Abstract
Background:
This study aimed to investigate correlation between adiponectin and waist-hip-ratio with severity of coronary artery disease (CAD). There is uncertainty about the association between circulating concentrations of adiponectin and CAD.
Methods:
We enrolled eighty-two consecutive patients undergoing non-urgent coronary angiography for CAD survey. According to the angiography results, the patients were divided into two groups in 1:1 ratio patients admitted with a diagnosis of CAD and non-CAD. We conducted hospital based research, involving study group with documented angiographically CAD, and control group without evidence of CAD. Angiograms were also quantified for the extent and severity of CAD by the Gensini scoring system. We measured baseline adiponectin levels in stored serum samples of all patients, anthropometric and biochemical risk factors were assessed in both groups.
Results:
The presence of CAD was associated with current smoking, male gender, waist–hip ratio (WHR) and left ventricular ejection fraction (LVEF). Baseline adiponectin concentrations correlated significantly in terms of the lipid parameters, positively with HDL cholesterol concentrations (r=0.327, P=0.028, P<0.05) and serum triglyceride concentrations were correlated negatively (r=-0.513, P<0.001). No significant difference between median adiponectin levels at baseline was observed between cases and controls.
Conclusion:
There is a significant positive correlation between waist–hip ratio and presence and severity of coronary artery disease. In conclusion, there is a significant positive correlation between adiponectin and Gensini score among Kosovar patients.
Keywords: adiponectin, waist–hip ratio, coronary artery disease, Gensini score
1. INTRODUCTION
Adiponectin (ARCP 30, AdipoQ, apM1 or GBP28), secreted by adipose tissue, is a 247-amino acid peptide which was discovered in 1995 (1, 2). The amounts of adiponectin account for 0.01% of total plasma protein. The concentration of adiponectin circulating in plasma is very high (2 to 20 µg/ml) (3). Plasma levels of adiponectin in the Japanese population is about 5 to 10 µg/ml (4) and serum adiponectin is lower in Indo-Asians when compared with Caucasians (median 3.3 vs. 4.9 µg/ml) (5). Women have about 40% higher circulating levels of adiponectin than men (3). Adiponectin has gained particular interest on account of its relation with insulin sensitivity, atherosclerosis, and inflammation (6). Current projections estimate that cardiovascular disease, especially atherosclerosis, will be the leading cause of all disease in 2020 (7). Recent findings suggest that high adiponectin concentration is an independent predictor of mortality in chronic heart failure patients, chronic kidney patients, and elderly patients, who are at high risk for cardiovascular events (8-12).
Obesity has been proven to be an independent risk factor for CAD in both genders, and is a growing health problem in most developed and some developing countries (13-15). Body weight, body mass index (BMI), waist circumference (WC) and WHR are primary methods to determine obesity. While BMI reflects general obesity, waist circumference and waist–hip ratio are related to central-type obesity, where body fat is primarily located in the abdomen. Prospective epidemiological studies have revealed that central obesity (determined by WC and WHR) is more relevant in CAD risk compared to general obesity (determined by BMI) (16-18). While WHR is commonly used in reflecting central obesity, waist circumference is shown to have a better correlation with abdominal fat localization (19-22). To our best knowledge, this is the first study performed in Kosovo focusing on this particular issue.
2. MATERIALS AND METHODS
2.1. Study subjects
The study population consisted of 82 consecutive patients who underwent coronary angiography for suspected or known coronary atherosclerosis at the University Clinical Center of Kosovo. With the exception of acute coronary syndromes, the patients had to present in a stable clinical condition without major concomitant non-cardiovascular disease. The study protocol was approved by the Ethical Committee of the Faculty of Medicine, University of Prishtina, and research was conducted in accordance with Declaration of Helsinki guidelines. Written informed consent was obtained from all study participants before inclusion in the study.
2.2. Coronary angiography
CAD was defined as more than 50% luminal stenosis identified in a minimum of two views, in the case of single-vessel involvement. Angiograms were scored by two experienced observers, unaware of the diagnosis. Gensini score assesses the severity of coronary artery disease: it grades narrowing of the lumen of the coronary artery and scores it as 1 for 1-25% narrowing, 2 for 26-50% narrowing, 4 for 51-75%, 8 for 76-90%, 16 for 91-99% and 32 for a completely occluded artery. This score is then multiplied by a factor, according to the importance of the coronary artery. The multiplication factor for a left main stem (LMS) lesion is 5, it is 2.5 for proximal left anterior descending artery (LAD) and proximal circumflex artery (CX) lesions, 1.5 for a mid-LAD lesion, and 1 for distal LAD, mid/distal CX and right coronary artery lesions. Gensini score was expressed as the sum of the scores for all three coronary arteries to evaluate the entire extent of coronary artery disease (23).
