Abstract
Introduction:
Psychological stress and changes in hypothalamic-pituitary-adrenal (HPA) axis in period after diagnosis of “de novo” Parkinson disease (PD) could be a big problem for patients.
Materials and Methods:
We measured psychological stress and changes in hypothalamic-pituitary-adrenal axis (HPA) in thirty patients (15:15) with “de novo” Parkinson’s disease, average age 64.17 ± 13.19 (28-82) years (Department of Neurology, University Clinical Center Tuzla). We used Impact of events scale (with 15 questions) to evaluate psychological stress. Normal level of morning cortisol was 201-681 nmol/l, and morning adrenocorticotropic hormone (ACTH) up to 50 pg/ml.
Results:
Almost 55% patients suffered from mild or serious psychological stress according to IES testing (Horowitz et al.). Non-iatrogenic changes in HPA axis were noticed at 30% patients. The differences between female and male patients regarding to the age (p=0.561), value of cortisol (p=0.745), value of ACTH (p=0.886) and IES testing (p=0.318) were not noticed. The value of cortisol was the predictor of value of ACTH (r=0.427).
Conclusion:
Psychological stress and changes in hypothalamic-pituitary-adrenal axis are present in patients with “de novo” PD. There is significant relation between values of cortisol and ACTH. Psychological stress is frequent problem for “de novo” PD patients.
Keywords: Psychological stress, HPA axis, “de novo”, Parkinson’s disease
1. INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the loss of the dopaminergic neurons in substantia nigra and the accumulation of alpha-synuclein and other proteins in intracellular proteinaceous aggregates called Lewy bodies (1). Stress is a non-specific consequence (physiological or physical) of any request on a organism that overcomes adoptive possibilities.(2). A word means a great pressure, strain, effort. The consequences are different changes manifested through a classic reaction such as “fight or escape” (3). The changes occur in the hypothalamic-pituitary-adrenal (HPA) axis. Cortisol and adrenocorticotropic hormone (ACTH) are usually intensely produced in order to reduce the harmful effect of stress on the organism. Also, a comparison of persons who suffered from extraordinary emotional and physical stress has been made with healthy peers. The higher risk of the appearance of PD was found in the first group. The potential explanation would be the growth of metabolic production of dopamine under the stress with the higher possibilities of „so called” free radicals as products of oxidated stress damage neurones in the nigra substance (4, 5). Other studies also showed that chronic stress and stressors may be risk for developing of PD (6, 7). A research made on the patients with PD and Alzheimer’s disease (AD) and healthy volunteers showed that the value of cortisol is significantly higher at the diseased subjects (8). The changes in the HPA axis are noticeable with the patients with ischaemic stroke and heart attack (9, 10).
2. AIM
Evaluate the existence of stress reaction of patients with “de novo” PD;
Discover the changes of hypothalamic-pituitary-adrenal (HPA) axis with patients with “de novo” PD.
3. MATERIAL AND METHODS
The research has been conducted at the Department of Neurology (University Clinical Center-UCC Tuzla) with the approval of the clinic criteria such as: tremor, bradykinesia, rigidity, posture difficulties, and a good reaction to levodopa substances. The diagnosis was announced by a doctor specialist in a simple language understandable by the patients. After the announcement, the basic characteristics were explained to the patients, also in a realistic and understandable way. The medical conditions that should be fulfilled for the participation in the groups were: the possibilities for adequate testing on psychological stress (speech disorder, concussion, and significant cognitive disorder, etc); therapy without corticosteroid substances and spironolactone, and absence of Cushing’s / Addison’s syndrome. For evaluation of existence of psychological stress was used Impact of events scale - IES, with 15 questions (11). A short and simple scale made for the quantitative evaluation of stress reaction. Up to 8 is subclinical, 9 to 25 indicates mild stress, 26 to 43 indicates mild to serious stress, and over 44 indicates serious stress. The subjects filled the scales by themselves. In a couple of cases, for legitimate reason (exhaustion, weakness in the upper limbs, illiteracy, hand shaking, partially sight problems, etc) the answers were filled by the physician. The answers were not suggested by the doctor. The blood was taken in the morning, around 7 a.m. on an empty stomach, before the examinees got up from the bed. For measuring the value of cortisol in serum, it was used fluorometric method with DELFIA® Cortisol fluoroimmunoassay. The instrument used for this test was LKB WALLAC 1230 Arcus fluorometer. The normal value of cortisol were 201-681 nmol/l (12). For measuring the value of adrenocorticotropic hormone (ACTH) in serum, it was used RIA (radioimunoessay) method, in vitro determination. The instrument used was “so called” gamma-counter PERKIN ELMER®. The concentration of ACTH in the samples was directly proportional to the level of the radioactivity. The normal value of ACTH were up to 50 pg/ml (13). The measurements were realized on the Department of Nuclear Medicine (UCC Tuzla). The testing on psychological stress and blood samples tested on measurement of cortisol and ACTH were made seven days after announcing the “de novo” PD patients. The following static parameters were used for the analysis: normal readings and standard deviation with using T-test and χ2 test for evaluation of significant differences and Pearson’s coefficient of correlation (r). The differences are acknowledged as significant with p<0.05.
