Abstract
Object:
The incidence of cerebrospinal fluid (CSF)-related complications after intradural spinal tumor (IST) surgery is high and reported in up to 18% of patients. However, no efficient way to prevent those complications has been reported so far. Treating these complications may require prolonged bed rest, re-exploration, external lumbar drain, use of antibiotics, and possible precipitation of other complications. To alleviate the risk of CSF-related complications, we prospectively adopted the intraoperative use of autologous fat grafting after IST surgery.
Methods:
This is a perspective analysis of 37 cases (out of 40 cases series) that a prospective use of abdominal fat autograft was applied during dural closure. After the tumor was resected and the dura closed, we used the Valsalva maneuver to ensure watertight closure. CSF leak was prevented with the enforcement of suture with a fat autograft as necessary. In addition a thin layer of fat tissue was then placed over the dura to obliterate any dead space. Fibrin glue was then applied over the graft. Filling the dead space with the fat graft prevented a low-pressure space in which CSF could pool and form a pseudomeningocele.
Results:
After adopting the fat autograft technique, we did not observe any post-surgery CSF-related complications in any of these patients.
Conclusions:
The prospective use of autologous fat grafting can ensure watertight dural closure and obliterate the dead space created during surgical exposure and bone removal. This technique significantly reduces, and may completely eliminate, postoperative CSF-related complications in patients with ISTs.
Keywords: spinal cord tumor, intradural extramedullary spinal tumor, intradural intramedullary spinal tumor, CSF leak, pseudomeningocele, postoperative complications
1. INTRODUCTION
The incidence of cerebrospinal fluid (CSF)-related complications (i.e., CSF leak, pseudomeningocele, and meningitis) after intradural spinal tumor surgery (IST) remains high following resection of intradural extramedullary spinal tumors (IEST) and intradural intramedullary spinal tumors (IIST) (1-17). Treating these complications is a vexing problem and often requiring prolonged postoperative bed rest, operative re-exploration, the placement of an external lumbar drain, and a prolonged use of antibiotics (18-30). Furthermore, these complications may lead to additional complications, and even the death of the patient.
Despite Mayfield’s (18) and Black’s (5) recommendations many years ago to use a fat graft to prevent potentially life-threatening CSF complications in patients undergoing posterior fossa and spinal surgery, the practice did not gain routine use for those undergoing surgery for ISTs. We were not able to find any reports of the use of intraoperative fat grafting in the IST literature or reports of any other efficient way that ensures preventing this vexing postoperative complication problem while CSF-related complications of surgical IST series have been regularly reported in up to 18% of patients (1, 2, 4, 6, 7, 9, 11-17, 19-21, 25, 28-30).
In our earlier practice, CSF-related complications were similar to those reported by other authors (1-3, 6, 7, 9, 11-14, 17-19, 22, 23, 25, 26). As a consequence, we have prospectively adopted the intraoperative use of autologous fat grafting. This report describes our technique and experience among patients in our IST series.
2. METHODS
This study was approved by the appropriate institutional review board and was a retrospective analysis of all patients treated operatively by the senior author for ISTs over 13-year period (September 2003 through September 2016). Medical records were reviewed for pre and postoperative exams, pre and postoperative magnetic resonance imaging (MRI), and follow-up visits. In June 2005 was noted because this was when a patient with a sacral dumbbell schwannoma developed a large pseudomeningocele postoperatively, prompted us for the prospective application of autograft fat during dural closure for all other patients. Any evidence of a CSF leak or pseudomeningocele was evaluated prospectively. The fat-tissue dynamics on postoperative MRIs were also assessed. The patients’ demographics, the tumors’ histology, the degree of tumor resection, and neurological outcomes were evaluated.
All patients underwent postoperative MRI scans with and without contrast in the hospital 2 months after surgery, yearly for 2 years, and then every 5 years during follow-up.
Fat Harvest and Application Technique
After endotracheal intubation and line placement in the supine patient, the left paraumbilical area is prepared and draped (Figure 1). Fat was harvested though a 2 cm paraumbilical incision (Figure 1). The harvested fat is placed in antibiotic saline in a sterile cup until needed for closure.
