Abstract
Importance
Although cardiorespiratory fitness (CRF) is prognostic in older adults, the effect of CRF during early adulthood on long-term cardiovascular structure, function, and prognosis is less clear.
Objective
To examine whether CRF in young adults is associated with long-term clinical outcome and subclinical cardiovascular disease (CVD).
Design, Setting, and Participants
Prospective study of 4872 US adults aged 18 to 30 years who underwent treadmill exercise testing at a baseline study visit from March 25, 1985, to June 7, 1986, and 2472 individuals who underwent a second treadmill test 7 years later. Median follow-up was 26.9 years, with assessment of obesity, left ventricular mass and strain, coronary artery calcification (CAC), and vital status and incident CVD. Follow-up was complete on August 31, 2011, and data were analyzed from recruitment through the end of follow-up.
Main Outcomes and Measures
The presence of CAC was assessed by computed tomography at years 15 (2000-2001), 20 (2005-2006), and 25 (2010-2011), and left ventricular mass was assessed at years 5 (1990-1991) and 25 (with global longitudinal strain). Incident CVD and all-cause mortality were adjudicated.
Results
Of the 4872 individuals, 273 (5.6%) died and 193 (4.0%) experienced CVD events during follow-up. After comprehensive adjustment, each additional minute of baseline exercise test duration was associated with a 15% lower hazard of death (hazard ratio [HR], 0.85; 95% CI, 0.80-0.91; P < .001) and a 12% lower hazard of CVD (HR, 0.88; 95% CI, 0.81-0.96; P = .002). Higher levels of baseline CRF were associated with significantly lower left ventricular mass index (β = −0.24; 95% CI, −0.45 to −0.03; P = .02) and significantly better lobal longitudinal strain (β = −0.09; 95% CI, −0.14 to −0.05; P < .001) at year 25. Fitness was not associated with CAC. A 1-minute reduction in fitness by year 7 was associated with 21% and 20% increased hazards of death (HR, 1.21; 95% CI, 1.07-1.37; P = .002) and CVD (HR, 1.20; 95% CI, 1.06-1.37; P = .006), respectively, along with a more impaired strain (β = 0.15; 95% CI, 0.08-0.23; P < .001). No association between change in fitness and CAC was found.
Conclusions and Relevance
Higher levels of fitness at baseline and improvement in fitness early in adulthood are favorably associated with lower risks for CVD and mortality. Fitness and changes in fitness are associated with myocardial hypertrophy and dysfunction but not CAC. Regular efforts to ascertain and improve CRF in young adulthood may play a critical role in promoting cardiovascular health and interrupting early CVD pathogenesis.
Cardiorespiratory fitness (CRF) is associated with clinical outcome, with greater levels of fitness associated with a decreased risk for cardiovascular disease (CVD).1-4 Despite a shift in the locus of CVD prevention to youth,5,6 little evidence currently exists on the role of CRF and its changes in young adulthood on long-term clinical cardiovascular outcomes and structure. Most large studies of CRF focus on middle-aged and older adults, demonstrating that fitness at a single point in time is associated with risk.1-4 Furthermore, understanding the implications of fitness early in life—before prevalent CVD or its risk factors—would provide important evidence about the mechanisms of the benefit of fitness for general health and the development of changes in the cardiovascular system linked to adverse long-term prognosis and use of health care resources (eg, heart failure or myocardial infarction). Evidence demonstrating the implications of CRF and its change early in life on mortality, cardiac structure, and cardiovascular events in a population of young adults at low clinical risk will be critical to shaping risk assessment, care delivery, and policy recommendations for fitness and exercise.
We sought to determine the importance of CRF and changes in CRF in young adults in relation to future CVD to reinforce a central hypothesis that fitness is a modifiable risk factor in young adulthood that reflects long-term CVD progression and prognosis. To this end, we examined the association of CRF in young adulthood with clinical and subclinical CVD during more than 25 years in the Coronary Artery Risk Development in Young Adults (CARDIA) study. We hypothesized that measures of CRF early in adulthood would be associated with long-term risks for mortality, CVD, and subclinical CVD independent of CVD risk factors, including obesity. In addition, we hypothesized that reductions in CRF during a period of 7 years in early adulthood would be associated with long-term clinical risk and markers of CVD progression.
Methods
Study Population
The CARDIA study is a longitudinal cohort designed to study determinants of CVD among 5115 young adults (aged 18-30 years) initially recruited from March 25, 1985, to June 7, 1986. Participants were recruited from 4 US sites (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). Recruitment balanced enrollment at each site by sex, age (18-24 vs 25-30 years), race, and education. Serial follow-up of participants at 2 (March 25, 1987, to July 13, 1988), 5 (May 18, 1990, to July 30, 1991), 7 (May 25, 1992, to June 30, 1993), 10 (May 18, 1995, to June 29, 1996), 15 (May 30, 2000, to June 30, 2001), 20 (June 1, 2005, to August 31, 2006), and 25 (June 1, 2010, to August 31, 2011) years after enrollment has been performed, with retention of 3547 and 3499 surviving participants of 4933 and 4849 participants (71.9% and 72.2%) at years 20 and 25, respectively. All participants provided written informed consent, with institutional review board approval at each field center (University of Alabama at Birmingham, Northwestern University, University of Minnesota, and Kaiser Permanente).