4. RESULTS
The analyzed group consisted of 30 examinees of average age 64.17±13.19 and 15 (50%) examinees were female. The average age of hospitalized female examinees were 62.73±10.69 (48-77), and age of hospitalized male examinees was 65.6±15.54 (28-82).
Almost 55% patients with „de novo” PD suffered from mild to serious stress (Figure 1). Every sixth patient had increased value of cortisol (16.7%), but also ACTH (16.7%) (Figure 2, 3). The change in the HPA axis, followed by the increased value of stress hormone(s) was present at (30%) of patients, or every third patient (Figure 4). No significant differences have been noticed with male and female patients regarded to the age (p=0.561), value of cortisol (p=0.745) and value of ACTH (p=0.886), as well as Horowitz’s test (p=0.318). The value of cortisol was predictor of ACTH value confirmed by Pearson’s coefficient of correlation (r=0.427).
Figure 1.
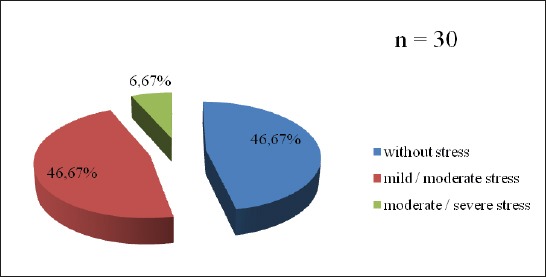
Distribution of patients with “de novo” Parkinson’s disease in relation to the psychological stress
Figure 2.
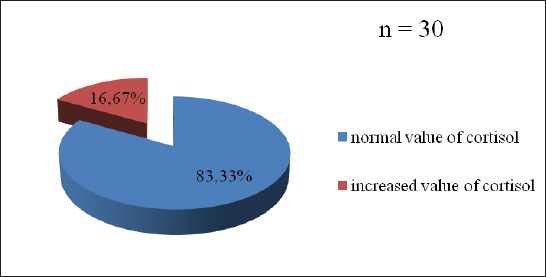
Distribution of patients with “de novo” Parkinson’s disease in relation to the level of cortisol
Figure 3.
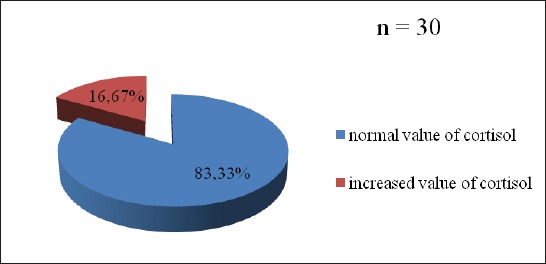
Distribution of patients with “de novo” Parkinson’s disease in relation to the level of adrenocorticotropic hormone (ACTH)
Figure 4.
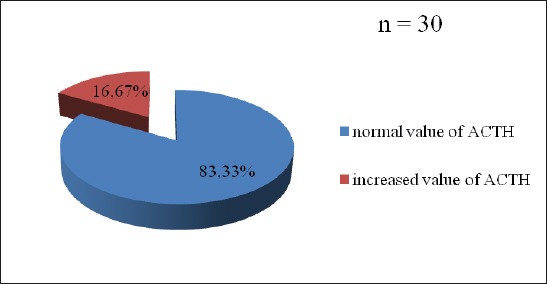
Distribution of patients with Parkinson’s disease in relation to the value of cortisol and/or adrenocorticotropic hormone
5. DISCUSSION
Stress is a condition of human experience and also an important factor in the onset of several diseases, including cardiovascular, metabolic and neuropsychiatric diseases (14, 15). Under stressful situation, humans mobilize psychological resources to respond to these situations in the so-called stress response (16). Due to that, two different systems are activated: sympathetic-adrenal-medullary (SMA) and hypothalamic-pituitary-adrenal (HPA). The patients with neurodegenerative diseases have mildly activated HPA axis. This is the possible due to the hypocampal degeneration which is present during exposure to stress or during steroid effect (17).
