Abstract
BACKGROUND
The discovery of potent and broadly neutralizing antibodies (bNAbs) against human immunodeficiency virus (HIV) has made passive immunization a potential strategy for the prevention and treatment of HIV infection. We sought to determine whether passive administration of VRC01, a bNAb targeting the HIV CD4-binding site, can safely prevent or delay plasma viral rebound after the discontinuation of antiretroviral therapy (ART).
METHODS
We conducted two open-label trials (AIDS Clinical Trials Group [ACTG] A5340 and National Institutes of Health [NIH] 15-I-0140) of the safety, side-effect profile, pharmacokinetic properties, and antiviral activity of VRC01 in persons with HIV infection who were undergoing interruption of ART.
RESULTS
A total of 24 participants were enrolled, and one serious alcohol-related adverse event occurred. Viral rebound occurred despite plasma VRC01 concentrations greater than 50 μg per milliliter. The median time to rebound was 4 weeks in the A5340 trial and 5.6 weeks in the NIH trial. Study participants were more likely than historical controls to have viral suppression at week 4 (38% vs. 13%, P = 0.04 by a two-sided Fisher’s exact test in the A5340 trial; and 80% vs. 13%, P<0.001 by a two-sided Fisher’s exact test in the NIH trial) but the difference was not significant at week 8. Analyses of virus populations before ART as well as before and after ART interruption showed that VRC01 exerted pressure on rebounding virus, resulting in restriction of recrudescent viruses and selection for preexisting and emerging antibody neutralization–resistant virus.
CONCLUSIONS
VRC01 slightly delayed plasma viral rebound in the trial participants, as compared with historical controls, but it did not maintain viral suppression by week 8. In the small number of participants enrolled in these trials, no safety concerns were identified with passive immunization with a single bNAb (VRC01). (Funded by the National Institute of Allergy and Infectious Diseases and others; ACTG A5340 and NIH 15-I-0140 ClinicalTrials.gov numbers, NCT02463227 and NCT02471326.)
Therapeutic administration of monoclonal antibodies has revolutionized treatment options in oncology, rheumatology, endocrinology, gastroenterology, neurology, and the field of infectious diseases.1,2 The use of broadly neutralizing antibodies (bNAbs) against human immunodeficiency virus (HIV) is a potential approach to the prevention of HIV infection and its therapy and cure.3,4 VRC01 is a bNAb that targets the CD4-binding site of the HIV envelope glycoprotein. VRC01 has been shown to neutralize approximately 90% of a broad panel of 190 group M HIV envelope pseudotyped viruses with a mean 50% inhibitory concentration (IC50) of 0.33 μg per milliliter.5 Passive administration of bNAbs, including VRC01, has been shown to prevent HIV transmission in animal models6–9 and is now being tested in clinical trials of vertical and horizontal transmission in humans.
Combination antiretroviral therapy (ART) potently suppresses HIV replication; however, it does not eradicate the persistent viral reservoir.10 In most HIV-infected persons, plasma viral rebound predictably occurs within days after treatment interruption.11–14 HIV-specific bNAbs hold potential advantages over current ART. First, bNAbs can be administered as long-acting agents by means of antibody engineering6 or vectored delivery. 15,16 Second, unlike classic ART, antibody Fc effector functions enable the killing of HIV-infected cells, which may assist in the clearance of the persistent viral reservoir.4,17 Finally, bNAbs engage the host immune system and may augment antiviral responses.18,19
Preclinical studies of single and combination bNAbs in animal models have shown virus suppression, enhanced viral killing, augmented anti-HIV immune responses, and reduction of the cellular reservoir.18,20,21 In clinical trials involving humans, no safety concerns have been identified thus far with passive administration of bNAbs targeting the CD4-binding site in healthy uninfected persons and in participants with chronic HIV infection who have either viremia or viral suppression.22–24 Passive administration of VRC01 to HIV viremic persons led to a reduction of 1.1 to 1.8 log10 in plasma HIV viremia, although it was ineffective in the quarter of study participants who had baseline resistance to the antibody.24 In addition, we previously found that HIV isolated from the latent viral reservoir of many, but not all, infected persons was inhibited by VRC01 ex vivo.17 Collectively, these findings suggest that passive immunotherapy with VRC01 could potentially prevent plasma viral rebound in HIV-infected persons after analytic treatment interruption.
Here, we report the results of two phase 1 clinical trials designed to investigate the feasibility of achieving sustained suppression of plasma viremia (virologic remission) in HIV-infected persons by means of multiple infusions of VRC01 after the discontinuation of ART that had been successfully suppressing plasma viremia below the detectable concentration. Our goals were to ascertain whether the passive administration of VRC01 is safe and has an acceptable adverse-event and side-effect profile, leads to high VRC01 plasma concentrations, can suppress plasma viremia after the discontinuation of ART, and could inform our understanding of recrudescent viruses after immunologic intervention.
METHODS
STUDY DESIGN AND OVERSIGHT
We conducted two clinical trials with similar designs to evaluate the safety, adverse-event and side-effect profile, pharmacokinetic characteristics, and antiviral activity of the human monoclonal antibody VRC-HIVMAB060-00-AB (VRC01) in HIV-infected persons who were undergoing analytic treatment interruption. The first trial, AIDS Clinical Trials Group (ACTG) A5340, was conducted at the clinical research sites of the University of Pennsylvania and University of Alabama (see the protocol, available with the full text of this article at NEJM.org). The second trial (NIH 15-I-0140, hereafter referred to as the National Institutes of Health [NIH] trial) was conducted at the National Institute of Allergy and Infectious Diseases, NIH, in Bethesda, Maryland (see the protocol).
Both trials had similar entry criteria and recruited participants who had chronic HIV infection with fully suppressed plasma viremia while receiving ART (details are provided in the Supplementary Methods section in the Supplementary Appendix, available at NEJM.org). Study participants were not prescreened for sensitivity of the virus to neutralization by VRC01 in either trial.
The trials were reviewed by the institutional review boards of each participating institution. All participants provided written informed consent. All the authors vouch for the accuracy and completeness of the data and analyses and the fidelity of the trial to the respective protocol. There was no commercial support for these trials.
