Abstract
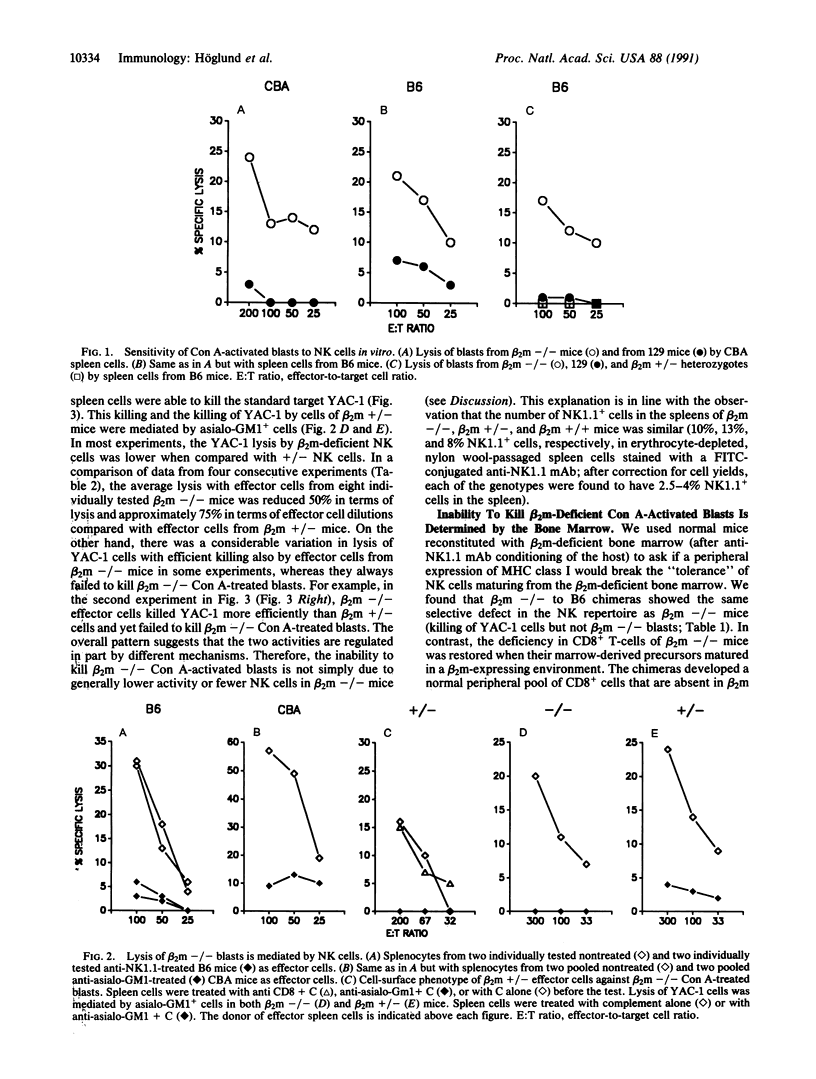
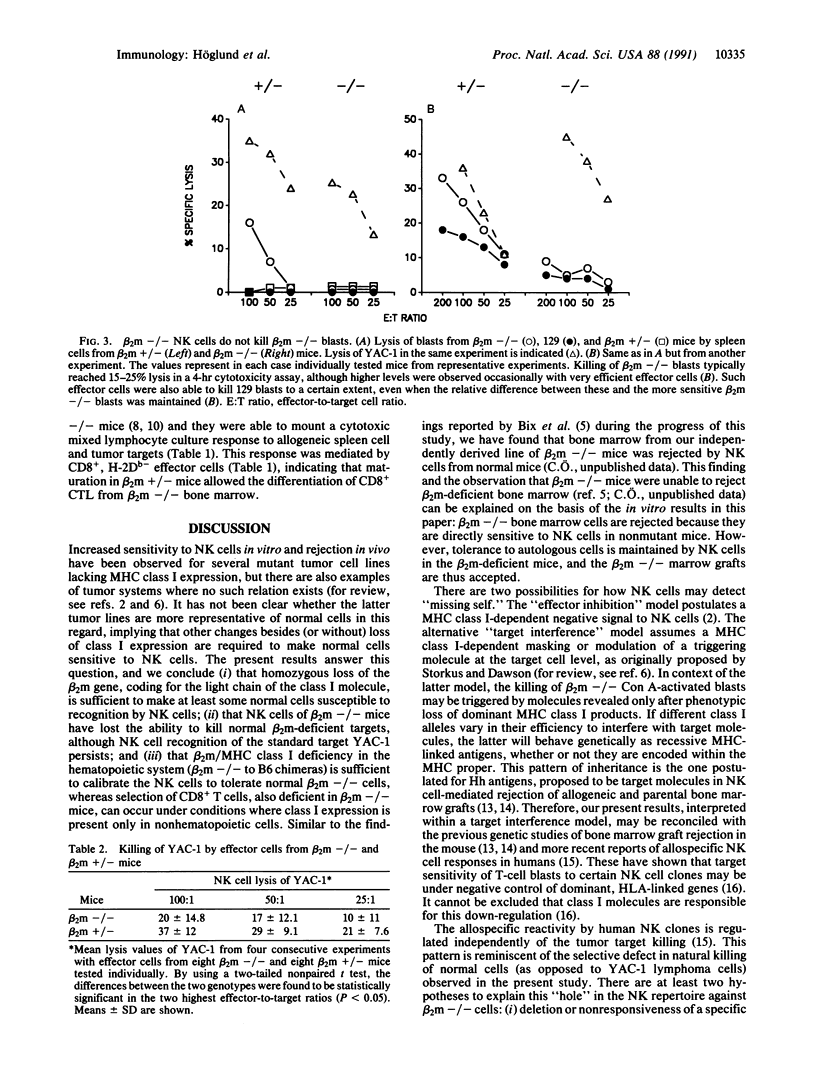
The role of major histocompatibility complex (MHC) class I expression in control of the sensitivity of normal cells to natural killer (NK) cells was studied by the use of mutant mice made deficient for expression of beta 2-microglobulin (beta 2m) through homologous recombination in embryonal stem cells. T-cell blasts from beta 2m-deficient (beta 2m -/-) mice were killed by NK cells from normal mice in vitro, while beta 2m +/- blasts were resistant. The beta 2m defect also affected the NK effector cell repertoire: NK cells from beta 2m -/- mice failed to kill beta 2m -/- blasts, while they retained the ability to kill the prototype NK cell target lymphoma YAC-1, although at reduced levels. The inability to recognize beta 2m -/- blasts could be transferred with beta 2m -/- bone marrow to irradiated beta 2m-expressing mice. In contrast, the development of CD8+ T cells (deficient in beta 2m -/- mice) was restored in such chimera. These results indicate that loss of MHC class I/beta 2m expression is sufficient to render normal cells sensitive to NK cells, and that the same defect in the hemopoietic system of a mouse renders its NK cells tolerant to beta 2m-deficient but otherwise normal cells. In the beta 2m -/- mice, NK cells may be selected or educated by other bone marrow cells to tolerate the MHC class I deficiency. Alternatively, the specificity may be controlled directly by the class I molecules on the NK cells themselves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bix M., Liao N. S., Zijlstra M., Loring J., Jaenisch R., Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991 Jan 24;349(6307):329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- Ciccone E., Colonna M., Viale O., Pende D., Di Donato C., Reinharz D., Amoroso A., Jeannet M., Guardiola J., Moretta A. Susceptibility or resistance to lysis by alloreactive natural killer cells is governed by a gene in the human major histocompatibility complex between BF and HLA-B. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9794–9797. doi: 10.1073/pnas.87.24.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone E., Pende D., Viale O., Tambussi G., Ferrini S., Biassoni R., Longo A., Guardiola J., Moretta A., Moretta L. Specific recognition of human CD3-CD16+ natural killer cells requires the expression of an autosomic recessive gene on target cells. J Exp Med. 1990 Jul 1;172(1):47–52. doi: 10.1084/jem.172.