Abstract
The present study demonstrates the in vitro effectiveness of the macrolide rokitamycin and the phenothiazine compound chlorpromazine against Acanthamoeba castellanii. Growth curve evaluations revealed that both drugs inhibit trophozoite growth in dose- and time-dependent ways. The effects of both drugs when they were used at the MICs at which 100% of isolates are inhibited were amoebistatic, but at higher doses they were amoebicidal as well as cysticidal. Experiments showed that when rokitamycin was associated with chlorpromazine or amphotericin B, rokitamycin enhanced their activities. Furthermore, low doses of rokitamycin and chlorpromazine, alone or in combination, blocked the cytopathic effect of A. castellanii against WKD cells derived from the human cornea. These results may have important significance in the development of new anti-Acanthamoeba compounds.
Acanthamoebas are small, ubiquitous, free-living amoebas which can exist in two forms: the motile trophozoite form and the double-walled cyst form (26, 32). In the encysted state they are protected from unfavorable environmental conditions and are resistant to extremes of temperature, desiccation, and microbial agents (3, 18, 19, 42). Human infections are generally site specific: corneal and neural tissues are the primary targets, although other tissues can be affected. These amoebas have been associated with amoebic keratitis, especially in contact lens wearers, and with chronic but fatal granulomatous amoebic meningoencephalitis (GAE) (27). They have also been identified as agents of cutaneous nodules and abscesses, arthritis, and rhinosinusitis (16, 24, 40, 41). Most recently, Acanthamoeba has been recognized as an opportunistic pathogen of humans and other animals and is known to cause a spectrum of infections in immunocompromised individuals, including those with AIDS (4, 6, 8, 20, 37). Eradication of these protozoa from infection sites is difficult because under adverse conditions the amoeba encyst and medical therapy is often less effective against cysts than against trophozoites. There are numerous reports of drugs that have the potential for the treatment of Acanthamoeba keratitis. Infections with this organism are usually treated with a combination of cationic antiseptics (polyhexamethylene biguanide), which inhibit the membrane, and aromatic diamidines (propamidine isethionate), which inhibit DNA synthesis (23, 25, 28, 31). More recently, chlorhexidine, alone or in combination with propamidine isethionate, has also been successfully applied (35). The therapeutic treatment for GAE or disseminated acanthamoebiasis is more problematic. Although two GAE cases were successfully treated by chemotherapy with ketoconazole or fluconazole and sulfadiazine after surgical excision of the lesions (29, 36), at present, no clinical trial of these treatments for Acanthamoeba systemic infections has yet been attempted. This is due to the low sensitivity of Acanthamoeba to many antiamoebic agents and, in the case of GAE, to the weak abilities of these agents to cross the blood-brain barrier into the central nervous system (CNS). Recent studies have indicated that alkylphosphocholine compounds, such as hexadecylphosphocholine, cross the blood-brain barrier and have antiamoebic activities in vitro (22, 44), but further studies are needed to determine their precise modes of action.
Therefore, sensitivity testing is useful for comparison of novel antimicrobial agents or combinations of agents for their effectiveness in vitro.
The aim of the present study was the comparative evaluation, under reproducible cultural conditions, of the efficacies of several antibiotics (rokitamycin, erythromycin, fusidic acid, pefloxacin, and polymyxin B), antifungal agents (amphotericin B and ornidazole), antiprotozoal agents (suramin), and antiviral agents (acyclovir and zidovudine [AZT]) as well as phenothiazines (chlorpromazine) on the growth rate, excystment, and cytopathogenicity of Acanthamoeba castellanii.
In this paper, we report that, among the pharmaceutical agents tested, rokitamycin and chlorpromazine are the drugs with the greatest potential for the treatment of Acanthamoeba infections.
MATERIALS AND METHODS
Amoeba cultivation.
Our study was performed with trophozoites of A. castellanii isolated from the corneal ulcer of a soft contact lens wearer (in Ancona, Italy), axenically grown at 25°C in peptone-yeast extract-glucose medium (9). The species identification of this isolate was based on cyst morphology and indirect immunofluorescence microscopy. Before the experiments, universal bacterial primers were used to detect any bacteria that may have been present, and protozoa were tested by PCR (21) and were found to be free of any contamination. Amoebas used as inocula were taken from logarithmic-phase cultures. Incubation at 37°C was routinely adopted for drug testing, as previous observations have shown that this Acanthamoeba isolate is able to grow at this temperature.
Pharmaceutical agents.
Rokitamycin, fusidic acid, and pefloxacin were kind gifts from Grunenthal Formenti Laboratories (Milan, Italy). Erythromycin, polymyxin B, amphotericin B, ornidazole, suramin, and chlorpromazine were obtained from Sigma-Aldrich (Milan, Italy). The antiviral drugs acyclovir and AZT were both a kind gift of Glaxo Wellcome (Greenford, United Kingdom).