We excluded participants who did not start the treadmill test or who were missing maximal exercise duration at baseline (n = 154), who were missing baseline covariates used for adjustment in statistical analysis (n = 88), or who withdrew study consent (n = 1), leaving 4872 CARDIA participants for analysis of baseline fitness and long-term risk. Exclusion criteria for treadmill testing included resting hypertension (systolic blood pressure ≥160 mm Hg; diastolic blood pressure ≥100 mm Hg); prior myocardial infarction, angina, or valvular heart disease; current use of cardiovascular medications (excluding antihypertensives and thiazide diuretics); and clinical heart failure. In secondary analyses examining the 7-year change in CRF, additional exclusions consisted of not attending the year 7 examination (n = 963), violation of the treadmill protocol (n = 1071, owing to participants holding the treadmill apparatus at 1 site), and missing the year 7 treadmill duration or not starting the test (n = 366). Thus, 2472 participants underwent analysis for the 7-year change in CRF.
Clinical Assessments
All clinical assessments were performed at the baseline CARDIA visit (for analyses with baseline CRF) and the year 7 visit (for longitudinal changes in CRF).7 Clinical and demographic features and physical activity were assessed by questionnaire.8-11 Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) was defined as normal (18.5-24.9), overweight (25.0-29.9), or obese (≥30.0) using standard conventions. Diabetes mellitus was defined as a fasting blood glucose level of at least 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) or receiving medication for diabetes mellitus.
Exercise Treadmill Testing
The graded, symptom-limited maximal exercise test followed a modified Balke treadmill protocol, which consisted of as many as nine 2-minute stages of gradually increasing difficulty.12 We considered maximal exercise duration (in minutes) as our primary measure of CRF. Participants underwent a second assessment at 7 years with treadmill exercise testing to determine the association of changes in CRF over time with prognosis.
Cardiovascular Imaging
We examined the coronary artery calcification (CAC) score (range, 0-5949.9, with greater scores indicating greater burden of atherosclerosis) at the most contemporary CARDIA examination (year 25) using multiple-detector computed tomography.13 CAC, also measured at years 15 and 20, was handled as nonzero and as a continuous variable (with logarithm-transformation) for positive CAC scores. Doppler and M-mode echocardiography were performed using an cardiac ultrasonographic scanner (Artida; Toshiba Medical Systems) with standardized protocols across all centers and offline imaging interpretation (Digisonics). Left ventricular (LV) mass was derived from the Devereux formula14 and indexed to height. Imaging for LV mass was performed at years 5 and 25. We defined LV hypertrophy, stratified by race and sex, as an LV mass at year 25 (indexing is to height raised to 2.7th power) that is at least 2 SDs above the year 5 echocardiographic LV mass (indexed by height raised to the 2.7 power). The cutoff was determined separately by race and sex. The speckle-tracking echocardiography images for myocardial strain measurements were analyzed in a 16-segment basis for the LV middle-wall layer using tracking software (Wall Motion 2-dimensional Tracking; Toshiba Medical Systems). Three cardiac cycles from each view were recorded for offline analyses. Strain was calculated as the change in segment length relative to its end diastolic length, and the peak systolic value was recorded.
Cardiovascular Outcomes
Definitions of all-cause mortality and cardiovascular events have been previously described.15-20 Participants were contacted annually to inquire about interim hospitalizations. For each event, medical records were obtained and adjudicated by 2 morbidity and mortality committee members. For CVD events, we included nonfatal myocardial infarction or stroke; hospitalization for angina pectoris, congestive heart failure, or transient ischemic attack; revascularization for or demonstration of obstruction of carotid arteries or peripheral arterial disease on angiographic or ultrasonographic findings; and fatal atherosclerotic coronary heart disease, stroke, atherosclerotic disease other than coronary or stroke, or nonatherosclerotic cardiac disease. Participants who did not have events and who did not drop out of the study were censored at 27 years after the initial examination.
Statistical Analysis
Data were analyzed from study enrollment through August 31, 2011. Exercise test duration was modeled continuously or categorized into tertiles (across the entire cohort) in survival analyses. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for CVD events and all-cause mortality. Logistic regression was used to obtain odds ratios for prevalent CAC at years 15, 20, and 25 and LV hypertrophy at year 25. Linear regression was used to model year 25 LV mass and global LV longitudinal strain and year 25 logarithm-transformed CAC (for participants with a CAC score >0) as a function of treadmill test duration. Statistical models were adjusted for baseline (study entry [year 0]) age, race, sex, parental history of myocardial infarction (at younger than 60 years), BMI, systolic blood pressure, diabetes mellitus, smoking exposure (cigarettes per day), and high-density lipoprotein and total cholesterol levels. Additional Cox proportional hazards regression models for CVD events and all-cause mortality were run replacing baseline BMI, systolic blood pressure, diabetes mellitus, smoking exposure, and high-density lipoprotein and total cholesterol levels with time-dependent covariates updated at the years 2, 5, 7, 10, 15, 20, and 25 examinations. In addition, given published data surrounding the importance of fitness in mitigating obesity-related risk,21 we included multiplicative interaction terms with categories of BMI to assess effect modification.
The 7-year change in CRF was computed by subtracting the baseline treadmill duration from the year 7 duration. Associations of fitness change with outcomes were assessed using similar statistical models as those described above but with additional adjustment for baseline treadmill duration and 7-year changes in BMI, systolic blood pressure, smoking exposure, high-density lipoprotein and total cholesterol levels, and incident diabetes mellitus. Participants who died before or did not attend the year 7 visit were excluded from the year 7 data analysis, and no remaining participants experienced a nonfatal CVD event before year 7.
All analyses were performed in SAS software (version 9.4; SAS Institute Inc). Two-tailed P values of less than .05 were considered statistically significant.