Figure 1.
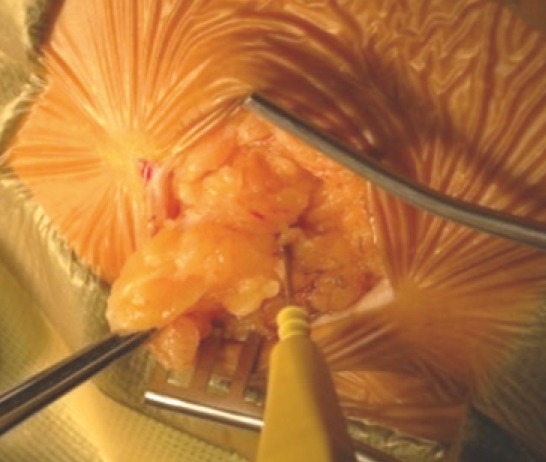
Left abdominal fat graft harvest-harvesting fat with monopolar cautery.
All patients had neurophysiologic monitoring throughout the operation. Once the tumor was resected, the dura was closed with 5-0 Prolen stitches in a running fashion. If the incision was in the midline, the Valsalva maneuver was done at 30 cm H20 for 5–10 seconds to ensure that it was watertight. If any area in the suture line leaked CSF, an additional suture was placed there and a piece of fat tissue was cut and positioned inside the stitch, which was then tightened. This maneuver reinforced the dural closure. The same Valsalva maneuver was repeated until there were no more leaks.
If the dural incision was T-shaped (i.e., for dumbbell, intra/extradural, foraminal tumors), or Y-shaped (i.e., for sacral canal/foraminal tumors), the dural incision in the midline and its T or Y extension over the nerve root were closed in running fashion with 5-0 Prolen. Multiple pieces of fat tissue were then incorporated into single additional dural sutures to achieve watertight closure and reinforce the dural suture.
When suturing was complete, a layer of fat tissue 6–8 mm thick was placed over the entire exposed dura to obliterate the dead space that remained after a laminectomy or facetectomy. The fat tissue allograft nicely conformed to the allotted space and serves as filler. The fat graft also created small pressure onto the dural suture line, lessening the chance of CSF seepage and pseudomenigocele. In other words, the graft prevented a potential low-pressure space into which CSF may migrate and form a pseudomeningocele, later producing a CSF leak. Fibrin glue was then applied over the graft. The muscle, fascia, subcutaneous tissue, and skin layers are then closed in the usual fashion (Figures 2–4).
Figure 2.
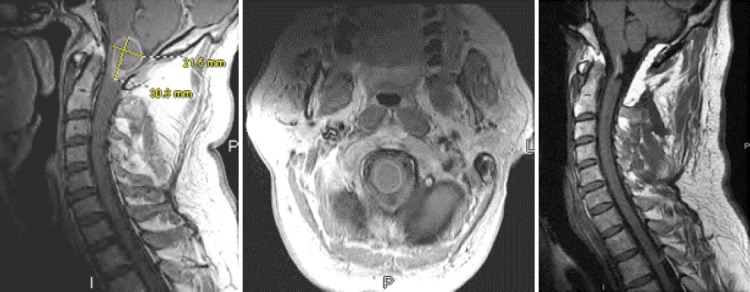
A man in his late 40s with an IIST (ependymoma) at C1. He presented with gait and balance problems and 4+/5 quadriparesis. A) Sagittal post-contrast T1 weighted MRI showing a peripherally enhancing tumor. B) Axial post-contrast T1-weighted MRI showing the partially enhancing tumor. C) Sagittal pre-contast T1-weighted MRI depicting the radical tumor resection. Note the fat graft overlying the dura dorsally and enforcing the patch graft/dural suture line. The patient regained his full strength postoperatively and his balance problems resolved.
Figure 3.