Rokitamycin, erythromycin, and AZT were made up as stock solutions containing 20, 8, and 1 mg of drug/ml, respectively, in 95% (vol/vol) ethanol. Fusidic acid (0.5 mg/ml), pefloxacin (1 mg/ml), polymyxin B (8 mg/ml), suramin (8 mg/ml), and chlorpromazine (10 mg/ml) were dissolved in PYG medium. Ornidazole (0.8 mg/ml) and amphotericin B (2.5 mg/ml) were dissolved in PYG medium containing 3 and 2% dimethyl sulfoxide (DMSO), respectively. An acyclovir stock solution was prepared directly in DMSO at a concentration of 20 mg/ml. All drug solutions were sterilized by filtration through a 0.22-μm-pore-size filter (Millex-GS; Millipore, Molsheim, France). Preliminary tests with ethanol and DMSO were performed to ensure that no trophozoite inhibition occurred at the concentrations used.
Experimental design.
Experiments were performed in sterile 96-well plates (Corning). Serial twofold dilutions of the drugs were prepared in PYG medium. Control wells received 100 μl of PYG medium in place of drug dilutions. The amoebas, which were washed twice in phosphate-buffered saline (PBS) buffer (pH 7.2), were suspended in PYG medium at a density of 8 × 103 cells/ml. One hundred microliters of the calibrated trophozoite suspension was added to each well, and then the plates were sealed and incubated at 37°C in a 5% CO2 atmosphere. Tests were performed in duplicate and were repeated at least three times.
Effects of drugs on Acanthamoeba growth.
At 2, 3, and 6 days of incubation, the plates were observed with a Zeiss (Tilaval 31) inverted microscope to detect and count the viable trophozoites in each well. The total well area was 32.1536 mm2, but to facilitate our task, we counted the trophozoites included in the rectangle corresponding to the photographic field (the area of which was 0.3215 mm2). For each well we considered at least six of these rectangles; then, the total number of trophozoites growing in each well was calculated by the following formula: (total number of trophozoites in each rectangle/number of rectangles considered) × 100.
The amoebistatic or amoebicidal effects of the drugs.
To investigate the types of actions exerted by the drugs, at the end of each experiment, the plates were centrifuged (at 100 × g for 2 min) and the culture medium was discarded, and after two washes with 200 μl of PBS, 200 μl of fresh PYG medium was added to each well. During the 21 days of incubation at 37°C, the plates were observed with the inverted microscope to determine whether the amoebal growth resumed and reached control levels. A set of experiments was performed to evaluate cell viability, which was determined by staining the amoebas with nigrosin and examining them with the inverted microscope in a hemacytometer (Nageotte chamber).
Effects of the drugs on cystic stage.
To determine the effects of the drugs on the cystic stage, microplates were prepared as described above for the trophozoite assays. Acanthamoeba cysts were obtained from 10-day subcultures of trophozoites starved in PBS containing 2 mM 2-amino-2-methyl-1,3-propanediol (Sigma-Aldrich) at 25°C (14, 42). After two washes in PBS, they were suspended in PYG medium at a density of 8 × 103 cysts/ml. One hundred microliters of the calibrated cyst suspension was added to each well, and the plates were sealed and incubated at 37°C. After 3 days the wells were checked microscopically with the inverted microscope to detect cysts or viable trophozoites. The plates were then centrifuged (at 100 × g for 2 min) and the culture medium was discarded, and after two washes with 200 μl of PBS, 200 μl of fresh PYG medium was added to each well. During 21 days of incubation at 37°C, the plates were observed with the inverted microscope to determine the wells in which the Acanthamoeba excystment was completely inhibited.
Efficacies of combined drug treatments.
The efficacies of the combined drug treatments were determined as described above by diluting the associated drugs contemporaneously, and then the calibrated trophozoite-cyst suspension was added to each well and the plates were incubated at 37°C and 5% CO2 and processed as described above.
Drug action on cytopathic effect of Acanthamoeba.
Tests for the determination of the actions of the drugs on the cytopathic effect of Acanthamoeba were performed in 96-well plates, with A. castellanii trophozoites incubated on semiconfluent WKD cell monolayers. Amoebas not exposed to drugs and amoebas exposed to rokitamycin (4.6 μg/ml) or chlorpromazine (1.56 μg/ml) alone or in combination for 24 h at 37°C were washed twice with sterile PBS and suspended in RPMI 1640 medium (Gibco-BRL/Life Technologies) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL/Life Technologies) at a density of 8 × 103/ml. Each cell suspension was seeded in aliquots of 100 μl/well into 20 wells of sterile microtiter plates that contained target cells. The plates were then incubated at 37°C in a 5% CO2 atmosphere. The plates were observed with an inverted microscope at time intervals of 24 h for 5 days in succession to detect the destruction of the WKD cell monolayer in each well. These experiments were repeated at least three times, and the protective effects of the drugs were evaluated by estimating the percentage of cell damage compared with that for the control group. This parameter was evaluated for each sample by counting the number of wells containing destroyed WKD cell monolayers compared with the total number of wells inoculated.
WKD cells derived from a human cornea were maintained in continuous culture in RPMI 1640 medium containing 10% FCS, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml and were grown in 25-cm2 sterile plastic flasks at 37°C in a humid atmosphere containing 5% CO2. In each experiment, 2 × 104 cells suspended in 100 μl of RPMI 1640 medium-10% FCS were seeded into each well of sterile 96-well plates; the cell viability was >95%, as determined by the nigrosin dye exclusion method. After 24 h of incubation at 37°C in a 5% CO2 atmosphere, the culture medium was aspirated and was replaced with the amoebic suspensions prepared as described above.
Statistics.
Statistical differences between groups were determined by a two-tailed Student's t test. The difference was considered significant when P was <0.05.