Results
Baseline characteristics of the 4872 study participants are shown in Table 1, stratified by tertile of baseline exercise duration. Overall, the mean age was 24.8 years, mean (SD) BMI was 24.5 (4.9), and 28 participants (0.6%) had diabetes mellitus. The CRF at baseline was associated with male sex, white race, greater self-reported physical activity, a lower BMI, and a favorable biochemical profile (lower fasting insulin and triglyceride levels at baseline).
Table 1. Baseline Characteristics of 4872 CARDIA Participants by Tertiles of Treadmill Duration.
| Covariate | Study Population (n = 4872) | Tertile of Treadmill Duration | P Value for Trend | ||
|---|---|---|---|---|---|
| 1 (n = 1631) | 2 (n = 1617) | 3 (n = 1624) | |||
| Duration of treadmill test, min | 0.18-8.01 | 8.02-11.02 | 11.03-18.00 | ||
| Age, mean (SD), y | 24.8 (3.6) | 24.8 (3.8) | 24.8 (3.6) | 24.9 (3.6) | .25 |
| Male, No. (%) | 2222 (45.6) | 182 (11.2) | 670 (41.4) | 1370 (84.4) | <.001 |
| Black race, No. (%) | 2497 (51.3) | 1150 (70.5) | 743 (45.9) | 604 (37.2) | <.001 |
| Educational attainment, mean (SD), y | 13.8 (2.3) | 13.1 (1.9) | 14.0 (2.3) | 14.3 (2.4) | <.001 |
| Current smoker, No. (%) | 1473 (30.2) | 583 (35.7) | 497 (30.7) | 393 (24.2) | <.001 |
| Physical activity at baseline, exercise units, median (IQR)a | 360 (197-576) | 233 (112-396) | 366 (214-558) | 518 (325-742) | <.001 |
| Alcohol consumption, median (IQR), mL/d | 4.8 (0-14.5) | 0 (0-7.9) | 4.8 (0-14.8) | 7.8 (0-21.6) | <.001 |
| BMI, mean (SD) | |||||
| Baseline | 24.5 (4.9) | 26.8 (6.6) | 23.5 (3.8) | 23.0 (2.7) | <.001 |
| Year 25 (n = 3357) | 30.1 (7.2) | 33.9 (8.7) | 29.1 (6.2) | 27.7 (4.9) | <.001 |
| Waist circumference, mean (SD), cm | |||||
| Baseline | 77.6 (11.2) | 79.7 (13.9) | 75.6 (10.7) | 77.5 (7.8) | <.001 |
| Year 25 | 94.3 (16.0) | 98.6 (17.8) | 91.7 (15.7) | 92.9 (13.6) | <.001 |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic | 110 (11) | 109 (10) | 109 (11) | 112 (10) | <.001 |
| Diastolic | 68 (9) | 68 (10) | 68 (10) | 69 (9) | <.001 |
| Medication use ever, No. (%) | |||||
| Antihypertensives | 1123 (23.1) | 541 (33.2) | 329 (20.3) | 253 (15.6) | <.001 |
| Lowering of lipid levels | 651 (13.4) | 257 (15.8) | 189 (11.7) | 205 (12.6) | .009 |
| Smoking, median (IQR), No. of cigarettes/d | 0 (0-5) | 0 (0-7) | 0 (0-5) | 0 (0-0) | <.001 |
| Parental history of MI before 60 years of age, No. (%) | 601 (12.3) | 222 (13.6) | 205 (12.7) | 174 (10.7) | .01 |
| Diabetes mellitus, No. (%) | 28 (0.6) | 18 (1.1) | 6 (0.4) | 4 (0.2) | .002 |
| Presence of any CAC at year 25, No. (%) (n = 3067) | 869 (28.3) | 241 (24.3) | 267 (25.9) | 361 (34.6) | <.001 |
| LV mass at year 5, mean (SD), g (n = 3933) | 149 (44) | 141 (44) | 144 (44) | 162 (42) | <.001 |
| LV mass index at year 5b | 35.14 (9.17) | 36.28 (10.26) | 34.13 (8.88) | 35.08 (8.23) | .001 |
| Biomarkers of cardiometabolic risk | |||||
| Glucose level, mean (SD), mg/dL | 82 (14) | 82 (16) | 82 (15) | 83 (9) | .001 |
| Insulin level, mean (SD), μIU/mL | 8 (4.0) | 9.7 (5.5) | 7.8 (3.5) | 6.9 (2.3) | <.001 |
| CRP level, median (IQR), mg/L (year 7) | 1.1 (0.5-3.2) | 2.2 (0.8-5.6) | 1.1 (0.5-2.8) | 0.7 (0.3-1.6) | <.001 |
| Total cholesterol level, mean (SD), mg/dL | 177 (33) | 179 (34) | 178 (34) | 173 (32) | <.001 |
| HDL cholesterol level, mean (SD), mg/dL | 53 (13) | 52 (13) | 54 (14) | 53 (13) | .39 |
| Triglyceride level, mean (SD), mg/dL | 72 (48) | 74 (49) | 74 (53) | 70 (39) | .002 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAC, coronary artery calcification; CARDIA, Coronary Artery Risk Development in Young Adults; CRP, C-reactive protein; HDL, high-density lipoprotein; IQR, interquartile range; LV, left ventricular; MI, myocardial infarction.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; CRP to nanomoles per liter, multiply by 9.524; glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945; and triglycerides to millimoles per liter, multiply by 0.0113.
