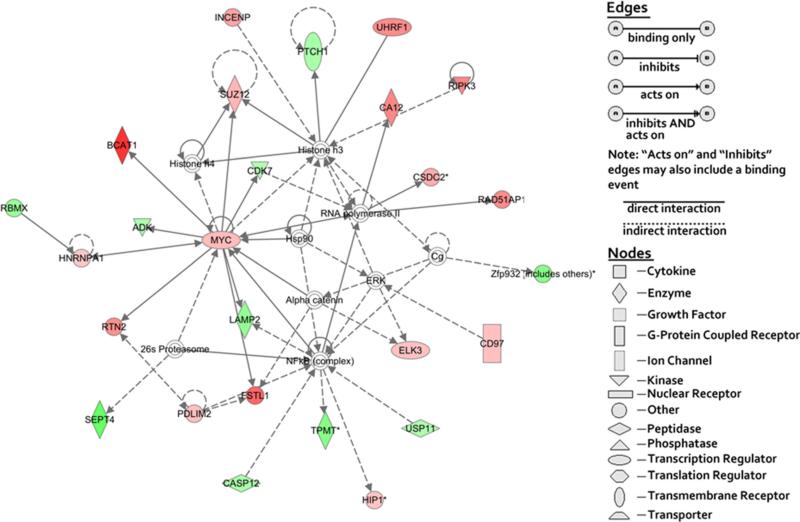
To identify functional gene networks, all 181 genes displayed in Tables 3 and 4 were analyzed for their functional pertinence using IPA tools. The network shown in Fig. 3 demonstrates that the overexpressed Myc gene is located in the center of the gene network and regulates (or is influenced by) many other genes either directly or indirectly. These genes in the network were related to cancer development, post-translational modification, and tumor morphology. Since the Myc gene is a quintessential oncogene [27] and overexpressed in highly aggressive ovarian cancer cell lines (our results), the association of the Myc gene with other genes in the network could reflect that the overexpression of the Myc gene was a critical factor that caused ovarian cancer cells with highly aggressive nature. In contrast to the affiliation of the Myc gene with highly aggressive ovarian cancer cell lines, androgen receptor (AR) gene network was found in moderately aggressive ovarian cancer cell lines (Fig. 3). High expression of the AR gene was reported to be associated with lower-stage ovarian cancer and better patient survival [28]. Besides Myc and AR genes, none of the other 181 genes were identified as critical genes located in the center of the network (data not shown). Our results suggest that the highly aggressive ovarian cancer cells are driven by the Myc gene network, and the moderately ovarian cancer cells are associated with the AR gene network.
Fig. 3.
Network of the genes related with post-translational modification, cancer, and tumor morphology. Ingenuity® bioinformatics pathway analysis tool was used to build a gene network relative to post-translational modification, cancer, and tumor morphology by using all relevant genes listed from Tables 3 and 4. Symbols for genes representing specific categories of cellular molecules as well as interactive relationships are depicted in the legend. Color gradations are based upon gene regulation at the fold change level. Red color represents the genes overexpressed in highly aggressive ovarian cancer cell lines. Green color represents the genes overexpressed in moderately aggressive ovarian cancer cell lines. No color represents the genes that did not show any differences between two cell groups
In the network results, we did not observed either ELK3 or PTCH1 genes located in the center of the networks, suggesting that both genes may not play a major regulative role in driving two groups of the ovarian cancer.