TREATMENT PROCEDURES, STUDY OBJECTIVES, AND STUDY OUTCOMES
In step 1 of the A5340 trial, 14 participants received up to three doses of VRC01 (40 mg per kilogram of body weight administered intravenously) at 3-week intervals (Fig. 1). One week after the first dose of VRC01, participants discontinued ART and were followed at weekly intervals until they had a confirmed CD4 T-cell count of less than 350 cells per cubic millimeter or a return of HIV viremia, which was defined as an HIV RNA level of 200 copies or more per milliliter followed by a confirmation level of 1000 copies or more per milliliter or three consecutive measurements of 200 copies or more per milliliter. On confirmation of viral rebound or a decrease in CD4 T cells, participants entered step 2, at which point ART was reinitiated and participants were followed until the HIV viral load was less than 50 copies per milliliter.
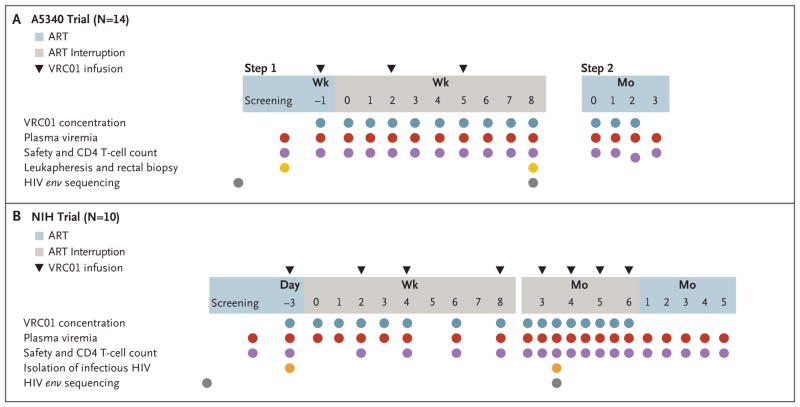
Figure 1. Trial Designs.
As shown in Panel A, the A5340 trial had two steps. In step 1, participants received an intravenous infusion of VRC01 (at a dose of 40 mg per kilogram of body weight) (triangles) 1 week before and 2 and 5 weeks after discontinuation of antiretroviral therapy (ART). ART was discontinued 1 week after the first VRC01 infusion. The treatment interruption was open-ended, and participants were monitored weekly until viral rebound. On confirmed plasma viral rebound, participants entered step 2 and ART was reinitiated. Participants were then followed until plasma viremia decreased below 50 copies per milliliter. HIV env sequencing (gray circles) was performed with the use of plasma samples obtained before the initiation of ART and after viral rebound. As shown in Panel B, in the National Institutes of Health (NIH) trial, participants received infusions of VRC01 (at a dose of 40 kg per kilogram) (triangles) 3 days before and 14 to 28 days after discontinuation of ART, and monthly thereafter. Infectious viral isolates (orange circles) were generated from samples obtained before VRC01 infusion and after plasma viral rebound. HIV env sequencing (gray circles) was performed with the use of plasma samples obtained before the initiation of ART and after viral rebound.
The primary objectives of the A5340 trial were to assess the safety and side-effect profile of multiple doses of VRC01 administered to persons with plasma viremia suppressed to below detectable levels and to estimate the proportion of participants with a return of viremia in the presence of high plasma levels of VRC01 at 8 weeks of analytic treatment interruption. Secondary objectives were the frequency of rebound viremia at 4 weeks and the evaluation of the pharmacokinetic characteristics of the product. Key exploratory objectives were the frequency of development of antibodies against VRC01 and the genetic and phenotypic characterization of the rebound virus. We calculated that 13 participants with data that could be evaluated would be required to provide this trial with 95% power to detect a difference of 40 percentage points (i.e., a return of viremia by 8 weeks in 50% of trial participants vs. 90% of historical controls)14 at a two-sided alpha level of 0.10.
In the NIH trial, 10 participants received infusions of VRC01 (at a dose of 40 mg per kilogram) intravenously 3 days before and 14 and 28 days after the discontinuation of ART and monthly thereafter (Fig. 1). Plasma viremia and CD4 T-cell counts were measured at baseline (day −3) and subsequently (Fig. 1). Participants who met any of the following criteria discontinued VRC01 infusions and resumed ART: a decrease of more than 30% in the baseline CD4 T-cell count or an absolute CD4 T-cell count below 350 cells per cubic millimeter, a sustained (≥2 weeks) HIV plasma viremia greater than 1000 copies per milliliter, any HIV-related symptoms, or pregnancy.
The primary end point of the NIH trial was safety, as defined according to the rate of occurrence of grade 3 or higher adverse events, including serious adverse events, that were possibly related to infusion of VRC01. The secondary end point was virologic efficacy, as defined according to the number of participants who met protocol-defined, virologic, immunologic, or clinical criteria to discontinue VRC01 infusions and restart ART. Post hoc analyses of the sequence diversity at the time of plasma viral rebound and the neutralization capacity of VRC01 and other bNAbs against autologous HIV before and after antibody infusions were performed.
STATISTICAL ANALYSIS
Statistical analysis of the time to the first confirmed HIV RNA level of 200 copies or more per milliliter during the analytic treatment interruption was performed in both trials (post hoc in the NIH trial). Measurements of HIV RNA levels that were taken closest to each scheduled week were obtained, and the cumulative probability of continued virologic suppression (i.e., no confirmed HIV RNA level ≥200 copies per milliliter) was calculated by means of Kaplan–Meier methods.
In both trials, a two-sided Fisher’s exact test was used to compare the percentage of trial participants with viral rebound at 4 and 8 weeks (post hoc in the NIH trial) with the percentage of historical controls with viral rebound in previous treatment-interruption trials conducted by the ACTG.14 In the A5340 trial, the proportion of participants with adverse events was estimated with an exact 95% confidence interval. The proportion of participants who had return of viremia at 8 weeks of analytic treatment interruption and who had data that could be evaluated was estimated with a prespecified exact 90% confidence interval. Details of the historical control group, participant monitoring, and laboratory and statistical methods are outlined in the Supplementary Methods section in the Supplementary Appendix.
RESULTS
STUDY PARTICIPANTS
The A5340 trial enrolled 14 participants, all of whom were male, with a median CD4 T-cell count at enrollment of 896 cells per cubic millimeter (interquartile range, 579 to 1053) and a median duration from the initiation of ART to study entry of 4.7 years (interquartile range, 3.8 to 6.0). One participant was excluded from the analyses of time to viral rebound because he discontinued ART before the administration of VRC01.