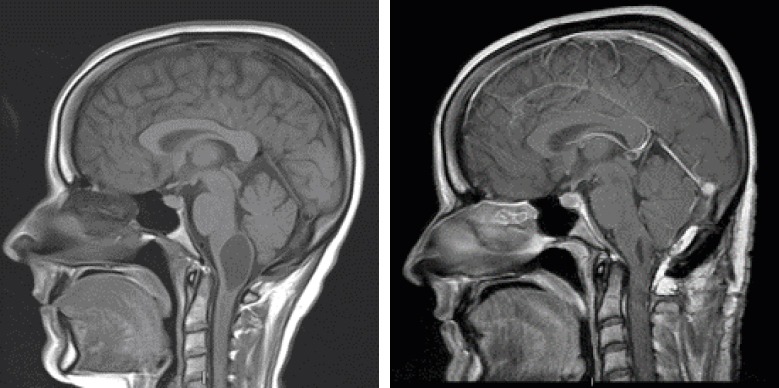
A woman in her early 30s with an IIST (hemangioblastoma) at C1. She presented with gait instability, swallowing problems, and a nasal voice. A) Sagittal post-contrast MRI showing a proximal intramedullary cyst. B) Postoperative post-contrast T1-weighted MRI showing the tumor resection and collapse of the cyst. Note the fat graft overlying the dura dorsally. The patient’s balance and swallowing problems resolved postoperatively.
Figure 4.
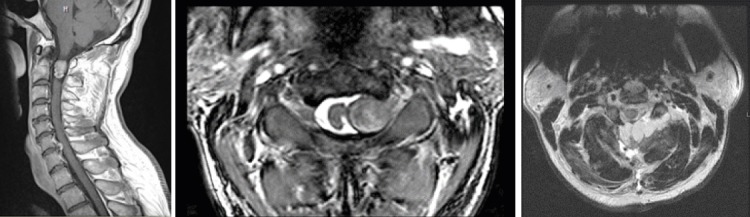
A man in his late 40s with a schwannoma at C1–C2. A) Post-contrast sagittal T1-weighted MRI showing the enhancing tumor. B) Axial T2-weighted MRI showing the left-sided tumor, which involved the C1–C2 intervertebral foramen and compressed the spinal cord. C) Postoperative T2-weighted MRI showing the radical tumor resection. Note the fat graft overlying the dura dorsally and reinforcing the dural closure.
3. RESULTS
Over the course of 13 years (September 2003–September 2016), 40 patients with an IST were operated on by the senior author. Of these, 34 were IEST, comprising 13 meningiomas, 14 schwannomas, 6 myxopapillary ependymomas, and 1 breast cancer metastasis. Six patients had IISTs: 3 astrocytomas (one high grade), 2 ependymoma, and 1 hemangioblastoma.
There were 11 men (28%) and 29 women (72%) with ages ranging from 24–89 years (mean 56 years). Thirteen patients (33%), all with IEST, were older than 65 years. The length of hospital stay ranged from 2 to 8 days (mean, 4 days), and follow-up ranged from 1 to 36 months (mean, 45 months). Details of the series are outlined in Table 1.
Table 1.