Gene enrichment analysis
The functional enrichment analysis of the identified 181 significant genes was focused on two gene subsets that were overexpressed in highly and moderately aggressive cell lines, respectively (Tables 3 and 4). The genes expressed in highly aggressive cell lines contained 109 genes in which 91 genes had a function annotation in the David database. Twenty-three GO terms were overrepresented (the Benjamini P values <0.05) by these genes (Table 7). The cancer-relevant significant GO terms included “GO:0007049~Cell cycle,” “GO:0006259~DNA metabolic process,” and “GO:0012501~Programmed cell death.” The numbers of the genes in cancer-relevant GO terms were 15, 12, and 11, respectively, as listed in Table 8. Some genes were involved in two categories and thus total 31 genes were displayed in three categories. In the case of the genes expressed in moderately aggressive cell lines, 72 member genes were not enriched significantly in any GO term (adjusted by the Benjamini P value>0.05). These results suggest that the genes overexpressed in highly aggressive ovarian cancer cell lines (which underexpressed in moderately aggressive cell lines) may play a major role in the networks relevant to enhancing cell division and controlling the cell proliferation. In addition, the oncogene MYC (but not tumor suppressor genes) is displayed in two important cancer-relevant GO term categories (see Table 8), indicating again that it played an important role in highly aggressive ovarian cancer cell lines.
Table 7.
Function enrichment analysis for the genes overexpressed in highly aggressive cell lines
| Category | Term/name | Gene counta | Fold enrichment | P value | Benjamini corrected P value |
|---|---|---|---|---|---|
| BP | GO:0007049~cell cycle | 15 | 4.83 | 1.55E-06 | 9.95E-04 |
| BP | GO:0012501~programmed cell death | 10 | 4.16 | 5.52E-04 | 2.92E-02 |
| BP | GO:0006259~DNA metabolic process | 12 | 5.15 | 8.00E-06 | 4.57E-03 |
| BP | GO:0022403~cell cycle phase | 11 | 6.60 | 4.92E-06 | 1.58E-03 |
| BP | GO:0000279~M phase | 10 | 6.96 | 1.09E-05 | 2.34E-03 |
| BP | GO:0022402~cell cycle process | 11 | 5.51 | 2.38E-05 | 3.81E-03 |
| BP | GO:0000278~mitotic cell cycle | 9 | 7.26 | 2.81E-05 | 3.61E-03 |
| BP | GO:0000087~M phase of mitotic cell cycle | 8 | 8.12 | 5.04E-05 | 4.62E-03 |
| BP | GO:0007067~mitosis | 7 | 7.26 | 3.68E-04 | 2.92E-02 |
| BP | GO:0000280~nuclear division | 7 | 7.26 | 3.68E-04 | 2.92E-02 |
| BP | GO:0048285~organelle fission | 7 | 7.00 | 4.46E-04 | 3.14E-02 |
| BP | GO:0006915~apoptosis | 10 | 4.24 | 4.88E-04 | 3.09E-02 |
| BP | GO:0051301~cell division | 8 | 5.61 | 4.95E-04 | 2.85E-02 |
| BP | GO:0006281~DNA repair | 7 | 6.21 | 8.37E-04 | 4.04E-02 |
| BP | GO:0008219~cell death | 10 | 3.88 | 9.07E-04 | 4.08E-02 |
| BP | GO:0016265~death | 10 | 3.79 | 1.07E-03 | 4.48E-02 |
| CC | GO:0044454~nuclear chromosome part | 5 | 11.04 | 1.00E-03 | 3.57E-02 |
| CC | GO:0005819~spindle | 5 | 10.43 | 1.24E-03 | 3.53E-02 |
| CC | GO:0000775~chromosome, centromeric region | 5 | 10.24 | 1.32E-03 | 3.14E-02 |
| CC | GO:0000792~heterochromatin | 4 | 17.83 | 1.37E-03 | 2.80E-02 |
| CC | GO:0044427~chromosomal part | 9 | 6.43 | 6.02E-05 | 8.69E-03 |
| CC | GO:0000228~nuclear chromosome | 6 | 11.27 | 1.69E-04 | 1.22E-02 |
| CC | GO:0005694~chromosome | 9 | 5.41 | 2.00E-04 | 9.60E-03 |
Genes that were overexpressed in highly aggressive ovarian cancer cell lines were selected and analyzed by David v6.7. Benjamini adjusted P value was smaller than 0.05
BP biological process, CC cellular component
Gene numbers were involved in each term/name
Table 8.