Calculated based on intensity, frequency, and consistency of participation. Engaging in exercise at 6 metabolic equivalents for 2 hours per week for 11 months per year corresponds to approximately 200 exercise units.
Calculated as LV mass, indexed by height raised to the 2.7th power.
During a median 26.9-year follow-up, 273 deaths (5.6%) and 193 CVD events (4.0%) occurred (the distribution of events is found in eTable 1 in the Supplement). Among the deaths, 200 (73.3%) were noncardiovascular in origin, with the greatest number of noncardiovascular deaths due to cancer (45 [22.5%]). In terms of subclinical CVD, 869 of 3067 individuals (28.3%) had any CAC by year 25, and 324 of 3001 (10.8%) had LV hypertrophy (defined as above).
After full adjustment for age, race, sex, obesity, CVD risk factors, and LV mass index, each additional minute of baseline exercise test duration was associated with a 15% lower hazard of death (HR, 0.85; 95% CI, 0.80-0.91; P < .001) and a 12% lower hazard of CVD (HR, 0.88; 95% CI, 0.81-0.96; P = .002) (model 2, Table 2). Exercise test duration was not associated with CAC at years 15, 20, and 25. After full adjustment, each 1-minute increase in exercise test duration was associated with a significantly reduced absolute LV mass index (β = −0.24; 95% CI, −0.45 to −0.03; P = .02) and significantly better global longitudinal strain, a measure of subclinical dysfunction (β = −0.09; 95% CI, −0.14 to −0.05; P < .001). Neither race nor sex modified the associations between exercise test duration and CAC, LV mass, or clinical outcome. Similarly, we found no interaction by baseline obesity status on most of these associations (eTable 2 in the Supplement; P > .05 for interaction).
Table 2. Unadjusted and Multivariable-Adjusted Associations of Outcomes With 1-Minute Increase in Treadmill Exercise Duration.
| Event | Association per Minute of Exercise Duration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model | |||||||||||
| No. of Participants | Effect Size (95% CI) | P Value | Model 1a | Model 2b | Model 3c | |||||||
| No. of Participants | Effect Size (95% CI) | P Value | No. of Participants | Effect Size (95% CI) | P Value | No. of Participants | Effect Size (95% CI) | P Value | ||||
| All-cause mortality, HRd | 4872 | 0.96 (0.92 to 1.00) | .08 | 4872 | 0.87 (0.82 to 0.92) | <.001 | 3918 | 0.85 (0.80 to 0.91) | <.001 | 4872 | 0.87 (0.82 to 0.92) | <.001 |
| Incident CVD, HRd | 4872 | 0.92 (0.87 to 0.96) | <.001 | 4872 | 0.91 (0.85 to 0.99) | .02 | 3918 | 0.88 (0.81 to 0.96) | .002 | 4872 | 0.90 (0.84 to 0.97) | .006 |
| Presence of CAC, ORe | ||||||||||||
| Year 15 | 2934 | 1.05 (1.01 to 1.10) | .02 | 2934 | 0.98 (0.91 to 1.05) | .50 | 2624 | 0.96 (0.89 to 1.04) | .30 | NA | NA | NA |
| Year 20 | 3016 | 1.05 (1.01 to 1.08) | .006 | 3016 | 0.99 (0.94 to 1.04) | .63 | 2685 | 0.98 (0.92 to 1.03) | .38 | NA | NA | NA |
| Year 25 | 3067 | 1.07 (1.04 to 1.10) | <.0001 | 3067 | 1.00 (0.95 to 1.05) | .86 | 2709 | 1.00 (0.95 to 1.05) | .99 | NA | NA | NA |
| β Value for CAC (logarithm-transformed, where CAC >0)f | 869 | −0.02 (−0.06 to 0.02) | .37 | 869 | −0.02 (−0.08 to 0.04) | .50 | 757 | −0.05 (−0.11 to 0.02) | .17 | NA | NA | NA |
| LV hypertrophy, ORe,g | 3001 | 0.84 (0.80 to 0.87) | <.001 | 3001 | 0.97 (0.90 to 1.04) | .40 | 2641 | 0.95 (0.88 to 1.03) | .24 | NA | NA | NA |
| β Value for LV mass index (year 25)f,h | 3001 | −0.69 (−0.84 to -0.54) | <.001 | 3001 | −0.12 (−0.33 to 0.08) | .23 | 2641 | −0.24 (−0.45 to −0.03) | .02 | NA | NA | NA |
| β Value for global longitudinal strain (year 25)f | 2915 | −0.04 (−0.07 to −0.01) | .01 | 2915 | −0.09 (−0.14 to −0.04) | <.001 | 2556 | −0.09 (−0.14 to −0.05) | <.001 | NA | NA | NA |
Abbreviations: CAC, coronary artery calcification; CVD, cardiovascular disease; HR, hazard ratio; LV, left ventricular; NA, not applicable; OR, odds ratio.
Adjusted for baseline age, race, sex, parental history of myocardial infarction (MI), body mass index (BMI), systolic blood pressure, diabetes mellitus, smoking exposure, and high-density lipoprotein (HDL) and total cholesterol levels.
Adjusted for model 1 covariates and year 5 LV mass index.
Adjusted for baseline age, race, sex, parental history of MI, time-dependent BMI, systolic blood pressure, diabetes mellitus, smoking exposure, and HDL and total cholesterol levels.
Modeling framework was Cox proportional hazards regression.
Modeling framework was logistic regression.
Modeling framework was general linear models.
Defined as year 25 LV mass index >2 SDs above the race-sex–specific year 5 echocardiographic LV mass, indexed by height raised to the 2.7th power.