Also, stress can be manifested not only before the development of PD, but also after making and announcing “ de novo” PD diagnosis. Significant number of our patients with „de novo” PD had a stress reaction (during psychological testing, as well as during measurement of changes in the HPA axis). It should be mentioned that the increase of the cortisol level and ACTH were non-iatrogenic. Values of cortisol were higher up to 17% in PD patients, but not at their relatives (18). Even dogs that were injected 1-metyl-4-phenyl-1,2,3,6-tetrahydropiridine (MPTP) as an experimental method for development of PD had a significant increase of the value of cortisol and ACTH for 60% and 40% (19).
Chronic fatigue, syndrome of chronic stress, can be found in up to 70% of PD patients at some stage of illness (20). Vital exhaustion can be a marker of neurodegeneration leading to PD (21). High levels of cortisol and ACTH, provoked by a dysfunctional HPA axis, have been associated to dopaminergic cell loss and found in PD patients (22-24). That could be another reason for PD progress. However, a circle appears in which dysfunctional HPA axis destroys dopaminergic neurones and inevitable worsening PD leads to the stress progress and impossibility to repair HPA axis.
In patients with PD, the HPA axis is unbalanced and the cortisol levels are significantly increased, implying a deregulation of glucocorticoid receptor (GR) function in immune cells. In experimental parkinsonism, the activation of microglial GR has a crucial effect in diminishing microglial cell activation and reducing dopaminergic degeneration. Moreover, glucocorticoids are also known to regulate human brain vasculature as well as blood brain barrier (BBB) permeability. So, any dysfunction in their actions may influence infiltration of cytotoxic molecules resulting in increased vulnerability of dopamine neurons (25).
However, the greatest risk factor appears to be age, since the symptoms of PD emerge preferentially after 65. It is interesting that the average age of our examinees was around 65. In this regard, it is important to note that dysfunctions in the stress response develop during the aging process. Hence, as an organism ages, the response of the HPA axis to stress becomes hyperactive and less efficient to return to former homeostatic conditions, thus exposing brain cells to higher levels of stress-hormones for longer periods of time (26).
Stress-related glucocorticoids may be an important contributing factor to modulate the long-term brain inflammatory response, including the appearance of neurotoxic microglia. Midbrain dopaminergic neurons are especially sensitive to pro-inflammatory microglia, so the impact of chronic stress in the etiology and course of PD is very important (1).
The additional value to our research yields information on the relationship between the prediction and the secretion of cortisol and ACTH. It shows the “be united of the stress hormone” in changes within the HPA axis and in our sample. However, we think it best to do the planned tests on psychological stress and changes in the HPA axis in twice–before and after the diagnosis of “de novo” PB. Such results would surely be complete, more exact and objective. It is necessary to continue with similar studies on a larger sample in order to obtain proper results.
6. CONCLUSION
Psychological problems and changes in HPA axis are present in patients with “de novo” PD. There is significant relation between values of cortisol and ACTH. Psychological stress is frequent problem for PD patients.
Footnotes
• Author’s statement: All authors were included in preparing of this article, including final proof reading.
• Conflict of interest: none declared.