Selected GO terms with gene list
| Category | Term/name | Count | Genes | Benjamini |
|---|---|---|---|---|
| BP | GO:0007049~Cell cycle | 15 | Skp2, Trip13, Nasp, Rbl1, Incenp, Kntc1, Hells, C79407, E2f7, Sgol2, Nusap1, Uhrf1, Cdca2, Hmga2, Dlg7 (Dlgap5) | 9.95E-04 |
| BP | GO:0006259~DNA metabolic process | 12 | Trip13, Pparbp (Med1), Myc, Prim1, Rad51ap1, Nasp, Slk, Rad54l, Uhrf1, Rad18, Pole, Hells | 4.57E-03 |
| BP | GO:0012501~Apoptosis and cell death | 11 | Myc, Gadd45g, Bag2, Bok, Slk, Phlda1, Hip1, Sh3kbp1, Ripk3 6030408C04Rik, Arf6 | 2.92E-02 |
Total 31 genes (some genes were involved in two categories) were only overexpressed in highly aggressive ovarian cancer cell lines and involved in the categories of cell cycle, DNA metabolic process, and apoptosis and cell death
Discussion
We have shown that epithelial ovarian cancer cell clones derived from the murine parental IG-10 cell line [9] displayed different aggressiveness in vivo, as indicated by the differences in mortality rates and long-term and short-term survival of the mice injected with relevant tumor cells (Table 2). Microarray analysis indicates that the 181 significant genes are expressed differentially in the cell lines that exhibit high and moderate aggressiveness in vivo. Using bioinformatics tools to analyze these genes reveals a significant relationship between cancer cell aggressiveness and biological functions of the gene networks.
In current study, the detected biomarkers are based on two groups of different aggressive ovarian cancer cell lines. We have not seen other published reports focusing on the different gene expression between different aggressive ovarian cancers. Of the 181 significant genes, 109 genes are overexpressed in highly aggressive cell lines and 72 genes are overexpressed in moderately aggressive cell lines. More than half of the 181 genes that had not been reported in ovarian cancer are identified in this study (see Tables 5 and 6), suggesting the necessity of our study in the discovery of hitherto unknown potential biomarkers for highly and moderately aggressive ovarian cancer. Why are so many biomarkers not identified by other investigators? The possible reason is different research objects investigated between our and other's studies. The studies by other investigators usually used normal samples as controls to compare with ovarian cancer samples ([29–31]) and thus the identified biomarkers reflected their relevance to most ovarian cancer samples. The biomarkers that are significantly different between highly and moderately aggressive ovarian cancer samples were negatively selected by other investigators.
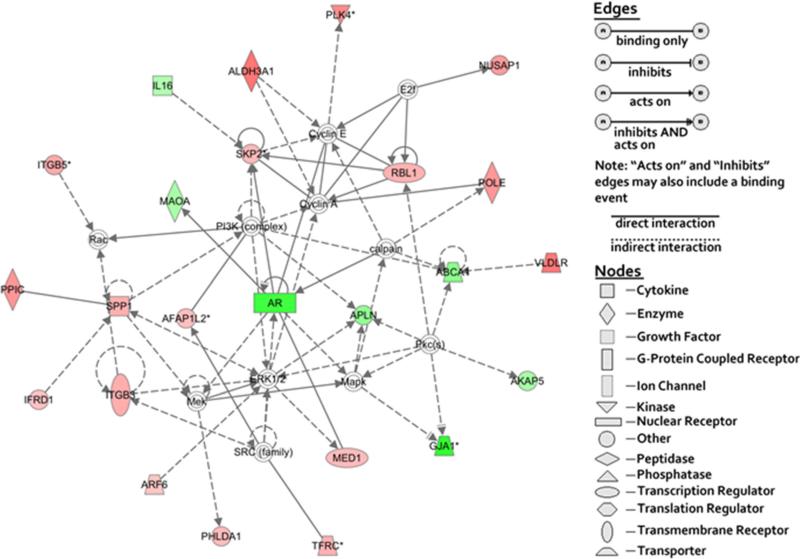
Many biological functions are provided by gene networks. All member genes in a particular network coordinate to support a biological function. Two gene networks that are affiliated with MYC gene and AR gene, respectively, have been found in highly or moderately aggressive cell lines (Figs. 3 and 4). MYC deregulated expression is usual in the development of many malignancies [32] and facilitates ovarian cancer development [33–35]. Levels of MYC gene expression have been found in the following evidence: the ovarian carcinoma higher than borderline tumors (that are low-potential malignancy tumors) and the borderline tumors higher than normal ovary [33]. Overexpression of MYC gene in ovarian cancer has been reported with worse patients’ survival compared to that of patients with normal levels [35]. Reducing MYC gene expression in ovarian adenocarcinoma cells results in less malignant cell phenotype in ovarian adenocarcinoma cells [34]. These reports support our finding and suggest that MYC gene can be used as a prognostic marker.