The NIH trial enrolled 10 participants (8 men and 2 women) with a median CD4 T-cell count of 724 cells per cubic millimeter (interquartile range, 630 to 926) (Fig. S1 in the Supplementary Appendix) and a median duration from the initiation of ART to study entry of 10.0 years (interquartile range, 7.7 to 13.3). In the NIH participants, the median frequency of CD4 T cells carrying HIV proviral DNA was 881 copies per 106 cells (range, 154 to 2079) (Fig. S2 in the Supplementary Appendix). Table 1 lists the baseline characteristics of the participants in both trials and the historical controls.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | A5340 Trial (N = 14) | NIH Trial (N = 10) | Historical Controls from Previous ACTG Studies (N = 61) |
|---|---|---|---|
| Sex — no. (%) | |||
| Male | 14 (100) | 8 (80) | 53 (87) |
| Female | 0 | 2 (20) | 8 (13) |
| Age — yr | |||
| Median (IQR) | 38 (34–44) | 51 (44–56) | 44 (40–50) |
| Range | 27–52 | 33–59 | 27–73 |
| Race or ethnic group — no. (%)† | |||
| White non-Hispanic | 6 (43) | 6 (60) | 41 (67) |
| Black non-Hispanic | 6 (43) | 3 (30) | 13 (21) |
| Hispanic, regardless of race | 2 (14) | 1 (10) | 7 (11) |
| Weight — kg | |||
| Median (IQR) | 86 (77–102) | 83 (78–89) | NA |
| Range | 60–115 | 75–100 | NA |
| HIV RNA — copies/no. (%) | |||
| <50 copies/ml | 13 (93) | 10 (100) | 61 (100) |
| ≥50 copies/ml | 1 (7) | 0 | 0 |
| CD4 T-cell count — cells/mm3 | |||
| Median (IQR) | 896 (579–1053) | 724 (630–926) | 852 (686–1048) |
| Range | 470–1586 | 577–1616 | 350–1667 |
| Nadir CD4 T-cell count — no. (%) | |||
| <201 cells/mm3 | 0 | 2 (20) | 3 (5) |
| 201–500 cells/mm3 | 12 (86) | 3 (30) | 39 (64) |
| >500 cells/mm3 | 2 (14) | 4 (40) | 16 (26) |
| Unknown | 0 | 1 (10) | 3 (5) |
| Duration from initiation of ART to study entry — yr | |||
| Median (IQR) | 4.7 (3.8–6.0) | 10.0 (7.7–13.3) | 5.6 (4.1–6.7) |
| Range | 2.7–14.5 | 7.0–17.2 | 0.7–16.8 |
| Duration of suppression — yr | |||
| Median (IQR) | NA | 8.3 (6.8–12.9) | NA |
| Range | NA | 3.0–16.8 | NA |
| ART regimen — no. (%) | |||
| Abacavir–lamivudine–dolutegravir | 4 (29) | 3 (30) | 0 |
| Abacavir–lamivudine–atazanavir | 0 | 1 (10) | 0 |
| Emtricitabine–tenofovir–ritonavir-boosted atazanavir | 1 (7) | 1 (10) | 0 |
| Emtricitabine–tenofovir–ritonavir-boosted darunavir | 3 (21) | 0 | 0 |
| Emtricitabine–tenofovir–dolutegravir | 2 (14) | 0 | 0 |
| Emtricitabine–tenofovir–efavirenz | 0 | 1 (10) | 0 |
| Emtricitabine–tenofovir–elvitegravir–cobicistat | 3 (21) | 2 (20) | 0 |
| Emtricitabine–tenofovir–raltegravir | 1 (7) | 0 | 0 |
| Emtricitabine–tenofovir–rilpivirine | 0 | 2 (20) | 0 |
| Zidovudine–lamivudine–nelfinavir | 0 | 0 | 15 (25) |
| Zidovudine–lamivudine–indinavir | 0 | 0 | 10 (16) |
| Zidovudine–lamivudine–ritonavir-boosted indinavir | 0 | 0 | 4 (7) |
| Stavudine–lamivudine–indinavir | 0 | 0 | 6 (10) |
| Stavudine–lamivudine–nelfinavir | 0 | 0 | 5 (8) |
| Stavudine–didanosine–nelfinavir | 0 | 0 | 2 (3) |
| Other protease inhibitor–based regimen | 0 | 0 | 19 (31) |
ACTG denotes AIDS Clinical Trials Group, ART antiretroviral therapy, HIV human immunodeficiency virus, IQR interquartile range, NA not available, and NIH National Institutes of Health.
Race or ethnic group was self-reported.
SAFETY
All participants completed VRC01 infusions per protocol. One serious adverse event occurred in a participant who required a brief hospital admission to recover from conscious sedation following upper gastrointestinal endoscopy to evaluate a history of possible hematemesis after alcohol ingestion. In the A5340 trial, 14 participants received from 1 to 3 infusions of VRC01, and none had a grade 3 or higher adverse event or a grade 2 VRC01-related adverse event (0%; 95% confidence interval [CI], 0 to 23). In the NIH trial, participants received 2 to 6 infusions (median, 3.5) of VRC01, and no adverse events occurred during the infusion or immediate postinfusion period. Complete data on adverse events are provided in the Supplementary Appendix. These safety results are consistent with those of much larger and ongoing studies of the same product.
In 24 participants, ART was reinitiated on confirmation of viral rebound and their plasma viremia was suppressed again. In the A5340 trial, participants had not received ART for a median of 6 weeks (range, 3 to 13). The median time from the first detectable HIV RNA level of 200 copies per milliliter or more to the first suppressed HIV level of less than 200 copies per milliliter after ART reinitiation was 6 weeks (range, 3 to 14). In the NIH trial, participants did not receive ART for a median of 8 weeks (range, 3 to 17). The median time from the first detectable HIV RNA level of 200 copies or more per milliliter to the first suppressed HIV level of less than 200 copies per milliliter after ART reinitiation was 11 weeks (range, 4 to 20). No participant had a confirmed decrease in the CD4 T-cell count below 350 cells per milliliter; 1 participant had a decrease of more than 30% from the baseline CD4 T-cell count; this led to the reinitiation of ART per protocol.