2.3. Anthropometric measurements
Were taken after the patients had removed their shoes and upper garments and had donned an examining gown. Each measurement was performed twice and the average was used in the analysis. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Weight was measured to the nearest 0.1 kg using a hospital balance beam scale. Waist height ratio (WHtR) is measured by division of weight circumference by their height, always expressed in same units. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). The WC was measured by tape in cm and was also categorized according to the World Health Organization (WHO) criteria (24). Blood pressure was measured by European Society of Hypertension-International Protocol 2 (ESH-IP2). With the WHR, the waist is measured at the narrowest part of the waist, between the lowest rib and iliac crest, and the hip circumference is taken at the widest area of the hips at the greatest protuberance of the buttocks. Then simply divide the waist measurement by the hip measurement. The WHO defines the ratios of >9.0 in men and >8.5 in women as one of the decisive benchmarks for metabolic syndrome.
2.4. Laboratory measurements
The 12-h fasting blood samples were taken in the morning and the sera were stored at -70 C immediately after centrifugation until being assayed. The laboratory was monitored for the precision and accuracy of glucose and lipid measurements by the surveillance program. Adiponectin serum concentrations were determined by a commercially available ELISA. The primary predictor variable was serum adiponectin level as determined by immunoassay of thawed fasting serum samples. Each sample was assayed in duplicate, and adiponectin level was calculated as the average of the two measurements. The laboratory technicians who performed the assays were blinded to the patient characteristics.
2.4. Statistical analysis
The data were entered and analyzed with SPSS Statistics, version 22. The continuous variables were primarily analyzed using a t-test, while the binary data were primary analyzed using a Chi-squared test. The continuous variables are presented in the tables with their means ± SD (Standard Deviation). Also for adiponectin relations with other variables were analyzed using a Pearson correlation. Additionally, single (unadjusted) and multiple (adjusted) binary logistic regressions were carried out with CAD group as the dependent variable, and the other significant variables as independent predictors. The cut p value of 0.1 was used as a criterion to identify the variables which need to be included in the Binary Logistic Regression Models. Adiponectin as the focusing variable of this study was included in the model regardless of its statistical significance. Other adjusting variables included are: diastolic blood pressure (DBP), WHR, LVEF, gender male, and smoking.
3. RESULTS
Of the eighty-two study patients, 41 patients comprised the CAD group (study subject) and 41 patients were classified as the non-CAD group (control subject). Study group (mean age 66.76+9.12) were on the average, 2 years older than the controls (mean age 64.80 ± 8.30).
The baseline characteristics of the two groups are depicted in Table 1. According to Table 1, in comparison with the controls, the CAD group patients were older and were more likely to be male and smokers. The number of male patients in the study group was 29 (70.7%) and in the control group was 17 (41.5%), which is significantly higher (p=0.008), also smokers were 27 (65.9%) vs 13 (31.7%) (p=0.002) significantly more numerous in the study group than the controls.
Table 1.
Demographic and clinical characteristics of non-CAD and CAD groups. BMI: basal metabolic index, WC, WHtR, WHR, systolic blood pressure (SBP), DBP.
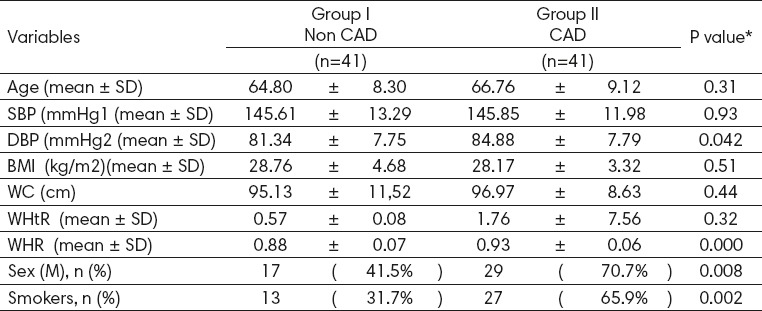
Fifteen patients (18.2%) had normal BMI (mean 23.22±1.4), 37 patients (45%) were overweight, 30 patients (43.4%) were obese. BMI in case group (mean 28.17± 3.32) and control (mean 28.76±4.68) and WC in case (96.97±8.63) vs control (95.13±11.52) they were no significant. WHtR in case (1.76 ±7.56) vs control (0.57±0.08), and WHR is significant between case and control (0.93±0.06) vs (0.88±0.07) (p-value: 0.0001) as seen in Table 1. A significant association of DBP and current status of smoking was found with CAD.