Fig. 4.
Network of the genes related with cancer and DNA replication, recombination, and repair. Ingenuity® bioinformatics pathway analysis tool were used to build a gene network relative to cancer. DNA replication, recombination, and repair using all relevant genes are listed in Tables 3 and 4. Symbols for genes representing specific categories of cellular molecules as well as interactive relationships are depicted in the legend. Color gradations are based upon gene regulation at the fold change level. Red color represents the genes overexpressed in highly aggressive ovarian cancer cell lines. Green color represents the genes overexpressed in moderately aggressive ovarian cancer cell lines. No color represents the genes that did not show any differences between two cell groups
AR expression has been found in more than 90 % of ovarian cancer [36–38], and high expression of the AR gene was reported to be associated with lower-stage ovarian cancer and better patient survival [28]. In normal human ovarian surface epithelial (HOSE) cells, AR gene expression is higher than that expressed in ovarian cancer cells [39]. One report shows that in serous ovarian cancer, low AR expression in patients had survival rate significantly better than high AR expression in patients [40]. Our cell lines are serous epithelial phenotype (data not shown).
Thus, the gene networks associated with MYC and AR may confer opposite biological functions: (1) the MYC network facilitates ovarian cancer aggressiveness and (2) the AR network reduces disease aggressiveness. In highly aggressive cell lines, the MYC gene is overexpressed and the AR gene is underexpressed. Thus, the biological function of the MYC network is enhanced while the biological function of the AR network is reduced. In moderately aggressive cell lines, the MYC gene and AR gene are expressed in an opposite manner, leading to biological functions that favor a reduced degree of aggressiveness.
Gene enrichment analysis of identified 109 genes that are overexpressed in highly aggressive cells and underexpressed in moderately aggressive cells revealed that cancer-relevant gene subgroups mainly fall under GO:0007049~Cell cycle, GO:0006259~DNA metabolic process, and GO:0012501~Programmed cell death (Table 8). The overexpressed genes in the GO:0007049~Cell cycle drive cell growth in vivo, while the overexpressed genes in the GO:0012501~Programmed cell death promote cell death in vivo. How do ovarian cancer cells develop a tendency toward “growth” or “death” in vivo? It is possible that (1) the levels of expression of the genes and (2) the interaction of the genes in the GO:0007049~Cell cycle provide a final biological function which determines if the cells are subject to cell cycle arrest. One example of a gene in this GO term is Cdca2. Overexpression of Cdca2 gene in human squamous cell carcinoma correlates with prevention of G1 phase arrest and downregulation of this gene expression results in high percentage of the cells subject to apoptosis [41]. Another example is E2f7 gene that has been reported to block cellular proliferation [42]. Thus, the expression and the interaction of these genes in the cell cycle category determine the abilities of growth of highly and moderately aggressive ovarian cancer cell lines in vivo. Similarly, the genes in the GO:0012501~Programmed cell death interact to regulate cell apoptosis, thereby tempering the degree of aggressiveness in their host animal. The genes with different functions in this GO term have been reported previously [43–45]. Some enhance apoptosis, some inhibit apoptosis, and some have double functions. For example, induction of the Gadd45g gene results in prostate tumor cell apoptosis [43], whereas the 6030408C04Rika (G2E3) gene is essential in preventing apoptosis during embryonic development [44]. MYC itself displays double functions in established tumor cells [45]. These documented findings indicate that expression of the genes in the GO:0012501~Programmed cell death can both motivate and prevent cell apoptosis and may explain why the genes enriched in this GO term can be overexpressed in highly aggressive cell lines. Taken together, in both sets of the genes (in the GO:0007049~Cell cycle and in the GO:0012501~Programmed cell death), each forces a unique function on highly and moderately ovarian cancer cell lines when they are overexpressed or underexpressed.