Calculated as LV mass divided by height raised to the 2.7th power.
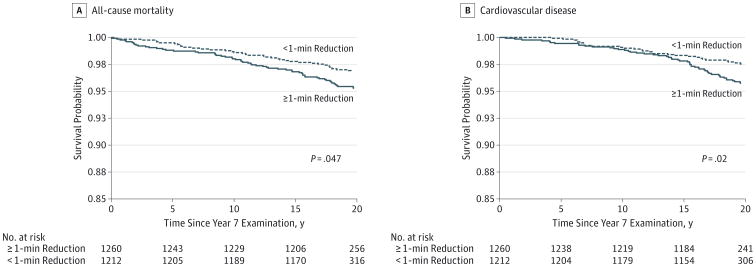
To quantify whether short-term changes in CRF are associated with long-term prognosis and subclinical CVD, we investigated a subgroup of 2472 individuals with exercise testing 7 years after initial treadmill testing. This subgroup undergoing a second treadmill test was representative of the overall study population in terms of clinical characteristics at study entry (eTable 3 in the Supplement). Median change in exercise test duration was −1.0 (interquartile range, −2.03 to 0.08) minutes, with 308 individuals (12.5%) achieving at least a 1-minute improvement (increase) in CRF. After full adjustment (including baseline exercise duration and time-dependent obesity and CVD risk), each 1-minute reduction in exercise test duration from baseline to year 7 was associated with a 21% increase in all-cause mortality in long-term follow-up (HR, 1.21; 95% CI, 1.07-1.37; P = .002) and a 20% increase in incident CVD (HR, 1.20; 95% CI, 1.06-1.37; P = .006). We did not observe any associations between changes in CRF during the 7 years and the presence of CAC at year 15, 20, or 25 or LV hypertrophy at year 25. On the other hand, each 1-minute reduction in treadmill exercise duration was associated with an increase (worsening) in global longitudinal strain (P < .001) (Table 3). When stratified around 1 minute of decline in exercise duration, individuals who had a decline in exercise duration of greater than 1 minute at year 7 had a significant reduction in long-term survival (P = .047) and CVD (P = .02; Figure).
Table 3. Unadjusted and Multivariable-Adjusted Associations of Every 1-Minute Reduction in Treadmill Exercise Duration Between Baseline and Year 7 Follow-up.
| Event | Association per Minute of Exercise Duration Reduction From Baseline to Year 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||||||
| No. of Participants | Effect Size, (95% CI) | P Value | No. of Participants | Effect Size (95% CI) | P Value | No. of Participants | Effect Size (95% CI) | P Value | |
| All-cause mortality, HRd | 2472 | 1.19 (1.06 to 1.33) | .002 | 2448 | 1.22 (1.08 to 1.38) | .002 | 2472 | 1.21 (1.07 to 1.37) | .002 |
| Incident CVD, HRd | 2472 | 1.24 (1.10 to 1.40) | <.001 | 2448 | 1.18 (1.02 to 1.37) | .03 | 2472 | 1.20 (1.06 to 1.37) | .006 |
| Presence of CAC, ORe | |||||||||
| Year 15 | 1803 | 0.98 (0.90 to 1.08) | .69 | 1792 | 0.98 (0.86 to 1.10) | .67 | NA | NA | NA |
| Year 20 | 1794 | 0.99 (0.93 to 1.06) | .82 | 1782 | 0.94 (0.86 to 1.03) | .19 | NA | NA | NA |
| Year 25 | 1789 | 1.01 (0.95 to 1.07) | .87 | 1779 | 0.97 (0.90 to 1.05) | .47 | NA | NA | NA |
| β Value for CAC at year 25 (logarithm-transformed, where CAC >0)f | 480 | −0.03 (−0.11 to 0.06) | .56 | 476 | −0.06 (−0.16 to 0.05) | .28 | NA | NA | NA |
| LV hypertrophy, ORe,g | 1796 | 1.18 (1.07 to 1.29) | <.001 | 1783 | 1.01 (0.90 to 1.14) | .83 | NA | NA | NA |
| β Value for LVmass index (year 25)f,h | 1796 | 0.97 (0.68 to 1.25) | <.001 | 1783 | 0.11 (−0.19 to 0.41) | .49 | NA | NA | NA |
| β Value for global longitudinal strain (year 25)f | 1690 | 0.20 (0.13 to 0.27) | <.001 | 1678 | 0.15 (0.08 to 0.23) | <.001 | NA | NA | NA |
Abbreviations: CAC, coronary artery calcification; CVD, cardiovascular disease; HR, hazard ratio; LV, left ventricular; NA, not applicable; OR, odds ratio.
Adjusted for baseline treadmill exercise duration.
Adjusted for baseline treadmill exercise duration, age, race, sex, parental history of myocardial infarction (MI), body mass index (BMI), systolic blood pressure, diabetes mellitus, smoking exposure, high-density lipoprotein (HDL) and total cholesterol levels, and 7-year changes in BMI, systolic blood pressure, smoking exposure, HDL and total cholesterol levels, and incident diabetes mellitus at year 7.
Adjusted for baseline treadmill exercise duration, age, race, sex, parental history of MI, and time-dependent BMI, systolic blood pressure, diabetes mellitus, smoking exposure, and HDL and total cholesterol levels.
Modeling framework was Cox proportional hazards regression.
Modeling framework was logistic regression.
Modeling framework was general linear models.
Defined as year 25 LV mass index >2 SDs above the race-sex–specific year 5 echocardiographic LV mass, indexed by height raised to the 2.7th power.