TIME TO VIRAL REBOUND
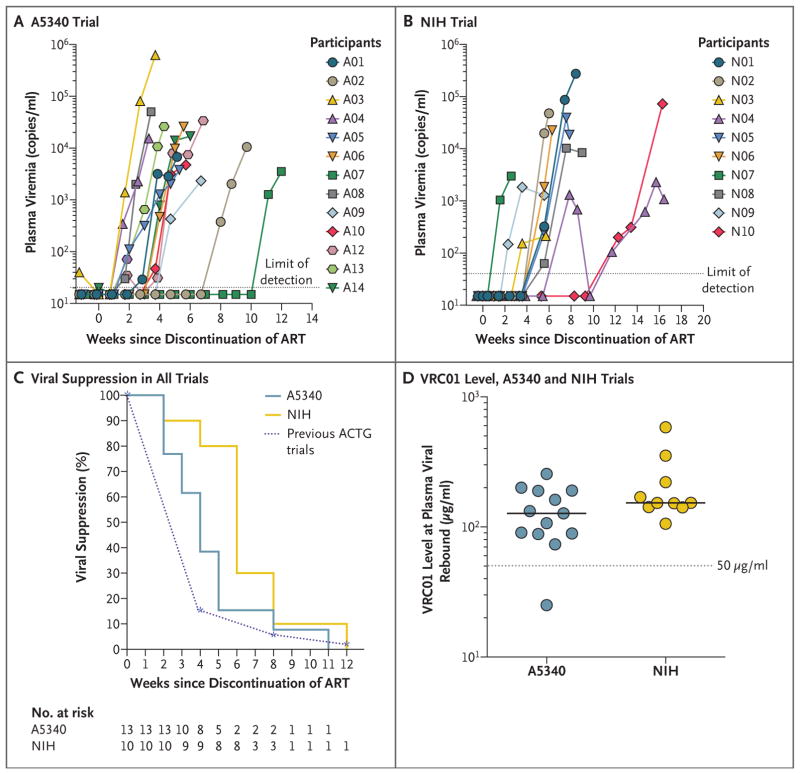
In both trials, the administration of VRC01 did not produce durable suppression of plasma viremia. In the A5340 trial, 12 of 13 participants with data that could be evaluated had viral rebound of more than 200 copies per milliliter at or before week 8 (92%; 90% CI, 68 to 100), with a median time to rebound of 4 weeks (interquartile range, 3 to 5) (Fig. 2A, and Fig. S3 in the Supplementary Appendix). This is a small delay in viral rebound as compared with the delay in historical controls from previous ACTG studies; 38% of the participants versus 13% of the controls had viral suppression at week 4 (P = 0.04 by a two-sided Fisher’s exact test) and 8% and 3%, respectively, had viral suppression at week 8 (P = 0.44 by a two-sided Fisher’s exact test) (Fig. 2C). One participant (Participant A07) in the A5340 trial had prolonged viral suppression, and detectable plasma viremia developed at week 11 of the analytic treatment interruption when plasma VRC01 levels had waned significantly (plasma VRC01 concentration, 25 μg per milliliter) (Fig. 2A, and Fig. S3 in the Supplementary Appendix). One participant (Participant A11) was excluded from the evaluation of time to viral rebound, since he had detectable plasma viremia at the time of VRC01 infusion (Fig. S3 in the Supplementary Appendix).
Figure 2. Plasma Viremia and Levels of VRC01 in Trial Participants after Discontinuation of ART.
Panel A shows the plasma viremia of participants in the A5340 trial after the interruption of therapy. The gray dotted line indicates the limit of detection of the assay (HIV RNA level, 20 copies per milliliter). Panel B shows the plasma viremia of NIH trial participants after interruption of therapy. The gray dotted line indicates the limit of detection of the assay (HIV RNA level, 40 copies per milliliter). Panel C shows the Kaplan–Meier curve of plasma viral suppression (<200 copies per milliliter) after VRC01 administration and analytic treatment interruption in A5340 and NIH trial participants as compared with historical participants in AIDS Clinical Trials Group (ACTG) trials who underwent interruption of therapy without other immunotherapeutic interventions. Panel D shows in vivo plasma levels of VRC01 at the first detectable plasma viremia. The limit of detection of VRC01 was less than 0.98 μg per milliliter.
In the NIH trial, viral rebound to more than 40 copies per milliliter occurred during VRC01 treatment in all 10 participants, with a median time to rebound of 39 days (interquartile range, 29 to 39) or 5.6 weeks (interquartile range, 4.1 to 5.6) (Fig. 2B, and Fig. S5 in the Supplementary Appendix). However, as compared with the time to plasma viral rebound (HIV RNA level, ≥200 copies per milliliter) in historical controls, VRC01 infusion led to a longer time to rebound (≥200 copies per milliliter), and 80% of the participants versus 13% of the controls had viral suppression at week 4 (P<0.001 by a two-sided Fisher’s exact test) and 10% and 3%, respectively, had viral suppression at week 8 (P = 0.37 by a two-sided Fisher’s exact test) (Fig. 2C). Nine of 10 study participants resumed ART because of virologic failure; ART was reinitiated in the remaining participant (Participant N03) because of a significant decrease in the CD4 T-cell count (>30%) (Figs. S1 and S5 in the Supplementary Appendix) associated with low-level plasma viremia (HIV RNA level, 471 copies per milliliter). One participant (Participant N04) self-administered antiretroviral drugs for 3 days off protocol after the first ART interruption; self-administration of antiretroviral drugs may have contributed to a brief period of aviremia (Fig. S5 in the Supplementary Appendix). However, his plasma viremia rebounded shortly after the second ART interruption.
VRC01 PHARMACOKINETIC CHARACTERISTICS
Plasma levels of VRC01 that were achieved by passive infusion were similar to levels reported in previous trials.23,24 In the A5340 trial, participants received 40 mg of VRC01 per kilogram every 3 weeks for three doses and maintained measured plasma VRC01 levels well above 50 μg per milliliter for 8 weeks (median, 175 μg per milliliter; range, 68 to 1494) (Fig. S6 in the Supplementary Appendix).
In the NIH trial, participants received 40 mg of VRC01 per kilogram every 2 weeks for the first three doses, then monthly for up to 6 months. NIH trial participants maintained levels of VRC01 in plasma above 100 μg per milliliter at almost all time points throughout the trial (Fig. S5 in the Supplementary Appendix).
Measured values of plasma VRC01 at the time of rebound were greater than 50 μg per milliliter in all trial participants, except for Participant A07 in the A5340 trial, who had delayed rebound at week 11 of analytic treatment interruption with VRC01 levels of approximately 25 μg per milliliter (Fig. 2D). No anti-VRC01 antibodies were identified in any trial participants.