In summary, the results of our current study show the different expression in gene transcription level between the highly and the moderately aggressive ovarian cancer cell lines which provide important potential biomarker information. This potential biomarkers need to be further studied and confirmed, and if successful, they will be useful for prognostic detection of advanced human ovarian cancer disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. Erik K. Flemington (Tulane University) for providing software and helping us to analyze microarray data. We also thank Mr. Reginald Starks (Xavier University) for taking care of the animals used in this study. RCMI and LCRC Core Facilities and RCMI Bioinformatics Facility are gratefully acknowledged for providing support for this study.
Grant support This study was supported by funding from NIH (RCMI, 2G12MD007595) and Louisiana Cancer Research Consortium (LCRC) to Dr. Qian-Jin Zhang.
Footnotes
Authorship Fengkun Du conducted most of the microarray analysis. Yan Li and Xiao-Lin Li did cell culture and animal works. Shubha P. Kale, Harris McFerrin, Ian Davenport, Guangdi Wang, Yuan-Xiang Meng, and Yong-Yu Liu analyzed data and participated in the many discussions on the findings and follow-up experiments. Elena Skripnikova did RT-PCR analysis. Nathan J. Bowen did part of microarray analysis. Leticia B. McDaniels is undergraduate student who participated in and assisted with the experiments. Paula Polk did microarray.
Electronic supplementary material The online version of this article (doi:10.1007/s13277-015-4518-4) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest None
References
- 1.Gloss BS, Samimi G. Epigenetic biomarkers in epithelial ovarian cancer. Cancer Lett. 2014;342:257–63. doi: 10.1016/j.canlet.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 5.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 6.Skirnisdottir IA, Sorbe B, Lindborg K, Seidal T. Prognostic impact of p53, p27, and C-MYC on clinicopathological features and outcome in early-stage (FIGO I-II) epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21:236–44. doi: 10.1097/IGC.0b013e31820986e5. [DOI] [PubMed] [Google Scholar]
- 7.Nikas JB, Boylan KL, Skubitz AP, Low WC. Mathematical prognostic biomarker models for treatment response and survival in epithelial ovarian cancer. Cancer Informat. 2011;10:233–47. doi: 10.4137/CIN.S8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamieniak MM, Rico D, Milne RL, Munoz-Repeto I, Ibanez K, Grillo MA, et al. Deletion at 6q24.2-26 predicts longer survival of high-grade serous epithelial ovarian cancer patients. Mol Oncol. 2015;9:422–36. doi: 10.1016/j.molonc.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 10.Li XL, Zhang DQ, Wang D, Knight DS, Yin L, Bao J, et al. In vivo survivors of transformed mouse ovarian surface epithelial cells display diverse phenotypes for gene expression and tumorigenicity. Tumour Biol. 2008;29:359–70. doi: 10.1159/000178981. [DOI] [PubMed] [Google Scholar]
- 11.Urzua U, Frankenberger C, Gangi L, Mayer S, Burkett S, Munroe DJ. Microarray comparative genomic hybridization profile of a murine model for epithelial ovarian cancer reveals genomic imbalances resembling human ovarian carcinomas. Tumour Biol. 2005;26:236–44. doi: 10.1159/000087378. [DOI] [PubMed] [Google Scholar]
- 12.D'Andrilli G, Giordano A, Bovicelli A. Epithelial ovarian cancer: the role of cell cycle genes in the different histotypes. Open Clin Cancer J. 2008;2:7–12. doi: 10.2174/1874189400802010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Andrilli G, Kumar C, Scambia G, Giordano A. Cell cycle genes in ovarian cancer: steps toward earlier diagnosis and novel therapies. Clin Cancer Res. 2004;10:8132–41. doi: 10.1158/1078-0432.CCR-04-0886. [DOI] [PubMed] [Google Scholar]