CAD patients had higher mean Gensini scores than non-CAD patients (53.16 ± 33.46 vs. 1.21 ±5.66, P<0.0001), but there was no statistically significant difference detected between CAD and non CAD for plasma adiponectin levels, presented in Table 2. Total cholesterol in the populations averaged (3.58 ± 1.07 mmol/l) in cases and (3.59 ± 1.19 mmol/l) in the control g
Table 2.
Biochemical characteristics of the two groups. CAD, AST:aspartate aminotransferase, CRP:C reactive protein, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol, LVEF.
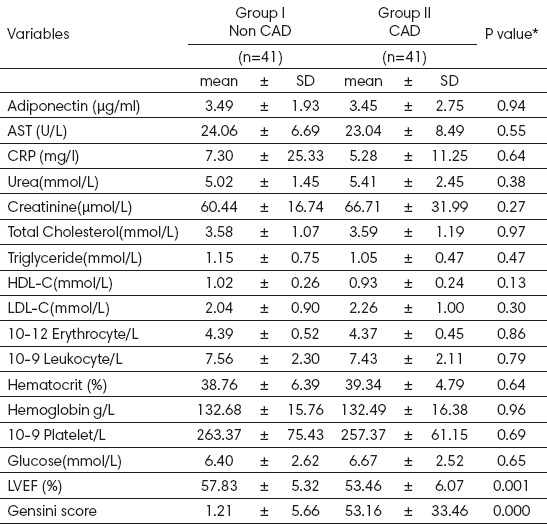
roups. Plasma HDL-cholesterol levels were (0.93 ± 0.24mmol/l) and (1.02±0.26mmol/l), respectively.
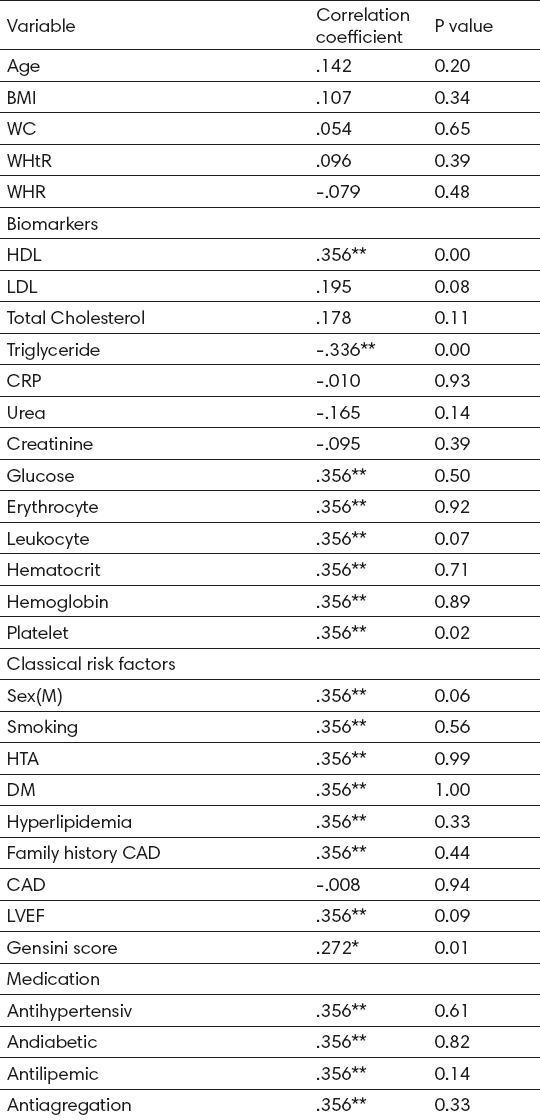
Baseline adiponectin concentrations correlated significantly in terms of the lipid parameters, positively with HDL cholesterol concentrations (r= 0.365, P=0.001) and serum triglyceride concentrations were correlated negatively (r=-0.336, P=0.001). Total cholesterol concentration and LDL cholesterol concentrations did not correlate significantly with plasma concentrations of adiponectin, shown in Table 3.