SEQUENCE EVIDENCE OF VRC01-MEDIATED VIRUS RESTRICTION
Different strategies were applied in each trial to elucidate the mechanisms leading to early viral rebound. To characterize rebounding viral populations in the A5340 trial, single-genome sequencing25 of plasma HIV env genes from pre-ART plasma samples (available in 8 participants) and rebound plasma samples from the first and second weeks of detectable viremia (available in 13 participants) was performed. When analyzed together in a maximum likelihood phylogenetic tree, the pre-ART and rebound sequences of the 13 participants with data that could be evaluated clustered independently, indicating the relatedness of pre-ART and rebound viruses (Fig. S7 in the Supplementary Appendix).
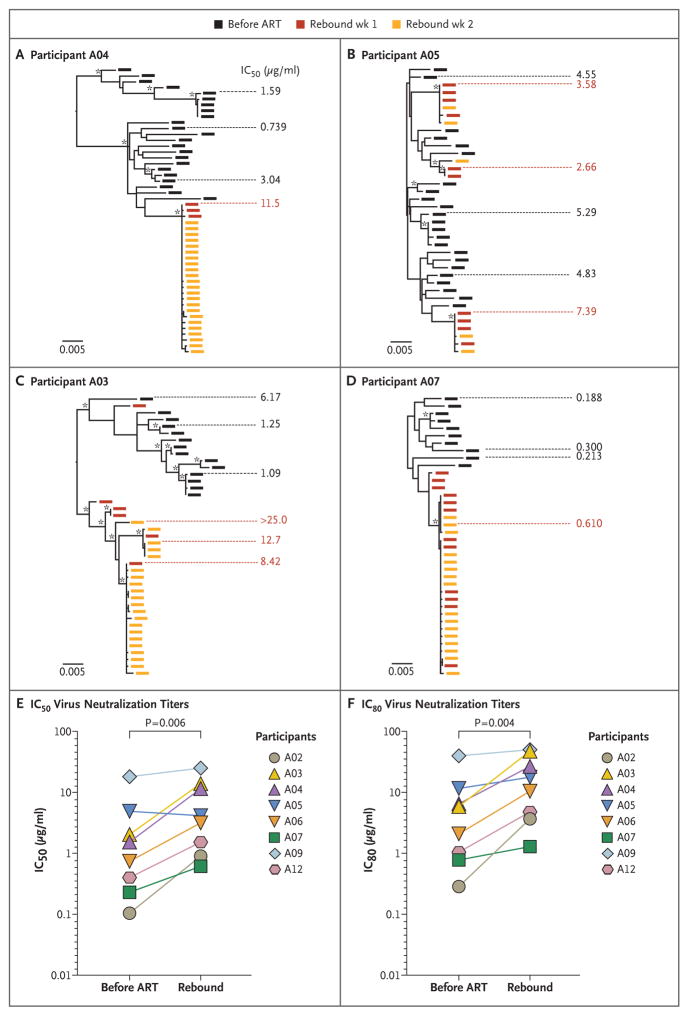
Three independent studies have recently shown that without any additional intervention besides ART, viral rebound after analytic treatment interruption is consistently polyclonal because of the reactivation of multiple latent viruses.26–28 Genetic evidence of VRC01-mediated restriction of viral rebound was assessed by analyzing the clonality of rebound virus or by enumerating genetically distinct virus populations that composed rebound viremia. In 3 of 12 participants (25%) in the A5340 trial who had viral rebound in the presence of high concentrations of VRC01, sequence evidence suggested VRC01-mediated restriction of viral rebound. As shown in Participant A04 in Figure 3A and Participants A02 and A12 in Figure S8 in the Supplementary Appendix, pre-ART plasma virus from persons with chronic infection formed characteristic diverse trees,29 whereas rebound virus clustered into single low-diversity lineages of nearly identical sequences (Figs. S9 and S10 in the Supplementary Appendix).
Figure 3. Rebound Virus Clonality and Resistance to VRC01.
Panels A through D show maximum likelihood phylogenetic trees of single-genome sequencing–derived env sequences from pre-ART and rebound plasma virus and neutralization titers to VRC01 from four participants. Participants A04, A05, and A03 had early viral rebound despite high levels of VRC01; Participant A07 had delayed rebound with lower plasma VRC01 levels. Black rectangles indicate pre-ART plasma env sequences, and red and orange rectangles indicate the env sequences from the first and second weeks of detectable viremia. The scale bar indicates genetic distance. Fifty percent inhibitory concentration (IC50) neutralization titers are shown to the side of each tree aligned to the specific envelope glycoprotein that was cloned and tested for phenotypic features. Asterisks indicate bootstrap support of greater than 80%. As shown in Panel A, Participant A04 had monoclonal rebound with VRC01-resistant virus. As shown in Panel B, Participant A05 had polyclonal rebound with VRC01-resistant virus. As shown in Panel C, Participant A03 had polyclonal rebound with VRC01-resistant virus. Multiple rebound lineages arise clustered within one area of the phylogeny. Sequences from Participant A03 were tested for clustering; Slatkin–Maddison and Hudson’s “nearest neighbor” tests support sequence compartmentalization (P<0.001 and P = 0.004, respectively). As shown in Panel D, Participant A07 had polyclonal rebound of VRC01-sensitive virus. As shown in Panels E and F, pre-ART and rebound Env pseudotyped virus from the eight participants with available samples were compared for changes in neutralization sensitivity by IC50 (truncated at 25 μg per milliliter) (Panel E) and 80% inhibitory concentration (IC80) (truncated at 50 μg per milliliter) (Panel F) with the use of multilevel random-effects models (random intercept and slope) to account for multiple clones per participant at each time point. A two-sided P value for the estimated difference in pre-ART and rebound resistance was calculated. Mean titers are shown for pre-ART virus on the left and rebound virus on the right.
The remaining 9 of 12 participants (75%) had polyclonal rebound akin to what is reported in historical analytic treatment interruption without intervention,26,27 suggesting possible preexisting resistance. As shown in Figure 3B, rebound virus in Participant A05 clustered into multiple genetically distinct rebound lineages that align throughout the pre-ART virus phylogeny, whereas multiple rebound lineages in Participant A03 clustered unevenly within the pre-ART virus population (Fig. 3C). The rebound sequences in Participant A07 in the A5340 trial (Fig. 3D), who had virus suppression maintained until week 11 of analytic treatment interruption and had rebound with lower VRC01 concentrations, formed two closely related lineages.