- 14.Saldanha SN, Tollefsbol TO. Pathway modulations and epigenetic alterations in ovarian tumorbiogenesis. J Cell Physiol. 2014;229:393–406. doi: 10.1002/jcp.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S, Furukawa N, Haruta S, Tanase Y, Kanayama S, Noguchi T, et al. Expression profiles of genes involved in poor prognosis of epithelial ovarian carcinoma: a review. Int J Gynecol Cancer. 2009;19:992–7. doi: 10.1111/IGC.0b013e3181aaa93a. [DOI] [PubMed] [Google Scholar]
- 16.Sirotkin AV. Transcription factors and ovarian functions. J Cell Physiol. 2010;225:20–6. doi: 10.1002/jcp.22248. [DOI] [PubMed] [Google Scholar]
- 17.Farley J, Ozbun LL, Birrer MJ. Genomic analysis of epithelial ovarian cancer. Cell Res. 2008;18:538–48. doi: 10.1038/cr.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F, Liu X, Li D, Wang Q, Zhang W, Li L. Tumor suppressor genes associated with drug resistance in ovarian cancer (review). Oncol Rep. 2013;30:3–10. doi: 10.3892/or.2013.2446. [DOI] [PubMed] [Google Scholar]
- 19.Materna V, Surowiak P, Markwitz E, Spaczynski M, Drag-Zalesinska M, Zabel M, et al. Expression of factors involved in regulation of DNA mismatch repair- and apoptosis pathways in ovarian cancer patients. Oncol Rep. 2007;17:505–16. [PubMed] [Google Scholar]
- 20.Baserga R, Porcu P, Sell C. Oncogenes, growth factors and control of the cell cycle. Cancer Surv. 1993;16:201–13. [PubMed] [Google Scholar]
- 21.Zimonjic DB, Popescu NC. Role of DLC1 tumor suppressor gene and MYC oncogene in pathogenesis of human hepatocellular carcinoma: potential prospects for combined targeted therapeutics (review). Int J Oncol. 2012;41:393–406. doi: 10.3892/ijo.2012.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado MD, Albajar M, Gomez-Casares MT, Batlle A, Leon J. MYC oncogene in myeloid neoplasias. Clin Transl Oncol. 2013;15:87–94. doi: 10.1007/s12094-012-0926-8. [DOI] [PubMed] [Google Scholar]
- 23.Tamai Y, Taketo M, Nozaki M, Seldin MF. Mouse Elk oncogene maps to chromosome X and a novel Elk oncogene (Elk3) maps to chromosome 10. Genomics. 1995;26:414–6. doi: 10.1016/0888-7543(95)80232-b. [DOI] [PubMed] [Google Scholar]
- 24.Pickeral OK, Li JZ, Barrow I, Boguski MS, Makalowski W, Zhang J. Classical oncogenes and tumor suppressor genes: a comparative genomics perspective. Neoplasia. 2000;2:280–6. doi: 10.1038/sj.neo.7900090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gailani MR, Bale SJ, Leffell DJ, DiGiovanna JJ, Peck GL, Poliak S, et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992;69:111–7. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- 26.Agren M, Kogerman P, Kleman MI, Wessling M, Toftgard R. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. 2004;330:101–14. doi: 10.1016/j.gene.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Enrietto PJ. The myc oncogene in avian and mammalian carcino-genesis. Cancer Surv. 1987;6:85–99. [PubMed] [Google Scholar]
- 28.Slotman BJ, Nauta JJ, Rao BR. Survival of patients with ovarian cancer. Apart from stage and grade, tumor progesterone receptor content is a prognostic indicator. Cancer. 1990;66:740–4. doi: 10.1002/1097-0142(19900815)66:4<740::aid-cncr2820660423>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Tonin PN, Hudson TJ, Rodier F, Bossolasco M, Lee PD, Novak J, et al. Microarray analysis of gene expression mirrors the biology of an ovarian cancer model. Oncogene. 2001;20:6617–26. doi: 10.1038/sj.onc.1204804. [DOI] [PubMed] [Google Scholar]