Table 3.
Correlations of adiponectin with other variables
Sixty-two patients (75%) had hypertension, 29 patients (35%) had diabetes, 23 patients (28%) dyslipidemia. Forty-two patients (51%) had family history of coronary heart disease and sudden non-accidental death or a combination of these.
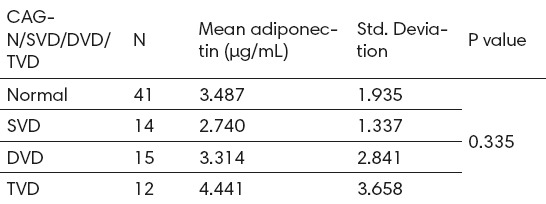
Of all patients 14.6% had triple-vessel disease, 18.3% had double-vessel disease and 17% single-vessel disease. Adiponectin levels show a non-significantly progressive decline as the angiographic severity of coronary artery stenosis increases (value=0.335) as shown in Table 4.
Table 4.
Angiographic severity of coronary artery stenosis and adiponectin levels. CAG:coronary angiography, SVD:single vessel disease, DVD:double vessel disease, TVD:triple vessel disease.
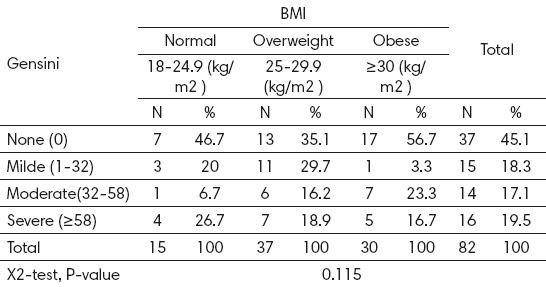
In control group 37 patients (45%) have (Gensini score =0), 15 patients (18%) had mild disease (Gensini score <32), 14 patients (17%) had moderate disease (Gensini score= 32-58), 16 patients (19.5%) had severe disease (Gensini score ≥58). BMI did not correlate with Gensini score by severity of coronary arteries, as seen in Table 5.
Table 5.
Distribution of Gensini in different BMI categories
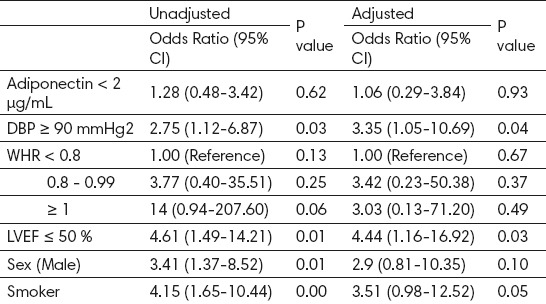
Significant differences were seen in Binary Logistic Regression models for CAD analysis for adiponectin, WHR, LVEF, DBP, male gender and for smoker. WHR (OR 14; 95% CI 0.94-207.60; p=0.06), LVEF (OR 4.61; 95% CI 1.49-14.21; p=0.01), DBP (OR 2.75; 95% 2.75 (1.12-6.87; p=0.03), smoking (OR 4.15; 95% CI 1.65-10.44; p=0.001), male patients (OR 3.41, 95% CI 1.37-8.52; p=0.01), all presented in Table 6.
Table 6.
Binary Logistic Regression Models for CAD (Unadjusted and Adjusted), DBP, WHR, LVEF.
4. DISCUSSION
The present study found that there was a strong association between adiponectin and coronary artery disease. These findings provide further evidence for an “adiponectin paradox” in which higher levels of adiponectin may be secreted as a protective or a compensatory response to worsening cardiovascular disease. In our study, we found medium significant positive correlation between Gensini and adiponectin (r= 0.272, P=0.01). This is in compliance with the SAPHIR study, which suggests that the role of adiponectin differs between early atherosclerosis and advanced stage vascular disease (25). Adiponectin protects against the development of disease, once the disease is established, adiponectin concentrations are elevated as a counter-regulatory response to protect against further inflammation and atherosclerosis (26). Findings of positive correlation between adiponectin and culprit lesion necrotic core (NC) content is consistent with the hypothesis that in advanced CAD, serum adiponectin levels are elevated in a counter-regulatory response (27). High serum adiponectin levels, instead of being protective, have been shown to be associated with increased mortality and adverse event rates. This paradoxical relation is further supported by study that included patients undergoing PCI for symptomatic CAD (28). Despite the potential association between heart failure and high plasma adiponectin shown by several clinical studies, other experimental studies have demonstrated a beneficial, protective effect of adiponectin on myocardium. Elevated plasma levels of adiponectin among patients with heart failure can be a reflection of accompanying renal dysfunction or “adiponectin resistance” including impaired adiponectin signal transduction in myocardium. Furthermore, several statins are also effective for elevating plasma adiponectin (29, 30).