Calculated as LV mass divided by height raised to the 2.7th power.
Figure. Kaplan-Meier Unadjusted Survival Curves.
The main study outcomes—all-cause mortality and cardiovascular disease—were stratified by 1-minute reduction in exercise duration. P values were calculated using the unadjusted log-rank test.
Discussion
In this large biracial cohort of young US adults with long-term follow-up, CRF was significantly associated with incident CVD and all-cause mortality independently of cardiometabolic and CVD risk factors. Lower LV mass and favorable global longitudinal strain (but not extent or development of CAC) 25 years after the index fitness evaluation were more likely in individuals with higher baseline fitness. The mean CRF declined during the initial 7 years in the CARDIA study, with each 1-minute decrease in fitness during 7 years in early adulthood associated with a significant increase in the risk for long-term mortality, CVD, and subclinical LV dysfunction (by strain) but not CAC. These results suggest that CRF in young adulthood is a modifiable, prognostic biomarker of long-term mortality and CVD risk, with links to the development of abnormal myocardial structure and function but not CAC. Although the importance of CRF in determining clinical outcomes is well established, most large cohort investigations in this field have included middle-aged to older individuals (late 30s to early 50s in most landmark studies1-4), among whom subclinical and overt CVD may already be established and may influence outcomes.22 Given that young adults do not necessarily manifest the same subclinical disease or adverse events as these older populations, investigating the burden of CVD in young adults requires careful long-term follow-up to witness the development of subclinical CVD and adverse clinical outcomes.
These results establish that early fitness at an age younger than previously described is associated with a future risk for mortality and adverse cardiovascular events. The finding that fitness is related to development of subclinical changes in myocardial structure and function, but not CAC, sheds light on potential mechanistic benefits of exercise in youth not captured by CAC. We found that the effect of fitness on outcome could not be explained completely by BMI, changes in weight, or advancing cardiometabolic disease, suggesting that reductions in obesity and its consequences may not be the only mechanism of benefit of being fit early in adulthood. Finally, short-term changes in fitness in young adulthood had ramifications on risks for CVD and mortality independently of race, sex, BMI, changes in weight, and CVD-related risk factors, suggesting modifiability of fitness in personalized prevention.
We were surprised to find that fitness (or its change over time) was not associated with the extent or the presence of CAC in long-term follow-up. Exercise and fitness have been linked to endothelial function and vascular disease23 and progression of dyslipidemia and obesity,24 which are risk factors for CVD. As such, the metrics of ideal cardiovascular health (eg, diet, risk factors, and physical activity) have been associated with a lower prevalence of CAC in cross-sectional25 and longitudinal studies,26 likely reflecting the cumulative burden of salutatory health habits on CVD pathogenesis. We did observe a prevalence of CAC (28.3% of study participants at year 25) similar to that of other studies in middle-aged adults (≤19% in individuals aged 40-46 years in the Cardiovascular Risk in Young Finns study27), which suggests a typical progression of subclinical CVD in the CARDIA study. Although published reports demonstrate heterogeneity regarding the effect of objectively measured CRF on CAC,28-30 the lack of association between CAC and point and serial measures of CRF in our study suggests that the cardiovascular benefits of fitness may extend beyond prevention of CAC and its progression. Exercise training may affect plaque composition31 and systemic inflammatory markers linked to plaque rupture (eg, matrix metalloproteinases32), suggesting a role for non–CAC-dependent protection from CVD. Even among individuals without CAC, coronary vasodilator function can be abnormal and associated with adverse events, underscoring the presence of prognostically important coronary artery disease in the absence of CAC.33 Finally, emerging data suggest that statins (which stabilize coronary plaques) may actually promote CAC development,34 more evidence that—although an important biomarker of subclinical CVD—CAC may not fully capture the benefits of fitness on cardiovascular health.
We also observed an association between fitness and its change early in adulthood with myocardial phenotypes late into follow-up—namely, cardiac mass and strain—which are both potent risk factors for incident CVD, specifically heart failure.35,36 Mechanistically, inflammation, insulin resistance, and endothelial dysfunction associated with lower levels of CRF have been linked to cardiac structural and functional remodeling,37 with increases in LV mass and reductions in strain predating heart failure.35 Although the younger CARDIA population is still accruing heart failure events, recognition of the effect of fitness in middle age on heart failure is emerging, with increments in exercise capacity in middle age associated with reduction in incident hospitalization for heart failure38 and lower levels of fitness associated with myocardial dysfunction.39 These data provide evidence that in the earliest stages of risk factor development, fitness may be a powerful, independent predictor of subclinical myocardial remodeling, suggesting a potential role for fitness as a prognostic and therapeutic tool against myocardial dysfunction and heart failure.40
The CARDIA study has a large biracial cohort with long follow-up and extensive data on confounding variables and detailed cardiovascular and metabolic phenotyping. Nevertheless, the results of this study have some limitations in light of its design. The possibility that the relationship between higher CRF and outcome may be confounded by unmeasured factors related to a generally more salutatory lifestyle (eg, improved diet41) may still exist; however, we performed time-dependent adjustments for hypertension, diabetes mellitus, and obesity—all metrics of cardiovascular and general health—to limit this possibility. Although these adjustments include confounders and mediators of fitness-related benefits for CVD, this approach yielded significant associations between CRF and cardiovascular structure and outcome, which suggests the robustness of CRF as an independent predictor. Although we used treadmill test duration as the primary measure of CRF (as opposed to assessments of peak aerobic capacity), this approach is widely available and clinically generalizable. We focused on serial fitness from baseline to 7 years as opposed to fitness assessments into middle age (eg, year 20) in the CARDIA study. Although this approach may limit our ability to detect the importance of evolution in CRF between early adulthood to middle age, our aim was to focus on CRF changes early in life in the CARDIA study during a time when increasing obesity and risk factors (but not clinical CVD) evolve. The CARDIA study assessed LV mass and strain at year 25, thereby potentially missing intermediate temporal changes during which more subtle phenotypes may emerge. However, the associations between CRF early in adulthood with very long-term cardiac structure and function are strong evidence of the long-lasting effect of CRF on myocardial disease.