RESULTS
Effects of the drugs on Acanthamoeba growth.
The method used to evaluate the Acanthamoeba growth curves during incubation in sterile 96-well plates was very sensitive and reproducible. The results were not significantly different from those obtained in preliminary experiments, performed by counting the amoebas grown in each well with a Nageotte chamber.
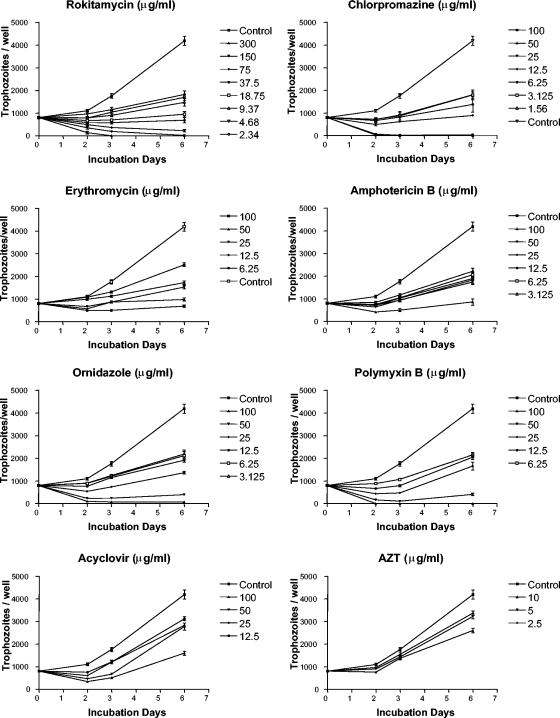
After 6 days of incubation at 37°C the control cultures of A. castellanii produced about 4.2 × 103 amoebas/well, with doubling times of about 58 h. Under the same experimental conditions, except for pefloxacin, all the drugs tested inhibited Acanthamoeba growth; but the novel macrolide rokitamycin and the antipsychotic agent chlorpromazine were distinctly inhibitory (Fig. 1). The antibiotic fusidic acid and the antiprotozoal drug suramin had marginal effects on A. castellanii growth (growth curves not shown).
FIG. 1.
Growth curves of A. castellanii suspended in PYG medium at a density of 8 × 103 trophozoites/ml in the absence (control) or in the presence of drugs and cultured in sterile microtiter plates at 37°C in a 5% CO2 atmosphere. Values are means ± the standard errors of at least six experiments.
The lowest drug concentrations that caused complete (100%) inhibition of trophozoite growth (MIC100) at days 2, 3, and 6 are shown in Table 1. The percentages of growth inhibition exerted by the 11 drugs at doses of ≤10 μg/ml, scored on days 2, 3, and 6, are shown in Table 2. Results are presented as percent inhibition of cell growth compared with the growth of the control cultures run at the same time. In general, drug activity was dose dependent and decreased with the increase in the incubation time. Chlorpromazine was the most effective compound and caused 100% growth inhibition after 48 h at a concentration of 1.56 μg/ml. At this time, rokitamycin at a dose of 2.34 μg/ml already caused about 95% growth inhibition, whereas the macrolide erythromycin produced the same effect at a concentration of >12.5 μg/ml (Fig. 1). Under the same conditions, amphotericin B at concentrations ranging from 3.125 to 6.25 μg/ml inhibited A. castellanii growth by about 87 to 100%, while ornidazole and polymyxin B at doses of 6.25 μg/ml exerted about 70% inhibition. At 48 h of incubation AZT also showed effective antiamoebic activity, inhibiting the amoebal growth by 66% at 5 μg/ml. At 3 and 6 days of incubation, the lowest doses of fusidic acid (15.625 μg/ml) and suramin (25 μg/ml) were able to inhibit amoebal growth by 48 to 61% and 28 to 48%, respectively.
TABLE 1.
MIC100s of the drugs tested for A. castellaniia
| Drug | MIC range (μg/ml) | MIC100 (μg/ml)
|
||
|---|---|---|---|---|
| Day 2 | Day 3 | Day 6 | ||
| Acyclovir | 1 00-12.5 | 12.50 | 50.00 | >100.00 |
| Amphotericin B | 100-0.05 | 6.25 | 100.00 | >100.00 |
| AZT | 10-0.02 | 10.00 | >10.00 | >10.00 |
| Chlorpromazine | 100-0.05 | 1.56 | 6.25 | 12.50 |
| Erythromycin | 100-0.05 | 25.00 | 100.00 | 100.00 |
| Fusidic acid | 125-0.06 | 125.00 | 125.00 | 125.00 |
| Ornidazole | 100-0.05 | 12.50 | 50.00 | 50.00 |
| Pefloxacin | 250-0.12 | >250.00 | >250.00 | >250.00 |
| Polymyxin B | 100-0.05 | 12.50 | 25.00 | 50.00 |
| Rokitamycin | 300-0.15 | 4.68 | 18.75 | 37.50 |
| Suramin | 100-0.05 | 50.00 | >100.00 | >100.00 |
Complete inhibition of trophozoite growth in PYG medium at 37°C in a 5% CO2 atmosphere was scored on days 2, 3, and 6.
TABLE 2.