Evidence of selective pressure exerted by VRC01 was also explored at the molecular level in the NIH trial by amplifying env genes by means of single-genome sequencing from pre-ART (a median of 1.8 months before the initiation of ART) and rebound plasma samples. As in the A5340 trial, the majority of NIH trial participants had phylogenetic evidence of multiple viral lineages in rebound plasma virus (Fig. S11 in the Supplementary Appendix). Specific amino acid changes within the VRC01 antibody-binding site were examined with the use of a neutralization-based epitope prediction algorithm.30 In four of the six NIH trial participants, changes were identified in or near the VRC01 epitope, mainly in the V5 loop and the CD4-binding loop (Fig. S12 in the Supplementary Appendix); this outcome suggested VRC01-mediated selective pressure on rebounding virus. Similar patterns were seen in the VRC01-binding site in participants in the A5340 trial (Fig. S10 in the Supplementary Appendix).
SELECTION FOR VRC01-RESISTANT REBOUNDING VIRUSES
The role of resistance to VRC01 at viral rebound in the presence of high VRC01 concentrations in the A5340 trial was assessed by cloning selected env genes from 46 quasi-species and lineages collected throughout the pre-ART and rebound periods of the trial (median, 3 pre-ART and 3 rebound env genes per participant). These env genes were cloned, expressed as pseudoviruses, and tested for sensitivity to neutralization by VRC01. Notably, nearly all participants who had viral rebound early with high concentrations of plasma VRC01 had rebound Env pseudoviruses with IC50 neutralization titers higher than 1 μg per milliliter (median, 4.1 μg per milliliter; range, 1.9 to >50.0), conferring what is generally perceived to be at least moderate resistance to VRC01.5,31,32 Only Participants A02 and A07, who had rebound at week 8 and 11 after analytic treatment interruption, respectively, had rebound viruses with IC50 neutralization titers below 1 μg per milliliter (Fig. 3E).
Similarly, all participants who had viral rebound early had preexisting resistant virus as either dominant or minor populations in the pre-ART virus, as shown in the phylogenetic trees of the four participants in Figure 3A through 3D. The prevalence of preexisting resistance predicted the pattern of rebound. In participants with VRC01-resistant virus in multiple pre-ART variants (e.g., Participants A05, A06, and A09) (Fig. 3, and Fig. S8 in the Supplementary Appendix), VRC01 therapy was followed by rapid, polyclonal rebound with highly resistant virus. In participants in whom there was a range of neutralization sensitivities in the baseline virus (e.g., Participants A03 and A04 [Fig. 3] and Participant A12 [Fig. S8 in the Supplementary Appendix]), VRC01 therapy led to monoclonal or compartmentalized rebound after variable durations of suppression (range, 2 to 6 weeks). Finally, Participants A02 and A07, who had highly sensitive virus throughout their tested pre-ART populations, had suppression maintained for 7 and 10 weeks, respectively, and had rebound with relatively sensitive monoclonal or oligoclonal virus.
Regardless of the time to rebound, resistance to VRC01 increased almost universally in participants in the A5340 trial during treatment with VRC01. In an exploratory analysis, pre-ART and rebound Env pseudoviruses were compared in the eight participants in whom both samples were available. This analysis showed significantly increased VRC01 resistance at rebound by IC50 (mean increase by a factor of 3.44, P = 0.006 by a two-sided random-effects model) and IC80 (mean increase by a factor of 3.79, P = 0.004 by a two-sided random-effects model) (Fig. 3E and 3F, and Fig. S13 in the Supplementary Appendix).
NEUTRALIZATION CAPACITY OF VRC01 AND OTHER BNABS AGAINST AUTOLOGOUS HIV BEFORE AND AFTER ANTIBODY INFUSIONS
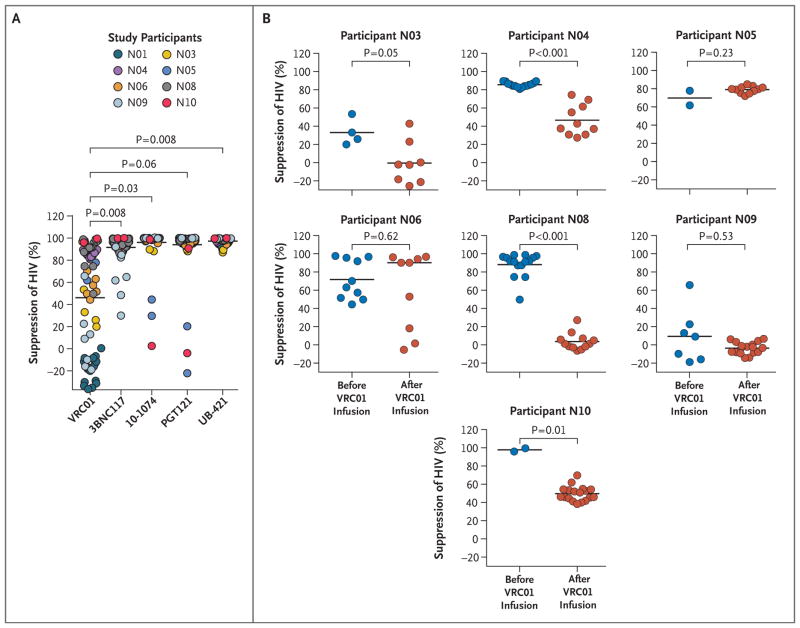
In the NIH trial, the role of resistance to VRC01 and other bNAbs was explored by testing fully replication-competent autologous HIV isolates recovered from the CD4 T cells of trial participants before and after antibody infusions. Multiple viral isolates (182 total) were generated from peripheral-blood mononuclear cells obtained from trial participants before infusions of VRC01 (eight participants, 75 isolates) and after infusions of VRC01 (nine participants, 107 isolates) and the discontinuation of ART. The susceptibility of the infectious isolates to neutralization by VRC01 and other bNAbs (3BNC117, 10–1074, and PGT121), and anti-CD4 antibody (UB-421) was then measured with the latter antibodies serving as controls, both for comparison with antibodies currently being tested in monotherapy trials as well as for their possible inclusion in future combination antibody trials. As shown in Figure 4A, the capacity of VRC01 to neutralize the preinfusion viral isolates was significantly lower than that of 3BNC117 (P = 0.008), 10–1074 (P = 0.03), and UB-421 (P = 0.008); this finding strongly suggests that the preexisting viral reservoir of the majority of NIH trial participants harbored VRC01-resistant HIV.