Conclusions
In a large study of young adults without clinical CVD, we demonstrate a strong association between absolute fitness and changes in fitness early in adult life on long-term mortality independently of obesity or other indices of metabolic or CVD risk. Fitness was associated with cardiac remodeling but not CAC, which suggests that traditional indices of atherosclerotic CVD progression may not completely explain fitness-related benefits on cardiovascular health. Early measures of CRF represent a quantifiable, prognostic, and mechanistically relevant biomarker of survival in early adulthood. Efforts to evaluate and improve fitness early in adulthood may affect long-term health at the earliest stages in CVD pathogenesis.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95168, N01-HC-95169, and HL098445-05 from the National Heart, Lung, and Blood Institute (NHLBI) (Dr Carr); by grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources; by a Fellow-to-Faculty Award from the American Heart Association (Dr Shah); by Centers of Biomedical Research Excellence grant 8P20 GM-1033528 from the National Institute of General Medical Sciences, National Institutes of Health (Dr Sarzynski); and by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the NHLBI, the Intramural Research Program of the National Institute on Aging (NIA), and intra-agency agreement AG0005 between the NIA and NHLBI (Coronary Artery Risk Development in Young Adults [CARDIA] study).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Ms Colangelo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Shah, Murthy, Colangelo, Abbasi, Goff, G. D. Lewis, Lima.
Acquisition, analysis, or interpretation of data: Shah, Murthy, Colangelo, Reis, Venkatesh, Sharma, Goff, Carr, Rana, Terry, Bouchard, Sarzynski, Eisman, Neilan, Das, Jerosch-Herold, C. E. Lewis, Carnethon, G. D. Lewis, Lima.
Drafting of the manuscript: Shah, Murthy, Colangelo, Eisman, G. D. Lewis, Lima.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Shah, Murthy, Colangelo, Sharma, Eisman, Carnethon.
Obtained funding: Reis, Goff, Carr, C. E. Lewis.
Administrative, technical, or material support: Reis, Sharma, Abbasi, Carr, Terry, Neilan, Das, C. E. Lewis, Lima.
Study supervision: Shah, Murthy, Carr, G. D. Lewis, Lima.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This article's abstract was presented as a poster at the 2015 Scientific Sessions of the American Heart Association; November 7-11, 2015; Orlando, Florida.
Additional Contributions: Mercedes Carnethon, PhD, Departments of Preventive Medicine and Epidemiology, Feinberg School of Medicine, Northwestern University, reviewed this manuscript for scientific content. She was not compensated for this role.
Contributor Information
Ravi V. Shah, Cardiovascular Division, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Venkatesh L. Murthy, Cardiovascular Medicine Division, Department of Medicine, University of Michigan, Ann Arbor; Nuclear Medicine Division, Department of Radiology, University of Michigan, Ann Arbor.
Laura A. Colangelo, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Jared Reis, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Bharath Ambale Venkatesh, Department of Medicine and Cardiology, Heart and Vascular Institute, Johns Hopkins Medical Institutions, The Johns Hopkins University, Baltimore, Maryland.
Ravi Sharma, Department of Medicine and Cardiology, Heart and Vascular Institute, Johns Hopkins Medical Institutions, The Johns Hopkins University, Baltimore, Maryland.
Siddique A. Abbasi, Division of Cardiology, Department of Internal Medicine, Rhode Island Hospital, Brown University, Providence, Rhode Island.
David C. Goff, Jr, Department of Epidemiology, Colorado School of Public Health, Aurora.
J. Jeffrey Carr, Department of Epidemiology, Vanderbilt University, Nashville, Tennessee.
Jamal S. Rana, Department of Cardiology, Kaiser Permanente Northern California, Oakland; Department of Medicine, University of California, San Francisco.
James G. Terry, Department of Epidemiology, Vanderbilt University, Nashville, Tennessee.
Claude Bouchard, Human Genomics Laboratory, Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Mark A. Sarzynski, Human Genomics Laboratory, Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Aaron Eisman, Department of Medicine, Massachusetts General Hospital, Boston.
Tomas Neilan, Department of Medicine, Massachusetts General Hospital, Boston.
Saumya Das, Cardiovascular Division, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts.
Michael Jerosch-Herold, Noninvasive Cardiovascular Imaging Section, Cardiovascular Division, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts; Department of Radiology, Brigham and Women's Hospital, Boston, Massachusetts.
Cora E. Lewis, Division of Preventative Medicine, Department of Medicine, University of Alabama at Birmingham.
Mercedes Carnethon, Department of Preventative Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Gregory D. Lewis, Human Genomics Laboratory, Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Joao A. C. Lima, Department of Medicine and Cardiology, Heart and Vascular Institute, Johns Hopkins Medical Institutions, The Johns Hopkins University, Baltimore, Maryland.