Percent inhibition of A. castellanii growth by the drugs tested at concentrations ≤10 μg/mla
| Drug | Concn range (μg/ml) | Concn (μg/ml) | % Inhibition ± SE
|
||
|---|---|---|---|---|---|
| Day 2 | Day 3 | Day 6 | |||
| Acyclovir | 100-12.50 | NDb | ND | ND | |
| Amphotericin B | 100-0.05 | 3.125 | 86.67 ± 2.2 | 61.42 ± 3.6 | 58.29 ± 4.2 |
| Amphotericin B | 6.25 | 100 | 74.45 ± 4.7 | 62.80 ± 3.8 | |
| AZT | 10-0.02 | 2.50 | 43.40 ± 1.8 | 22.52 ± 3.2 | 24.25 ± 2.5 |
| AZT | 5.00 | 66.13 ± 5.2 | 33.88 ± 1.9 | 28.55 ± 2.8 | |
| AZT | 10.00 | 100 | 59.49 ± 1.3 | 46.78 ± 3.6 | |
| Chlorpromazine | 100-0.05 | 1.56 | 100 | 87.99 ± 4.1 | 70.41 ± 5.3 |
| Chlorpromazine | 3.125 | 100 | 92.12 ± 3.3 | 70.59 ± 3.7 | |
| Chlorpromazine | 6.25 | 100 | 100 | 82.83 ± 2.6 | |
| Erythromycin | 100-0.05 | 6.25 | 70.80 ± 1.4 | 46.82 ± 4.0 | 49.50 ± 3.4 |
| Fusidic acid | 125-0.06 | 7.812 | 0 | 0 | 0 |
| Ornidazole | 100-0.05 | 3.125 | 64.50 ± 4.9 | 54.33 ± 4.6 | 59.06 ± 3.5 |
| Ornidazole | 6.125 | 67.93 ± 3.7 | 57.93 ± 4.2 | 61.13 ± 5.0 | |
| Pefloxacin | 250-0.12 | ND | ND | ND | |
| Polymyxin B | 100-0.05 | 6.25 | 70.83 ± 3.4 | 72.19 ± 4.1 | 56.34 ± 4.7 |
| Rokitamycin | 300-0.15 | 2.34 | 94.73 ± 1.2 | 64.30 ± 3.6 | 69.94 ± 3.2 |
| Rokitamycin | 4.68 | 100 | 75.08 ± 2.8 | 73.54 ± 2.8 | |
| Rokitamycin | 9.37 | 100 | 89.70 ± 4.3 | 80.15 ± 3.6 | |
| Suramin | 100-0.05 | 6.25 | 0 | 0 | 0 |
Inhibition of growth in PYG medium at 37°C in a 5% CO2 atmosphere was scored on days 2, 3, and 6. Values are the means ± standard errors (SEs) of at least three different experiments.
ND, not determined.
Amoebistatic or amoebicidal effects of the drugs.
The effects of all drugs when they were used at the MIC100 were amoebistatic, with recovery eventually occurring (data not shown).
The trophozoite minimum amoebicidal concentration was defined as the lowest concentration of drug that did not cause complete growth resumption after 3 weeks of incubation. When amoebas in the wells were washed free of the drug-containing medium and resuspended in fresh PYG medium, it was observed that only rokitamycin and chlorpromazine, at concentrations of 75 and 50 μg/ml, respectively, exerted trophozoite amoebicidal activities. Nigrosin dye exclusion test experiments, carried out during trophozoite incubation with the drugs, confirmed these data. In fact, on day 6, amoebas incubated with rokitamycin (300 to 75 μg/ml) and chlorpromazine (100 to 50 μg/ml) showed a cell mortality rate of about 98%.
Effects of the drugs on the cystic stage.
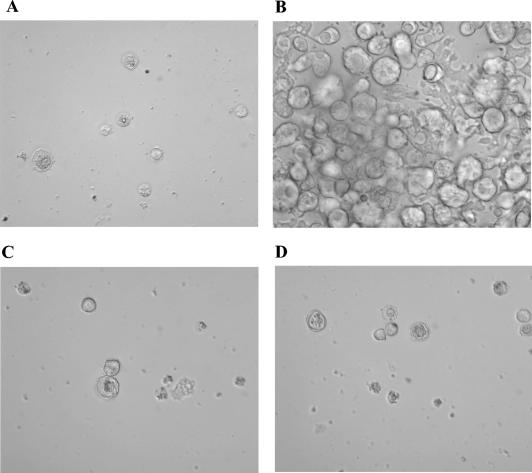
The drugs' abilities to prevent the excystment of A. castellanii were also investigated. In general, by day 3 of incubation all the drugs tested inhibited amoebic excystment, and this also occurred at the lowest concentrations tested. The minimum cysticidal concentration was defined as the lowest concentrations of the drugs that completely prevented amoeba excystment after 3 weeks of cyst reincubation in fresh PYG medium. Under these experimental conditions, only rokitamycin and chlorpromazine exerted complete cysticidal action, and this occurred at doses of 300 and 100 μg/ml, respectively (Fig. 2).
FIG. 2.
Light microscopic images showing the inhibitory effects of rokitamycin and chlorpromazine on A. castellanii excystment. Cysts (8 × 103/ml) were exposed to drugs for 3 days, washed, and reincubated in fresh PYG medium at 37°C for 21 days. (A) Cysts before treatment; (B) trophozoites from untreated cysts; (C and D) partial excystment after treatment with subinhibitory doses of rokitamycin (150 μg/ml) (C) and chlorpromazine (50 μg/ml) (D); (E) complete inhibition of excystment induced by the combination rokitamycin (75 μg/ml) and chlorpromazine (25 μg/ml).