There were no significant differences in plasma concentrations of adiponectin between patients with stable angina pectoris (SAP) and control patients (11.3 v 12.8 µg/ml) (31). Our study findings were similar to this study, where plasma concentrations of adiponectin between patients with SAP and control group (3.45 v 3.49 µg/ml) had no significant differences. In the present study, patients in our SAP group had already been receiving some treatment with antianginal and antiatherosclerotic drugs. As certain medical treatments may confound interpretation of plasma concentrations of adiponectin measurements and there may not be significant differences in plasma concentrations of adiponectin between patients with SAP and control patients.
Another important clinical finding showed that plasma concentrations of adiponectin were considerably increased in older and female patients with CAD (31). As a consequence of their findings they suggested that unstable plaque may stabilize as the patient grows older or that accumulation of adiponectin in atherosclerotic vascular walls may suppress its elimination half-life from plasma, resulting in an increase in plasma concentrations of adiponectin in older patients with CAD. Although a significant relation between serum adiponectin levels and age was not found in our study, it is known that age has an effect on serum adiponectin levels and that there is a positive relationship between age and serum adiponectin level (32). Including both individuals with and without cardiovascular disease, higher adiponectin has protecting role in those without cardiovascular disease and predictive of worse outcomes in those with existing disease (33). Moreover, it has been suggested that normal and even higher serum adiponectin levels may prevent the development of cardiovascular diseases and complications in healthy individuals (34). Male patients with hypoadiponectinemia (<4.0 µg/ml) had a two-fold increase in CAD prevalence, independent of well-known CAD risk factors (35). Similar to our study in male gender with hypoadiponectinemia (<2.0 µg/ml) increase the odds of having CAD (Table 6).
Our current study did not show a significant correlation between BMI and CAD (p-value 0.115), despite the presence of significant association between WHR and CAD (p-value= 0,0001). This may be explained by the fact that BMI quantifies general adiposity, although person who is overweight or obese is likely to have excess fat but BMI does not give an indication as to how this fat is distributed in the body. However, fat distribution is an important determinant of CAD independent of BMI and other classic risk factors of CAD (36). Welborn and Dhaliwal (37) and Srikanthan, Seeman, and Karlamangla (38) confirmed, and cited several other investigations that show WHR being the superior clinical measurement for predicting all cause and cardiovascular disease mortality. Welborn and Dhaliwal added that the hip circumference indicated a lower risk for body fat accumulation, and thus including it into the waist-to-hip equation enhances the accuracy of this measurement technique.
Relatively small sample of patients that were selected randomly from the general population, may have limited the ability to detect significant relationship between adiponectin and coronary artery disease, thus making it difficult to indicate the results to the general population.
5. CONCLUSION
There is a significant positive correlation between waist - hip ratio and severity of coronary artery disease. Also, there is a significant positive correlation between adiponectin and Gensini score among Kosovar patients.