IST Series. Abbreviations: IEST=intradural extramedullary spinal tumors; IIST=intradural intramedullary spinal tumors; IST=intradural spinal tumors
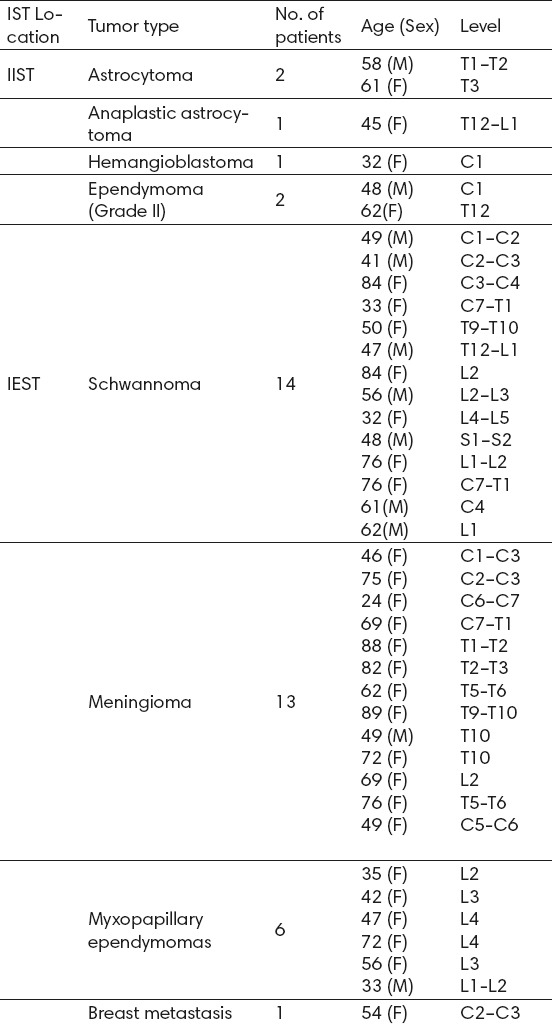
Except for 5 patients with schwannomas who presented with pain and numbness in the involved nerve areas, all other patients presented with motor, sensory, sphincter, and neurological deficits in various combinations. Six patients (15%) had dumbbell tumors that extended into the corresponding intervertebral neural foramen; 5 of these had schwannomas and 1 had a meningioma.
All but one patient was alive and neurologically intact during follow-up. One patient, a woman in her mid 40s with a grade III conus/cauda astrocytoma, died 3.5 years later as a result of disease progression and CSF seeding throughout her neuraxis despite postoperative irradiation and chemotherapy. No evidence of tumor recurrence during follow up was noted in any other patient.
The third patient in the series, a man in his late 40s with perineal numbness and tingling, impotence, and severe low back pain harbored a dumbbell schwannoma at S1–S2 and underwent resection. Ten days after surgery, he presented with a large subcutaneous swelling in the sacral area, consistent with a pseudomeningocele, which was verified with computed tomography. He underwent a second surgery, during which the dural closure was revised with a fat autograft along with external lumbar drainage for 5 days bed rest. All patients after this patient received a fat autograft at the time of dural closure, and no evidence of a CSF leak or a pseudomeningocele was noted on physical exam or neuroradiologic follow-up in these other patients.
No evidence of complications related to the fat harvest site was noted. On the second postoperative MRI, 2 months after surgery, the fat tissue routinely showed signs of significant resorption, and the 1-year follow-up MRI scans in all patients showed that the fat was totally reabsorbed.
4. DISCUSSION
Efficacy of the technique
In their classic work, Yasargil and colleagues (34) reported the successful radical removal of ISTs using microsurgical techniques with favorable neurological outcomes. Since then, numerous other reports of series of ISTs with good results followed (1-9, 11-31, 35). Nonetheless, the incidence of CSF-related complications (i.e., CSF leak, pseudomeningocele, and meningitis) after IST tumor surgery remains high and in reports from major high volume centers ranges between 5% up to 18% (1-4, 6, 7, 9, 11, 13-17, 19-22, 25, 28-30). Treating these complications may often require prolonged postoperative bed rest, external lumbar drainage, operative re-exploration, and the prolonged use of antibiotics. These complications may frequently lead to additional complications, such as deep vein thrombosis, pulmonary embolism, pneumonia, urinary tract infection, skin breakdown, subdural or cerebellar hematomas, and even death. They also significantly increase medical expenses and the length of the hospital stay, which in turn may worsen the outcome scores of both the hospital and individual surgeons, as well as patient satisfaction surveys. Weber et al. (32) reported a 50% increase in mean hospital cost per case with CSF-related postoperative complications after elective spinal surgery.
The effect of CSF-related complications on outcomes is particularly true as patients undergoing this surgery may be older (> 65 years) and have medical co-morbidities, as we found in one-third of the patients in our series. A higher number of elderly patients with ISTs who require surgical treatment has also been reported by Sacko and colleagues (25). While such patients can have a successful surgery with radical tumor resection and frequently no neurological consequences, they may not tolerate the prolonged bed rest required to repair the CSF-related postoperative complication. The additional complications mentioned earlier can also jeopardize an otherwise successful surgery and rehabilitation process.