- 30.Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A. 2004;101:2434–9. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grisaru D, Hauspy J, Prasad M, Albert M, Murphy KJ, Covens A, et al. Microarray expression identification of differentially expressed genes in serous epithelial ovarian cancer compared with bulk normal ovarian tissue and ovarian surface scrapings. Oncol Rep. 2007;18:1347–56. [PubMed] [Google Scholar]
- 32.Morgenbesser SD, DePinho RA. Use of transgenic mice to study myc family gene function in normal mammalian development and in cancer. Semin Cancer Biol. 1994;5:21–36. [PubMed] [Google Scholar]
- 33.Watson JV, Curling OM, Munn CF, Hudson CN. Oncogene expression in ovarian cancer: a pilot study of c-myc oncoprotein in serous papillary ovarian cancer. Gynecol Oncol. 1987;28:137–50. doi: 10.1016/0090-8258(87)90207-1. [DOI] [PubMed] [Google Scholar]
- 34.Somay C, Grunt TW, Mannhalter C, Dittrich C. Relationship of myc protein expression to the phenotype and to the growth potential of HOC-7 ovarian cancer cells. Br J Cancer. 1992;66:93–8. doi: 10.1038/bjc.1992.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsaros D, Theillet C, Zola P, Louason G, Sanfilippo B, Isaia E, et al. Concurrent abnormal expression of erbB-2, myc and ras genes is associated with poor outcome of ovarian cancer patients. Anticancer Res. 1995;15:1501–10. [PubMed] [Google Scholar]
- 36.Kuhnel R, de Graaff J, Rao BR, Stolk JG. Androgen receptor predominance in human ovarian carcinoma. J Steroid Biochem. 1987;26:393–7. doi: 10.1016/0022-4731(87)90106-3. [DOI] [PubMed] [Google Scholar]
- 37.Cardillo MR, Petrangeli E, Aliotta N, Salvatori L, Ravenna L, Chang C, et al. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohisto-chemical and semiquantitative image analysis study. J Exp Clin Cancer Res. 1998;17:231–7. [PubMed] [Google Scholar]
- 38.Ilekis JV, Connor JP, Prins GS, Ferrer K, Niederberger C, Scoccia B. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecol Oncol. 1997;66:250–4. doi: 10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- 39.Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci U S A. 1999;96:5722–7. doi: 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nodin B, Zendehrokh N, Brandstedt J, Nilsson E, Manjer J, Brennan DJ, et al. Increased androgen receptor expression in serous carcinoma of the ovary is associated with an improved survival. J Ovarian Res. 2010;3:14. doi: 10.1186/1757-2215-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida F, Uzawa K, Kasamatsu A, Takatori H, Sakamoto Y, Ogawara K, et al. Overexpression of CDCA2 in human squamous cell carcinoma: correlation with prevention of G1 phase arrest and apoptosis. PLoS One. 2013;8:e56381. doi: 10.1371/journal.pone.0056381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bruin A, Maiti B, Jakoi L, Timmers C, Buerki R, Leone G. Identification and characterization of E2F7, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem. 2003;278:42041–9. doi: 10.1074/jbc.M308105200. [DOI] [PubMed] [Google Scholar]
- 43.Liebermann DA, Hoffman B. Prostate cancer: JunD, Gadd45a and Gadd45g as therapeutic targets. Cell Cycle. 2011;10:3428. doi: 10.4161/cc.10.20.17528. [DOI] [PubMed] [Google Scholar]
- 44.Brooks WS, Helton ES, Banerjee S, Venable M, Johnson L, Schoeb TR, et al. G2E3 is a dual function ubiquitin ligase required for early embryonic development. J Biol Chem. 2008;283:22304–15. doi: 10.1074/jbc.M803238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.