Efficacies of combined drug treatments.
We also evaluated the effects of rokitamycin in combination with each of the other drugs tested on Acanthamoeba growth and excystment.
The MIC100s showed that the combination of rokitamycin and amphotericin B and the combination of rokitamycin and chlorpromazine exerted a higher level of efficacy than the single drugs, but only from day 3 of incubation (Table 3). Under our experimental conditions, the combination of rokitamycin and chlorpromazine also improved the amoebicidal activities of the drugs combined (trophozoite minimum amoebicidal concentrations, 37.5 and 12.5 μg/ml, respectively). In addition, this drug combination also showed synergistic cysticidal activity, which was observed at concentrations of 75 and 25 μg/ml, respectively (Fig. 2).
TABLE 3.
MIC100s of drug combinations for A. castellaniia
| Drug combination | Concn range (μg/ml) | MIC100 (μg/ml)
|
|
|---|---|---|---|
| Day 3 | Day 6 | ||
| Rokitamycin-amphotericin B | 300-0.15/100-0.05 | 9.37/3.125 | 18.75/6.25 |
| Rokitamycin-chlorpromazine | 300-0.15/100-0.05 | 9.37/3.125 | 9.37/3.125 |
Complete inhibition of trophozoites growth in PYG medium at 37°C in a 5% CO2 atmosphere was scored on days 3 and 6.
Actions of drugs on Acanthamoeba cytopathic effect.
To further investigate the actions of rokitamycin and chlorpromazine on A. castellanii, we studied the cytopathic effect exerted by trophozoites on human WKD cell monolayers after they were exposed to inhibitory drug concentrations for 24 h. The results indicated that short exposure to 4.6 μg of rokitamycin per ml and 1.56 μg of chlorpromazine ml, alone or in combination, significantly inhibited the cytopathic effects of A. castellanii trophozoites for at least 5 days.
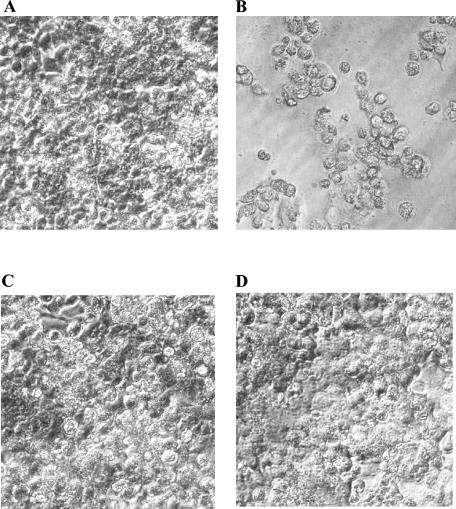
These experiments showed, in fact, that at 24 h of incubation untreated amoebas already caused monolayer destruction in all wells inoculated with target cells, whereas those pretreated with rokitamycin (4.6 μg/ml) or chlorpromazine (1.56 μg/ml), alone or in combination, did not induce cell damage. The protective effects of the drugs were also evident at successive incubation times, as indicated in Fig. 3 and Table 4, which show the morphological features of the target cell monolayers (at 96 h) and the percentage of cell damage compared with that for the control cells scored on day 5 of incubation, respectively. In this case, rokitamycin and chlorpromazine did not show any synergism. This lack of synergy was probably due to the short time (24 h) of coincubation of Acanthamoeba and the drugs. In fact, our data showed that both combinations, rokitamycin with amphotericin B and rokitamycin with chlorpromazine, exerted synergic effects only from day 3 of incubation.
FIG. 3.
Morphological features of WKD cells cultured for 4 days at 37°C in a 5% CO2 atmosphere in the absence of A. castellanii trophozoites (8 × 102/well) (A) or the presence of A. castellanii trophozoites (8 × 102/well) untreated (B) or treated for 24 h with 4.6 μg of rokitamycin per ml (C) or 1.56 μg of chlorpromazine per ml (D).
TABLE 4.
Percent inhibition of A. castellanii cytopathic effect on WKD cells by rokitamycin and chlorpromazinea
| Drug(s) | Concn (μg/ml) | % Inhibition ± SE
|
|
|---|---|---|---|
| Day 1 | Day 5 | ||
| Rokitamycin | 4.68 | 100 | 94.66 ± 0.27b |
| Chlorpromazine | 1.56 | 100 | 76.00 ± 0.47b |
| Rokitamycin-chlorpromazine | 4.68/1.56 | 100 | 87.00 ± 0.94b |
The cytopathic effect in RPMI 1640 medium-10% FCS at 37°C in 5% CO2 atmosphere was scored on days 1 and 5. Results are means ± standard errors (SEs) of at least three different experiments.
P < 0.01 compared with the results for the control.