Two recent studies (10, 33) evaluated the efficacy of polyethylene glycol sealants in an attempt to decrease the rate of CSF-related complications after intradural spinal surgery. Goodwin and colleagues (10) reported that CSF leaks occurred in 5% of patients and meningitis occurred in 1%. Similarly, Wright and associates (33) compared the use of polyethylene glycol sealant to the standard of care dural closure. CSF-related complications that required re-operation in the sealant treated group was 7% compared with 13% in the standard dural closure cohort. In addition, each cohort had additional 4% rate of pseudomeningocele formation that did not require re-operation.
Thirty-five and 15 years ago, respectively, both Mayfield (18) and Black (5) described and recommended the use of a fat graft to prevent potentially life-threatening CSF complications after posterior fossa and spinal surgery. However, we were not able to find any reports of the use of intraoperative fat grafting in the literature or reports of any other efficient way that ensures preventing this vexing postoperative complication problem.
Our own experience in the surgical series of ISTs treated at our institution mirrored the experience of others. As a consequence, we prospectively adopted the intraoperative use of fat grafting after the third patient in our series developed a large pseudomeningocele, was readmitted to the hospital, and underwent a second surgery with revision of the dural closure and placement of an autologous fat graft and external lumbar drainage for 5 days. Since then, we have operated on an additional 37 patients with ISTs prospectively using this graft at dural closure and have not seen any CSF-related complications in these patients.
Technical considerations
The spinal dura appears to be thickest dorsally in the midline and thins laterally, particularly along the dural sleeves of the spinal nerves. We use the running dural midline suture to achieve watertight closure after the tumor is removed and then use the Valsalva maneuver to ensure watertight closure. Still, there is no guarantee that the suture line won’t weaken or that a leak won’t occur after the patient is mobilized postoperatively, when CSF fully replenishes and patient engages in full, physiologic CSF pressure challenges of the dural suture line. CSF may still seep into the low pressure dead space created after a laminectomy, leading to a pseudomeningocele or CSF leak. We found that reinforcing the dural suture line with autologous fat is useful particularly in cases when the Valsalva maneuver revealed CSF seepage. In addition, the fat graft obliterates the dead space created by a laminectomy and muscle dissection, and creates gentle pressure to the dural suture line that may prevent the formation of a pseudomeningocele and a CSF leak.
In some cases, it may be impossible to achieve watertight dural closure, and these cases are particularly at risk for CSF leak and therefore clearly benefit from this concept and technique: 1) an IST in a sacral location (one of our cases); 2) a craniospinal IST requiring a Y-shaped dural incision and patch grafting (Figures 2 and 3, 27% of cases in our series); 3) when the tumor invades the dura (e.g., meningioma) and necessitates dural excision to achieve radical resection and subsequent dural patching; and 4) if the tumor is a dumbbell IEST extending into the intervertebral foramen and beyond (Figures 4 and 5). Eighteen percent of patients in our series had dumbbell tumors (5 schwannomas and 1 meningioma) and after radical tumor resection, it was not possible to achieve a watertight dural closure without incorporating a fat graft into the sutures.
Fat tissue was harvested at the beginning of surgery via a horizontal incision of approximately 2 cm while the patient was supine. It was routinely done on the left side to prevent confusion in the case of a future appendectomy or other abdominal surgery on the right. Fat deposits are abundant in the abdominal area even in patients with a low BMI. In our experience, the tissue harvest did not add any significant time or expense to surgery, nor did it lead to infection, hematoma, or cosmetic problems for our patients. In addition, fat autograft carries neither the risk of hypersensitivity reaction nor risk of infectious disease transmission.
Finally, we favored a separate incision for the tissue removal, rather than harvesting fat from the subcutaneous tissue in the area of the primary incision. This approach prevents the development of additional tissue pouches in the primary surgical area that may favor a pseudomeningocele or hematoma formation and jeopardize wound healing.