REFERENCES
- 1.Herrera AJ, Espinosa-Oliva AM, Carrillo-Jiménez A, Oliva-Martín MJ, García-Revilla J, García-Quintanilla A, et al. Relevance of chronic stress and two faces of microglia in Parkinson’s disease. Front Cell Neurosci. 2015;14(9):312. doi: 10.3389/fncel.2015.00312. doi:10.3389/fncel.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selye H. Hystory of stress concept. In: Goldberg L, Breznitz S, editors. Handbook of stress. New York: The Free Press; 1993. [Google Scholar]
- 3.Cannon WB. The wisdom of human body. New York: Norton. 1947 [Google Scholar]
- 4.Gibberd FB, Simmons JP. Neurological disease in ex-Far-East prisoners of war. Lancet. 1980;11:135–8. doi: 10.1016/s0140-6736(80)90015-x. [DOI] [PubMed] [Google Scholar]
- 5.Spina MB, Cohen G. Dopamine turnover and glutathion oxidation: implications for Parkinson’s disease. Proc Nat Acad Sci USA. 1989;88:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon TW. Mental hygiene. Am J Public Health. 2006;96:1740–2. doi: 10.2105/ajph.96.10.1740. doi. 10.2105/ AJPH.96.10.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmerle AM, Herman JP, Seroogy KB. Stress depression and Parkinson’s disease. Exp Neurol. 2012;233:79–86. doi: 10.1016/j.expneurol.2011.09.035. doi 10.1016/j.expneurol.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18(3):285–9. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahimagić OĆ, Sinanović O, Čičkušić A, Smajlović Dž. Ishemijski cerebrovaskularni inzult kao akutna reakcija na stres. Med Arch. 2005;59(2):79–82. [PubMed] [Google Scholar]
- 10.Ibrahimagić OĆ, Sinanović O, Smajlović Dž, Vidović M. Promjene u metabolizmu kortizola nakon ishemijskoga cerebrovaskularnog inzulta i infarkta miokarda. Medicus. 2006;15(1):189–94. [Google Scholar]
- 11.Horowitz M, Wilner N, Alvarez W. Impact of events scale (IES): A measure of subjective stress. Psychosom Med. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. DELFIA®Cortisol (Instructions for use) Turku, Finland: Wallac Oy; 2001. [Google Scholar]
- 13.Anonymous. IRMA ACTH ®(Direction for use) Marseille, France: Immunotech SA; 2003. [Google Scholar]
- 14.Renard GM, Suarez MM, Levin GM, Rivarola MA. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiol Behav. 2005;85:363–9. doi: 10.1016/j.physbeh.2005.05.003. doi 10.1016/j.physbeh.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010 doi: 10.1371/journal.pone.0008566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front. Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. doi 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 17.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–94. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Charlett A, Dobbs RJ, Purkiss AG, Wright DJ, Peterson DW, Weller C, et al. Cortisol is higher in parkinsonism and associated with gait deficit. Acta Neurol Scand. 1998;97(2):77–85. doi: 10.1111/j.1600-0404.1998.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 19.Mizobuchi M, Hineno T, Kakimoto Y, Hiratani K. Increase of plasma adrenocorticotropic and cortisol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropiridine (MPTP)-treated dogs. Brain Res. 1993;612(1-2):319–21. doi: 10.1016/0006-8993(93)91678-l. [DOI] [PubMed] [Google Scholar]
- 20.Friedman JH, Abrantes A, Sweet LH. Fatigue in Parkinson’s disease. Expert Opin Pharmacother. 2011;12:1999–2007. doi: 10.1517/14656566.2011.587120. doi 10.1517/14656566.2011.587120. [DOI] [PubMed] [Google Scholar]
- 21.Clark AJ, Ritz B, Prescott E, Rod NH. Psychosocial risk factors, pre-motor symptoms and first-time hospitalization with Parkinson’s disease: a prospective cohort study. Eur J Neurol. 2013;20:1113–20. doi: 10.1111/ene.12117. doi 10.1111/ene.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller T, Muhlack S. Acute levodopa intake and associated cortisol decrease in patients with Parkinson disease. Clin Neuropharmacol. 2007;30:101–6. doi: 10.1097/01.WNF.0000240954.72186.91. doi 10.1097/01. wnf.0000240954.72186.91. [DOI] [PubMed] [Google Scholar]
- 23.Müller T, Muhlack S. Impact of endurance exercise on levodopa-associated cortisol release and force increase in patients with Parkinson’s disease. J Neural Transm. 2008;115:851–5. doi: 10.1007/s00702-008-0018-7. doi 10.1007/s00702-008-0018-7. [DOI] [PubMed] [Google Scholar]
- 24.Djamshidian A, Lees AJ. Can stress trigger Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2014;85:878–81. doi: 10.1136/jnnp-2013-305911. doi 10.1136/jnnp-2013-305911. [DOI] [PubMed] [Google Scholar]
- 25.Herrero MT, Estrada C, Maatouk L, Vyas S. Inflammation in Parkinson’s disease: role of glucocorticoids. Front Neuroanat. 2015;9:32. doi: 10.3389/fnana.2015.00032. doi:10.3389/fnana.2015.00032. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein-Behrens BA. Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–35. doi: 10.1111/j.1471-4159.1992.tb10047.x. doi 10.1111/j.1471-4159.1992.tb10047. [DOI] [PubMed] [Google Scholar]