DISCUSSION
Since the early 1960s, Acanthamoeba has been recognized as an opportunistic human pathogen capable of causing infections in both immunocompetent and immunocompromised hosts. Acanthamoeba infections are difficult to control. Substantial control problems occur for two main reasons: (i) the frequent absence of supporting documentation of amoebic infection by the diagnostic laboratory and (ii) the higher levels of resistance of both Acanthamoeba trophozoites and Acanthamoeba cysts to the usual antiprotozoal drugs. Because our aim was to find effective drugs that inhibit or eliminate amoebas, we have evaluated in vitro the efficacies of several antimicrobials and antiviral agents on the growth rate, excystment, and cytopathic effect of A. castellanii.
Our study suggests that some of the compounds screened might have potential for the treatment of Acanthamoeba chronic infections.
In particular, we found that the new oral macrolide rokitamycin was strongly inhibitory for A. castellanii. Macrolide antibiotics comprise a large group of drugs characterized by large lactonic cycles with 12, 14, 15, or 16 atoms to which sugars and/or amino sugars are bound. They have been extensively used for the treatment of bacterial infections, as they are able to interfere selectively with protein biosynthesis by binding to the 50S component of the prokaryotic ribosome. The macrolides have broad-spectrum activities against gram-positive and gram-negative bacteria, including actinomycetes and mycobacteria, as well as treponemes, mycoplasmas, chlamydiae, rickettsiae, and some protozoa (7, 11-13). Our data do not indicate any mechanism by which rokitamycin exerted its effect on Acanthamoeba. In general, its antiamoebic activity might be due to either the direct action on the protozoan or the inhibition of symbiotic bacterial flora. In our study, however, the second hypothesis must be discarded because the PCR results clearly indicated the absence of prokaryotic DNA in A. castellanii trophozoites (data not shown). It has been shown that the active 16-membered-ring macrolide rokitamycin, differently from the 14-membered-ring macrolide erythromycin, is rapidly accumulated by eukaryotic cells in large amounts at 37°C (15, 39). Our results show that erythromycin exerted a modest inhibitory effect on A. castellanii. The higher level of rokitamycin accumulation in the amoebas might explain the different antiamoebic activities of these two drugs. In addition, we observed that rokitamycin enhances the in vitro activity of amphotericin B against A. castellanii. Because this compound is sometimes used for the treatment of Acanthamoeba infections, these data suggest that the use of the combination of rokitamycin and amphotericin B might facilitate the inhibition and the elimination of these amoebas from host tissue.
Other investigators (34) have also reported that the 15-membered-ring macrolide azithromycin is effective against pathogenic Acanthamoeba in vitro. In that study, drug concentrations that were inhibitory for A. castellanii (at 30°C) were similar to those obtained in our experiments (at 37°C). In addition, azithromycin as well as rokitamycin exerted amoebistatic activities at their MICs. Furthermore, both of these macrolides were able to inhibit the cytopathic effect caused by Acanthamoeba trophozoites on different human target cells. On the whole, these results led to the in vitro screening of the activities of both macrolides, rokitamycin and azithromycin, against clinical Acanthamoeba isolates in order to further evaluate their possible use for the treatment of systemic infections.
Our study also demonstrates the in vitro effectiveness of chlorpromazine against A. castellanii. There is renewed interest in this phenothiazine compound, which was previously used as an antipsychotic agent (2), as an antibacterial, antifungal, and antiprotozoal drug (1, 5, 10, 17, 30, 38, 43).
Since 1984 it has been shown that phenothiazines have in vitro activities against the pathogenic free-living amoebas Naegleria fowleri, Acanthamoeba culbertsoni, and Acanthamoeba polyphaga (33). The mechanism of drug action is unclear. It may be due to the sensitivities of amoeba calcium regulatory proteins to the phenothiazine compounds, or it may be due to the lipophilic actions of the drugs on the amoeba plasma membrane.
Although accumulation of these compounds in the CNS makes them potentially useful chemotherapeutic agents for the treatment of amoebic meningoencephalitis caused by N. fowleri and Acanthamoeba spp. in humans, their use was hampered by their toxicities. Our data show that the combination chlorpromazine and rokitamycin exhibited synergistic amoebistatic, amoebicidal, and cysticidal activities against A. castellanii. This is very interesting, because it suggests that the contemporaneous use of both of these chemotherapeutic agents might allow a chlorpromazine dosage reduction and, hence, a reduction of the side effects associated with the drug. Moreover, additional studies on the pharmacokinetics of rokitamycin are needed to clearly demonstrate if this compound can cross the blood-brain barrier and enter the CNS, since data on this are apparently not available in the literature. However, the contemporaneous use of rokitamycin and chlorpromazine could be suggested for the treatment of both Acanthamoeba keratitis and Acanthamoeba systemic infections.
We point out that the MICs presented in this work were obtained after a single drug exposure and that the protective effects of rokitamycin and chlorpromazine observed at lower doses can be extended to as long as 48 h by replacing the medium and adding fresh compounds (data not shown). Moreover, the same concentrations were also able to inhibit the cytopathic actions of A. castellanii trophozoites against human WKD cells for at least 5 days from the time of removal of chlorpromazine and rokitamycin from the culture medium. These findings indicate that the protective effects of these compounds in vitro are maintained for a rather long time.
Further in vitro and in vivo studies are in progress to analyze the susceptibilities of other pathogenic Acanthamoeba species to both of these drugs, alone and in combination, and the interaction of rokitamycin with other phenothiazine compounds not associated with severe side effects, such as thioridazine. Nevertheless, on the basis of all data reported here, rokitamycin and chlorpromazine appear to have great potential for the treatment of Acanthamoeba infections.