Acknowledgments
The authors would like to thank Alban Fejza for statistical analysis.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Trujillo ME, Scherer PE. Adiponectin-journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–75. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 2.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 3.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decreased of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome Arterioscler. Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 5.Valsamakis G, Chetty R, McTernan PG, Al-Daghri NM, Barnett AH, Kumar S. Fasting serum adiponectin concentration is reduced in Indo-Asian subjects and is related to HDL cholesterol. Diabetes Obes Metab. 2003;5:131–5. doi: 10.1046/j.1463-1326.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow –up in elderly men. J Clin Endocrinol Metab. 2007;92:571–6. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 7.Masic I, Dilic M, Raljevic E, Vulic D, Mott D. Trends in cardiovascular disease in Bosnia and Herzegovina and perspectives with HeartScore Programme. Med Arh. 2010;64(5):260–3. doi: 10.5455/medarh.2010.64.260-263. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Pessina AC, Zanchetta M, De Toni R, Avogaro A, Pedon L, et al. Low plasma adiponectin is associated with coronary artery disease but not with hypertension in high-risk nondiabetic patients. J Intern Med. 2006;260:474–83. doi: 10.1111/j.1365-2796.2006.01714.x. [DOI] [PubMed] [Google Scholar]
- 9.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerk A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 10.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari T, et al. Circulating adiponectin concentration in patients with congestive heart failure. Heart. 2006;92:1420–4. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, et al. Adiponectin and mortality in patients with chronic kidney disease. Jam Soc Nephrol. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 12.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulation adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–7. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 13.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, et al. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–9. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 15.Rosengren A, Wedel H, Wilhelmsen L. Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality. A prospective population study. Eur Heart J. 1999;20:269–77. [PubMed] [Google Scholar]
- 16.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–27. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 17.Prineas RJ, Folsom AR, Kaye SA. Central adiposity and increased risk of coronary artery disease mortality in older women. Ann Epidemiol. 1993;3:35–41. doi: 10.1016/1047-2797(93)90007-q. [DOI] [PubMed] [Google Scholar]
- 18.Folsom AR, Kaye SA, Sellers TA, Hong CP, Cerhan JR, Potter JD, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269:483–7. [PubMed] [Google Scholar]
- 19.Ferland M, Despres JP, Tremblay A, Pinault S, Nadeau A, Moorjani S, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–48. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 20.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–9. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 21.Despres JP, Prud'homme D, Pouliot MC, Tremblay A, Bouchard C. Estimation of deep abdominal adipose - tissue accumulation from simple anthropometric measurements in men. Am J Clin Nutr. 1991;54:471–7. doi: 10.1093/ajcn/54.3.471. [DOI] [PubMed] [Google Scholar]
- 22.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 23.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation. Geneva, 8-11 December 2008. Geneva, Switzerland: WHO Press, World Health Organization; 2011. [Google Scholar]
- 25.Iglseder B, Mackevics V, Stadlmayer A, et al. Plasma adiponectin levels and sonographic phenotypes of subclinical carotid artery atherosclerosis: data from the SAPHIR study. Stroke. 2005;36:2577–82. doi: 10.1161/01.STR.0000190834.00284.fd. [DOI] [PubMed] [Google Scholar]
- 26.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–9. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 27.Steffes MW, Gross MD, Lee DH, Schreiner PJ, Jacobs DR Jr. Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: the Coronary Artery Risk Development in Young Adults Study. Obesity (Silver Spring) 2006;14:319–26. doi: 10.1038/oby.2006.41. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Hau WK, Tai BC, et al. Adiponectin profile in Asian patients undergoing coronary revascularization and its association with plaque vulnerability: IDEAS-ADIPO study. Obesity. 2012;20:2451–7. doi: 10.1038/oby.2012.92. [DOI] [PubMed] [Google Scholar]
- 29.Takagi T, Matsuda M, Abe M, et al. Effect of pravastatin on the development of diabetes and adiponectin production. Atherosclerosis. 2008;196(1):114–21. doi: 10.1016/j.atherosclerosis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Inami N, Nomura S, Shouzu A, et al. Effects of pitavastatin on adiponectin in patients with hyperlipidemia. Pathophysiology of Haemostasis and Thrombosis. 2008;36(1):1–8. doi: 10.1159/000112633. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, et al. Implication of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004 May;90(50):528–33. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel JV, Abraheem A, Dotsenko O, Creamer J, Gunning M, Hughes EA, et al. Circulating serum adiponectin levels in patients with coronary artery disease: relationship to atherosclerotic burden and cardiac function. J Intern Med. 2008;264:593–8. doi: 10.1111/j.1365-2796.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 33.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489–96. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 34.Giannessi D, Maltinti M, Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol Res. 2007;56:459–67. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 36.Després JP. CVD risk assessment: Do we need the metabolic syndrome or better global cardiometabolic risk calculators? Int J Obes. 2008;32(Suppl. 2):S1–4. doi: 10.1038/ijo.2008.27. [DOI] [PubMed] [Google Scholar]
- 37.Welborn TA, Dhaliwal SS. Preferred clinical measures of central obesity for predicting mortality. European Journal of Clinical Nutrition. 2007;61:1373–9. doi: 10.1038/sj.ejcn.1602656. [DOI] [PubMed] [Google Scholar]
- 38.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high functioning older adults. Annals of Epidemiology. 2009;19:724–31. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