References
- 1.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 2.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: the Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319(21):1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 5.Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation. 2011;124(15):1673–1686. doi: 10.1161/CIRCULATIONAHA.110.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125(8):996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis JP, Allen N, Gibbs BB, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: the CARDIA study. Obesity (Silver Spring) 2014;22(11):2434–2440. doi: 10.1002/oby.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenknecht LE, Perkins LL, Cutter GR, et al. Cigarette smoking behavior is strongly related to educational status: the CARDIA study. Prev Med. 1990;19(2):158–169. doi: 10.1016/0091-7435(90)90017-e. [DOI] [PubMed] [Google Scholar]
- 9.Dyer AR, Cutter GR, Liu KQ, et al. Alcohol intake and blood pressure in young adults: the CARDIA study. J Clin Epidemiol. 1990;43(1):1–13. doi: 10.1016/0895-4356(90)90050-y. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Jacobs DR, Jr, Sidney S, Haskell WL, Anderssen N, Oberman A. Physical activity in young black and white women: the CARDIA study. Ann Epidemiol. 1993;3(6):636–644. doi: 10.1016/1047-2797(93)90087-k. [DOI] [PubMed] [Google Scholar]
- 11.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol. 1991;133(12):1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 12.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–183. [PubMed] [Google Scholar]
- 13.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Luepker RV, Apple FS, Christenson RH, et al. AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Madden KP, Karanjia PN, Adams HP, Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study: Trial of ORG 10172 in Acute Stroke Treatment. Neurology. 1995;45(11):1975–1979. doi: 10.1212/wnl.45.11.1975. [DOI] [PubMed] [Google Scholar]
- 19.Easton JD, Saver JL, Albers GW, et al. American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology andIntervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Möhlenkamp S, McClelland R, et al. Multi-Ethnic Study of Atherosclerosis and the Investigator Group of the Heinz Nixdorf RECALL Study. A comparison of outcomes with coronary artery calcium scanning in unselected populations: the Multi-Ethnic Study of Atherosclerosis (MESA) and Heinz Nixdorf RECALL study (HNR) J Cardiovasc Comput Tomogr. 2013;7(3):182–191. doi: 10.1016/j.jcct.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juraschek SP, Blaha MJ, Whelton SP, et al. Physical fitness and hypertension in a population at risk for cardiovascular disease: the Henry Ford Exercise Testing (FIT) Project. J Am Heart Assoc. 2014;3(6):e001268. doi: 10.1161/JAHA.114.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minder CM, Shaya GE, Michos ED, et al. Relation between self-reported physical activity level, fitness, and cardiometabolic risk. Am J Cardiol. 2014;113(4):637–643. doi: 10.1016/j.amjcard.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Saleem Y, DeFina LF, Radford NB, et al. Association of a favorable cardiovascular health profile with the presence of coronary artery calcification. Circ Cardiovasc Imaging. 2015;8(1):e001851. doi: 10.1161/CIRCIMAGING.114.001851. [DOI] [PubMed] [Google Scholar]
- 26.Spring B, Moller AC, Colangelo LA, et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130(1):10–17. doi: 10.1161/CIRCULATIONAHA.113.005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartiala O, Magnussen CG, Kajander S, et al. Adolescence risk factors are predictive of coronary artery calcification at middle age: the Cardiovascular Risk in Young Finns study. J Am Coll Cardiol. 2012;60(15):1364–1370. doi: 10.1016/j.jacc.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 28.DeFina L, Radford N, Leonard D, Gibbons L, Khera A. Cardiorespiratory fitness and coronary artery calcification in women. Atherosclerosis. 2014;233(2):648–653. doi: 10.1016/j.atherosclerosis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Lee CD, Jacobs DR, Jr, Hankinson A, Iribarren C, Sidney S. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA study. Atherosclerosis. 2009;203(1):263–268. doi: 10.1016/j.atherosclerosis.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakka TA, Laukkanen JA, Rauramaa R, et al. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle-aged men. Ann Intern Med. 2001;134(1):12–20. doi: 10.7326/0003-4819-134-1-200101020-00008. [DOI] [PubMed] [Google Scholar]
- 31.Madssen E, Moholdt T, Videm V, Wisløff U, Hegbom K, Wiseth R. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am J Cardiol. 2014;114(10):1504–1511. doi: 10.1016/j.amjcard.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Niessner A, Richter B, Penka M, et al. Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: impact on plaque stabilization? Atherosclerosis. 2006;186(1):160–165. doi: 10.1016/j.atherosclerosis.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Naya M, Murthy VL, Foster CR, et al. Prognostic interplay of coronary artery calcification and underlying vascular dysfunction in patients with suspected coronary artery disease. J Am Coll Cardiol. 2013;61(20):2098–2106. doi: 10.1016/j.jacc.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65(13):1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort: the Framingham Heart study. Ann Intern Med. 1989;110(2):101–107. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 36.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart study. N Engl J Med. 1990;322(22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 37.Shah RV, Abbasi SA, Heydari B, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61(16):1698–1706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey A, Patel M, Gao A, et al. Changes in mid-life fitness predicts heart failure risk at a later age independent of interval development of cardiac and noncardiac risk factors: the Cooper Center Longitudinal Study. Am Heart J. 2015;169(2):290–297.e1. doi: 10.1016/j.ahj.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinker SK, Pandey A, Ayers CR, et al. Association of cardiorespiratory fitness with left ventricular remodeling and diastolsic function: the Cooper Center Longitudinal Study. JACC Heart Fail. 2014;2(3):238–246. doi: 10.1016/j.jchf.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8(1):33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation. 2015;132(9):804–814. doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.