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971 Dec 1;134(6):1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Höglund P., Glas R., Ohlén C., Ljunggren H. G., Kärre K. Alteration of the natural killer repertoire in H-2 transgenic mice: specificity of rapid lymphoma cell clearance determined by the H-2 phenotype of the target. J Exp Med. 1991 Aug 1;174(2):327–334. doi: 10.1084/jem.174.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Ljunggren H. G., Ohlén C., Ahrlund-Richter L., Scangos G., Bieberich C., Jay G., Klein G., Kärre K. Natural resistance against lymphoma grafts conveyed by H-2Dd transgene to C57BL mice. J Exp Med. 1988 Oct 1;168(4):1469–1474. doi: 10.1084/jem.168.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Levitsky H. I., Golumbek P. T., Pardoll D. M. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J Immunol. 1991 Feb 15;146(4):1113–1117. [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Sturmhöfel K., Wolpert E., Hämmerling G. J., Kärre K. Transfection of beta 2-microglobulin restores IFN-mediated protection from natural killer cell lysis in YAC-1 lymphoma variants. J Immunol. 1990 Jul 1;145(1):380–386. [PubMed] [Google Scholar]
- Ohlén C., Kling G., Höglund P., Hansson M., Scangos G., Bieberich C., Jay G., Kärre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989 Nov 3;246(4930):666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- Rembecki R. M., Bennett M., Kumar V., Potter T. A. Expression of hemopoietic histocompatibility antigens on H-2-loss variants of F1 hybrid lymphoma cells: evidence consistent with trans gene regulation. J Immunol. 1987 Apr 15;138(8):2734–2738. [PubMed] [Google Scholar]
- Seaman W. E., Sleisenger M., Eriksson E., Koo G. C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987 Jun 15;138(12):4539–4544. [PubMed] [Google Scholar]
- Sentman C. L., Hackett J., Jr, Kumar V., Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkus W. J., Dawson J. R. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol. 1991;10(5):393–416. [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Yankelevich B., Knobloch C., Nowicki M., Dennert G. A novel cell type responsible for marrow graft rejection in mice. T cells with NK phenotype cause acute rejection of marrow grafts. J Immunol. 1989 May 15;142(10):3423–3430. [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]