Figure 4. Characterization of Autologous, Replication-Competent HIV Isolates before and after Infusions of VRC01 and Discontinuation of ART in the NIH Trial.
Panel A shows neutralization of preinfusion autologous viral isolates by VRC01 and other monoclonal antibodies. Susceptibility of preinfusion infectious isolates obtained from eight trial participants to neutralization by VRC01 and other broadly neutralizing antibodies (3BNC117, 10–1074, and PGT121) and anti-CD4 antibody (UB-421) is shown. The percent suppression of HIV was calculated with the use of the following formula: (1 − [luciferase activity in the presence of test antibody ÷ luciferase activity in the presence of control antibody IgG]) × 100. Luciferase activity was expressed in relative light units. Gray horizontal bars indicate mean values. P values were computed with the use of a paired permutation test. Panel B shows neutralization of preinfusion and postinfusion viral isolates by VRC01 in seven trial participants from whom infectious isolates could be recovered at both time points. Gray horizontal bars indicate mean values. The P value for each participant was computed with the use of the Wilcoxon–Mann–Whitney test.
Next, the capacity of VRC01 to suppress the preinfusion and postinfusion autologous virus was evaluated in the seven participants from whom infectious isolates could be recovered at both time points (Fig. 4B, and Figs. S14 and S15 in the Supplementary Appendix). Notably, virus sensitivity to VRC01 diminished significantly after multiple infusions of VRC01 during analytic treatment interruption in several participants. Participant-to-participant variability prevented us from concluding that the observed decrease in sensitivity was universal (P = 0.08). Nonetheless, in Participants N03, N04, N08, and N10, there was strong evidence of the emergence of HIV isolates that were less sensitive to VRC01, highly resistant to VRC01, or both (Fig. 4B). It is noteworthy that the preinfusion isolates obtained from Participant N09 were already highly resistant to VRC01 and remained resistant after infusion. In contrast, there were no detected changes in susceptibility of pre-ART versus rebound viral isolates to neutralization by 3BNC117, 10–1074, PGT121, and UB-421 (Figs. S14 and S15 in the Supplementary Appendix). The rebound VRC01-resistant isolates from two participants (Participants N04 and N08) were also resistant to the CD4-binding site bNAb 3BNC117 (Figs. S14 and S15 in the Supplementary Appendix). Collectively, the analyses of viral isolates in the NIH trial corroborate the sequence-based analysis observed in the A5340 trial and show selection for preexisting VRC01-resistant virus and the capacity for VRC01 to further drive resistance during analytic treatment interruption.
DISCUSSION
In two similarly designed clinical trials, we found that the passive administration of multiple doses of VRC01 monotherapy generated high plasma VRC01 concentrations, and no safety concerns were identified. In persons with chronic HIV infection who were undergoing analytic treatment interruption, as compared with historical controls, VRC01 therapy slightly delayed plasma viral rebound; however, viral suppression beyond 8 weeks was not achieved. Sequence-based and neutralization analyses suggest that VRC01 can restrict the clonality of rebounding virus in some participants, select for preexisting resistance, and drive the emergence of VRC01-resistant virus. Baseline resistance to VRC01 was common in both trials, suggesting that persons with chronic infection may frequently harbor archived resistant virus to this antibody.
Our results suggest that the prevalence of clinically significant archived resistance to VRC01 may present a considerable challenge in the use of bNAbs as therapeutic agents for HIV infection. Preexisting resistance to bNAbs is biologically plausible, since before the initiation of ART, persons with chronic HIV infection have extensive exposure to a polyclonal autologous B-cell response that results in archived escape variants to many antibody specificities, including those of bNAb target epitopes.33–36 Indeed, a previous study tested replication-competent viral isolates derived from the latent viral reservoir and showed resistance to VRC01 in a substantial proportion of persons in an autologous culture system.17 In participants with only sensitive pre-ART virus (tested as infectious isolates or envelope-pseudo-typed virus) who had rebound with VRC01-resistant virus, it is unclear whether this rebound indicates selection for low-frequency resistant viruses that were not sampled or the emergence of new VRC01 resistance. The development of methods to characterize the phenotypic characteristics (e.g., neutralization sensitivity) of the persistent replication-competent viral reservoir will be needed to distinguish between these different mechanisms of failure of HIV-specific bNAbs.
Although extensive preexisting resistance limited the efficacy of VRC01 in both trials, it is notable that in a previous study of viral isolates17 and the present NIH clinical trial, other tested bNAbs appeared to have less prevalent archived resistance (Fig. 4A). The efficacy of any given bNAbs in persons with chronic HIV infection will be dependent in part on whether these persons have resistant virus to that bNAb, even at very low frequencies, in persistent viral reservoirs. Future clinical trials may consider prescreening for resistance, although this is a complex task.
The emergence of VRC01-resistant HIV after infusions of VRC01 and discontinuation of ART was observed in both trials. However, a fraction of infectious HIV isolates in some trial participants remained sensitive to VRC01 despite viral rebound in the presence of high levels of VRC01 in plasma. This could be explained by a possible artifact of culture whereby isolates from the persistent viral reservoir37–39 were induced by the ex vivo conditions needed to stimulate cells into producing replication-competent viral isolates, but they may not have been actively replicating after discontinuation of ART. In the A5340 trial, virus that rebounded early in the presence of high concentrations of VRC01 was almost universally resistant to VRC01. Only two participants (including Participant A07, who had viral rebound after plasma VRC01 concentrations had waned substantially) had VRC01-sensitive envelope glycoproteins.
Our findings highlight an important consideration for the design of future clinical trials of passive immunotherapy in HIV-infected persons. During the early years of development of antiretroviral drugs for HIV infection, the nucleoside reverse transcriptase inhibitor zidovudine used as a single agent resulted in a decrease of approximately 0.5 log copies per milliliter in plasma viremia that almost invariably rebounded40 with zidovudine-resistant mutants.41 The advent of additional antiretroviral drugs directed at different viral targets and used in combinations led to more potent viral suppression for longer durations of time.42,43 Analogous to current regimens of highly successful combination ART that targets multiple HIV gene products,44 our data suggest that immunotherapy will probably require multiple bNAbs that target different sites on the HIV envelope glycoprotein.