Acknowledgments
We thank Grunenthal Formenti Laboratories and Glaxo Wellcome for the kind gifts of their products.
This work was supported by a grant (ex 60%) from the University of Sassari, Sassari, Italy.
REFERENCES
- 1.Amaral, L., J. E. Kristiansen, M. Viveiros, and J. Atouguia. 2001. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy. J. Antimicrob. Chemother. 47:505-511. [DOI] [PubMed] [Google Scholar]
- 2.Baldessarino R. J. 1980. Drugs and treatment of psychiatric disorders, p. 391-447. In L. S. Goodmann and A. Gilman (ed.), The pharmacological basis of therapeutics, 6th ed. McMillan Publishing Co., New York, N.Y.
- 3.Biddick, C. J., L. H. Rogers, and T. J. Brown. 1984. Viability of pathogenic and nonpathogenic free-living amoebae in long-term storage at a range of temperatures. Appl. Environ. Microbiol. 48:859-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Gregorio, C., F. Rivasi, M. Mongiardo, B. De Rienzo, S. Wallace, and G. S. Visvesvara. 1992. Acanthamoeba meningoencephalitis in an AIDS patient: first report from Europe. Arch. Pathol. Lab. Med. 116:1363-1365. [PubMed] [Google Scholar]
- 5.Eilam, Y., I. Polacheck, G. Bengigi, and P. Chernichovsky. 1987. Activity of phenothiazines against medically important yeasts. Antimicrob. Agents Chemother. 31:834-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante, A. 1991. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 13:31-47. [DOI] [PubMed] [Google Scholar]
- 7.Fichera, M. E., M. K. Bhopale, and D. S. Ross. 1995. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob. Agents Chemother. 39:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedland, L. R., S. A. Raphael, E. S. Deutsch, J. Johal, L. J. Martin, G. S. Visvesvara, and H. Lischner. 1992. Disseminated Acanthamoeba infection in a child with symptomatic human immunodeficiency virus infection. Pediatr. Infect. Dis. 11:404-407. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, L. S., and D. A. Bruckner. 1993. Diagnostic medical parasitology, 2nd ed., p. 601-605. American Society for Microbiology, Washington, D.C.
- 10.Gayatri, R., and S. Chatterjee. 1991. Effects of chlorpromazine on growth and development of Dictyostelium discoideum. Microbios 68:97-107. [PubMed] [Google Scholar]
- 11.Georgopoulos A., K. F. Linnau, A. Buxbaum, C. Coste, M. A. Ramirez de Los Santos, A. Shabpar, and W. Graninger. 2001. Efficacy of macrolides vs. metronidazole against Entamoeba histolytica clinical isolates. Wien. Klin. Wochenschr. 113:593-596. [PubMed] [Google Scholar]
- 12.Giacometti, A., O. Cirioni, F. Barchiesi, F. Ancarani, and G. Scalise. 2000. Activity of nitazoxanide alone and in combination with azithromycin and rifabutin against Cryptosporidium parvum in cell culture. J. Antimicrob. Chemother. 45:453-456. [DOI] [PubMed] [Google Scholar]
- 13.Goswick, S. M., and G. M. Brenner. 2003. Activities of azithromycin and amphotericin B against Naegleria fowleri in vitro and in a mouse model of primary amoebic meningoencephalitis. Antimicrob. Agents Chemother. 47:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori, N., M. O. Nakajiama, K. O'Hara, and T. Sawai. 1998. Novel antibiotic susceptibility tests by the ATP-bioluminescence method using filamentous cell treatment. Antimicrob. Agents Chemother. 42:1406-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro, M., H. Koga, S. Kohno, T. Hayashi, K. Yamaguchi, and M. Hirota. 1989. Penetration of macrolides into human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 24:719-729. [DOI] [PubMed] [Google Scholar]
- 16.Jeansson, S., and T. K. Kvien. 2001. Acanthamoeba polyphaga in rheumatoid arthritis: possibility for a chronic infection. Scand. J. Immunol. 53:610-614. [DOI] [PubMed] [Google Scholar]
- 17.Kalkanidis, M., N. Klonis, L. Tilley, and L. W. Deady. 2002. Novel phenothiazine antimalarials: synthesis, antimalarial activity, and inhibition of the formation of beta-haematin. Biochem. Pharmacol. 63:833-842. [DOI] [PubMed] [Google Scholar]
- 18.Kilvington, S. 1989. Moist-heat disinfection of Acanthamoeba cysts. Lett. Appl. Bacteriol. 9:187-189. [DOI] [PubMed] [Google Scholar]
- 19.Kim, B. G., P. P. McCann, and T. J. Byers. 1987. Inhibition of multiplication in Acanthamoeba castellanii by specific inhibitors of ornithine decarboxylase. J. Protozool. 34:264-266. [DOI] [PubMed] [Google Scholar]
- 20.Koide, J., E. Okusawa, T. Ito, S. Mori, T. Takeuchi, S. Itoyama, and T. Abe. 1998. Granulomatous amoebic encephalitis caused by Acanthamoeba in a patient with systemic lupus erytrematosus. Clin. Rheumatol. 17:329-332. [DOI] [PubMed] [Google Scholar]
- 21.Kotilainen, P., J. Jalava, O. Meurman, O. P. Lehtonen, E. Rintala, O. P. Seppala, E. Eerola, and S. Nikkari. 1998. Diagnosis of meningoccocal meningitis by broad-range bacterial PCR with cerebrospinal fluid. J. Clin. Microbiol. 36:2205-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotting, J., M. R. Berger, C. Unger, and H. Eibl. 1992. Alkylphosphocholines: influence of structural variations on biodistribution of antineoplastically active concentrations. Cancer Chemother. Pharmacol. 30:105-112. [DOI] [PubMed] [Google Scholar]
- 23.Larkin, D. F. P., S. Kilvington, and J. K. G. Dart. 1992. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 99:185-191. [DOI] [PubMed] [Google Scholar]
- 24.Levine, S., A. E. Goldstein, M. Dahdouh, P. Blank, C. Hoffman, and C. A. Gropper. 2001. Cutaneous Acanthamoeba in a patient with AIDS: a case study with a review of new therapy. Cutis 67:377-380. [PubMed] [Google Scholar]
- 25.Lim, L., D. J. Coster, and P. R. Badenoch. 2000. Antimicrobial susceptibility of 19 Australian corneal isolates of Acanthamoeba. Clin. Exp. Ophthalmol. 28:119-124. [DOI] [PubMed] [Google Scholar]
- 26.Ma, P., G. S. Visvesvara, A. J. Martinez, F. H. Theodore, P. M. Daggett, and T. K. Sawyer. 1990. Naegleria and Acanthamoeba infections: review. Rev. Infect. Dis. 12:490-513. [DOI] [PubMed] [Google Scholar]
- 27.Marciano-Cabral, F., R. Puffenbarger, and J. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch, D., T. B. Gray, R. Cursons, and D. Parr. 1998. Acanthamoeba keratitis in New Zealand, including two cases with in vivo resistance to polyhexamethylene biguanide. Aust. N. Z. J. Ophthalmol. 26:231-236. [DOI] [PubMed] [Google Scholar]
- 29.Ofori-Kwakye, S. K., D. G. Sidebottom, J. Herbert, E. G. Fischer, and G. S. Visvesvara. 1986. Granulomatous brain tumor caused by Acanthamoeba. Case report. J. Neurosurg. 64:505-509. [DOI] [PubMed] [Google Scholar]
- 30.Ondarza, R. N., E. Hernandez, A. Iturbe, and G. Hurtado. 2000. Inhibitory and lytic effects of phenothiazine derivatives and related tricyclic neuroleptic compounds, on Entamoeba histolytica HK9 and HM1 trophozoites. Biotechnol. Appl. Biochem. 32:61-67. [DOI] [PubMed] [Google Scholar]
- 31.Perrine, D., J. P. Chenu, P. Georges, J. C. Lancelot, C. Saturnino, and M. Robba. 1995. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob. Agents Chemother. 39:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saragoza, R. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 33.Schuster, F. L., and N. Mandel. 1984. Phenothiazine compounds inhibit in vitro growth of pathogenic free-living amoebae. Antimicrob. Agents Chemother. 25:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster, F. L., and G. S. Visvesvara. 1998. Efficacy of novel antimicrobials against clinical isolates of opportunistic amoebas. J. Eukaryot. Microbiol. 45:612-618. [DOI] [PubMed] [Google Scholar]
- 35.Seal, D. V., J. Hay, and C. M. Kirkness. 1995. Chlorhexidine or polyhexamethylene biguanide for Acanthamoeba keratitis. Lancet 345:136. [DOI] [PubMed] [Google Scholar]
- 36.Seijo-Martinez M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez de Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amoebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selby, D., R. S. Chandra, T. A. Rakusan, B. Loechelt, B. M. Markle, and G. S. Visvesvara. 1998. Amoebic osteomyelitis in a child with acquired immunodeficiency syndrome: a case report. Pediatr. Pathol. Lab. Med. 18:89-95. [PubMed] [Google Scholar]
- 38.Sharma, S., H. Kaur, and G. K. Khuller. 2001. Cell cycle effects of the phenothiazines: trifluoperazine and chlorpromazine in Candida albicans. FEMS Microbiol. Lett. 199:185-190. [DOI] [PubMed] [Google Scholar]
- 39.Tasaka, Y., M. Sumi, Y. Niki, and R. Soejima. 1988. Rokitamycin uptake by alveolar macrophages. Jpn. J. Antibiot. 41:836-840. [PubMed] [Google Scholar]
- 40.Teknos, T. N., M. D. Poulin, A. M. Laruentano, and K. K. Li. 2000. Acanthamoeba rhinosinusitis: characterization, diagnosis, and treatment. Am. J. Rhinol. 14:387-391. [DOI] [PubMed] [Google Scholar]
- 41.Torno, M. S., Jr., R. Babapour, A. Gurevitch, and M. D. Witt. 2000. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 42:351-354. [DOI] [PubMed] [Google Scholar]
- 42.Turner, N. A., A. D. Russell, J. R. Furr, and D. Lloid. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 46:27-34. [DOI] [PubMed] [Google Scholar]
- 43.Viveiros, M., and L. Amaral. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225-228. [DOI] [PubMed] [Google Scholar]
- 44.Walochnik, J., M. Duchene, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, H. Eibl, and H. Aspock. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]