Supplementary Material
Acknowledgments
Supported by an award from the National Institute of Allergy and Infectious Diseases (NIAID) (U01AI068636); grants from the Penn Center for AIDS Research (P30 AI045008), the Penn Clinical Trials Unit (AI069534), the University of Alabama at Birmingham (UAB) Center for AIDS Research (P30 AI027767), the UAB Clinical Trials Unit (AI069452), the AIDS Clinical Trials Group Statistical and Data Analysis Center (UM1 AI068634), the NIAID (1-R21-AI118431, for A5340 viral analyses); and a Ruth L. Kirschstein National Research Service Award (F30 AI112426, to Dr. Kreider). The National Institutes of Health (NIH) study was funded by the Intramural Research Program of the NIAID, NIH.
We thank Kathleen Gittens, Olivia Fankuchen, Jennifer Bell, Christian Geisler, and the NIAID HIV Outpatient Clinic staff for their assistance in the execution of the NIH trial, the NIH AIDS Reagent Program for providing HIV-specific mononuclear antibodies, Dr. Sarah Read for helpful discussion, and the study volunteers of both trials for their participation.
Appendix
The authors’ full names and academic degrees are as follows: Katharine J. Bar, M.D., Michael C. Sneller, M.D., Linda J. Harrison, M.Sc., J. Shawn Justement, B.S., Edgar T. Overton, M.D., Mary E. Petrone, B.S., D. Brenda Salantes, B.S., Catherine A. Seamon, R.N., Benjamin Scheinfeld, B.A., Richard W. Kwan, P.A.-C., Gerald H. Learn, Ph.D., Michael A. Proschan, Ph.D., Edward F. Kreider, M.S., Jana Blazkova, Ph.D., Mark Bardsley, B.S.N., Eric W. Refsland, Ph.D., Michael Messer, R.N., Katherine E. Clarridge, M.D., Nancy B. Tustin, B.S., Patrick J. Madden, B.S., KaSaundra Oden, Ph.D., Sijy J. O’Dell, M.Sc., Bernadette Jarocki, B.S., Andrea R. Shiakolas, B.A., Randall L. Tressler, M.D., Nicole A. Doria-Rose, Ph.D., Robert T. Bailer, Ph.D., Julie E. Ledgerwood, D.O., Edmund V. Capparelli, Pharm.D., Rebecca M. Lynch, Ph.D., Barney S. Graham, M.D., Ph.D., Susan Moir, Ph.D., Richard A. Koup, M.D., John R. Mascola, M.D., James A. Hoxie, M.D., Anthony S. Fauci, M.D., Pablo Tebas, M.D., and Tae-Wook Chun, Ph.D.
From the University of Pennsylvania (K.J.B., D.B.S., B.S., G.H.L., E.F.K., M.B., J.A.H., P.T.) and Children’s Hospital of Philadelphia (N.B.T.) — both in Philadelphia; the Laboratory of Immunoregulation (M.C.S., J.S.J., M.E.P., J.B., E.W.R., K.E.C., S.M., A.S.F., T.-W.C.), Biostatistics Research Branch (M.A.P.), Vaccine Research Center (P.J.M., S.J.O., A.R.S., N.A.D.-R., R.T.B., J.E.L., R.M.L., B.S.G., R.A.K., J.R.M.), and Division of AIDS (R.L.T.), National Institute of Allergy and Infectious Diseases and National Institutes of Health (NIH), and the Critical Care Medicine Department, Clinical Center, NIH (C.A.S., R.W.K.), Bethesda, the AIDS Clinical Trials Group, Silver Spring (K.O.), and Columbus Technologies and Services, Greenbelt (R.L.T.) — all in Maryland; Harvard T.H. Chan School of Public Health, Boston (L.J.H.); the University of Alabama at Birmingham, Birmingham (E.T.O., M.M.); Frontier Science and Technology Research Foundation, Amherst, NY (B.J.); and the University of California, San Diego, La Jolla (E.V.C.).
Footnotes
References
- 1.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–34. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 3.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–6. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halper-Stromberg A, Nussenzweig MC. Towards HIV-1 remission: potential roles for broadly neutralizing antibodies. J Clin Invest. 2016;126:415–23. doi: 10.1172/JCI80561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–5. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegu A, Yang ZY, Boyington JC, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 9.Gautam R, Nishimura Y, Pegu A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–9. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol. 2015;16:584–9. doi: 10.1038/ni.3152. [DOI] [PubMed] [Google Scholar]
- 11.Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–5. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 13.The Strategies for Management of Anti retroviral Therapy (SMART) Study Group. CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30:343–53. doi: 10.1097/QAD.0000000000000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PR, Schnepp BC, Zhang J, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–6. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun TW, Murray D, Justement JS, et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci USA. 2014;111:13151–6. doi: 10.1073/pnas.1414148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–8. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoofs T, Klein F, Braunschweig M, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science. 2016;352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shingai M, Nishimura Y, Klein F, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–80. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–22. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caskey M, Klein F, Lorenzi JC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–91. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood JE, Coates EE, Yamshchikov G, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 25.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. 2015;112(10):E1126–34. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney MF, Wiegand A, Shao W, et al. Origin of rebound plasma HIV includes cells with identical proviruses that are transcriptionally active before stopping of antiretroviral therapy. J Virol. 2015;90:1369–76. doi: 10.1128/JVI.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bednar MM, Hauser BM, Zhou S, et al. Diversity and tropism of HIV-1 rebound virus populations in plasma level after treatment discontinuation. J Infect Dis. 2016;214:403–7. doi: 10.1093/infdis/jiw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrish NF, Gao F, Li H, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–33. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong R, Louder MK, Wagh K, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol. 2015;89:2659–71. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouvin-Pley M, Morgand M, Meyer L, et al. Drift of the HIV-1 envelope glycoprotein gp120 toward increased neutralization resistance over the course of the epidemic: a comprehensive study using the most potent and broadly neutralizing monoclonal antibodies. J Virol. 2014;88:13910–7. doi: 10.1128/JVI.02083-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–68. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch RM, Tran L, Louder MK, et al. The development of CD4 binding site antibodies during HIV-1 infection. J Virol. 2012;86:7588–95. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray ES, Madiga MC, Moore PL, et al. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J Virol. 2009;83:11265–74. doi: 10.1128/JVI.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bar KJ, Tsao CY, Iyer SS, et al. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog. 2012;8(5):e1002721. doi: 10.1371/journal.ppat.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 39.Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 40.Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:185–91. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 41.Larder BA, Darby G, Richman DD. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–4. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 42.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 43.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 44.Delaney M. The development of combination therapies for HIV infection. AIDS Res Hum Retroviruses. 2010;26:501–9. doi: 10.1089/aid.2010.0064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.