5. CONCLUSIONS
The reported incidence of CSF related complications after surgery for IST remains high in published series. The prospective use of autologous fat grafting ensures watertight dural closure and obliterates the dead space created during surgical exposure, muscle dissection, and bone removal. This technique appears to significantly reduce, if not completely eliminate, postoperative CSF-related complications in patients with ISTs, without adding any significant operative time, expense or complications.
Acknowledgments
The author thanks Ms. Julie Yamamoto for her editorial assistance and Mr. Andrew J. Gienapp (Department of Medical Education, Methodist University Hospital, Memphis, TN and Department of Neurosurgery, University of Tennessee Health Science Center, Memphis, TN) for technical and copy editing, preparation of the manuscript and figures for publishing, and publication assistance with this manuscript.
Footnotes
• Abbreviations: CSF=cerebrospinal fluid; IEST=intradural extramedullary spinal tumors; IIST=intradural intramedullary spinal tumors; IST=intradural spinal tumor surgery; MRI=magnetic resonance imaging
• Disclosures: No financial or material support was accepted as part of this study.
• Financial relationships: The author reports no financial relationships or conflicts of interest concerning the materials or methods used in this study or the findings specified in the paper.
REFERENCES
- 1.Ahn DK, Park HS, Choi DJ, Kim KS, Kim TW, Park SY. The surgical treatment for spinal intradural extramedullary tumors. Clinics in orthopedic surgery. 2009;1:165–72. doi: 10.4055/cios.2009.1.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese V, Platania N. Spinal intradural extramedullary tumors. Personal experience. Journal of neurosurgical sciences. 2002;46:18–24. [PubMed] [Google Scholar]
- 3.Arnautovic A, Arnautovic K. Extramedullary intradural spinal tumors. Contemp Neurosurg. 2014;36:1–8. [Google Scholar]
- 4.Arnautovic K, Arnautovic A. Extramedullary intradural spinal tumors: a review of modern diagnostic and treatment options and a report of a series. Bosnian Journal of Basic Medical Sciences. 2009;9(Suppl 1):40–5. doi: 10.17305/bjbms.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black P. Cerebrospinal fluid leaks following spinal or posterior fossa surgery: use of fat grafts for prevention and repair. Neurosurgical focus. 2000;9:e4. doi: 10.3171/foc.2000.9.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury FH, Haque MR, Sarker MH. High cervical spinal schwannoma;microneurosurgical management: an experience of 15 cases. Acta neurologica Taiwanica. 2013;22:59–66. [PubMed] [Google Scholar]
- 7.el-Mahdy W, Kane PJ, Powell MP, Crockard HA. Spinal intradural tumours: Part I - Extramedullary. British journal of neurosurgery. 1999;13:550–7. doi: 10.1080/02688699943042. [DOI] [PubMed] [Google Scholar]
- 8.Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. Journal of neurosurgery. 1993;79:204–9. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 9.Ferroli P, Acerbi F, Broggi M, Schiariti M, Albanese E, Tringali G, Franzini A, Broggi G. A novel impermeable adhesive membrane to reinforce dural closure: a preliminary retrospective study on 119 consecutive high-risk patients. World neurosurgery. 2013;79:551–7. doi: 10.1016/j.wneu.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin CR, Recinos PF, Zhou X, Yang JX, Jallo GI. Evaluation of complication rates of pediatric spinal procedures in which a polyethylene glycol sealant was used. Journal of neurosurgery Pediatrics. 2014;13:315–8. doi: 10.3171/2013.12.PEDS13456. [DOI] [PubMed] [Google Scholar]
- 11.Hanbali F, Fourney DR, Marmor E, Suki D, Rhines LD, Weinberg JS, McCutcheon IE, Suk I, Gokaslan ZL. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2. 0012;51:1162–72. doi: 10.1097/00006123-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Jeon JH, Hwang HS, Jeong JH, Park SH, Moon JG, Kim CH. Spinal schwannoma;analysis of 40 cases. Journal of Korean Neurosurgical Society. 2008;43:135–8. doi: 10.3340/jkns.2008.43.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, Chung CK. Surgical outcome of a posterior approach for large ventral intradural extramedullary spinal cord tumors. Spine. 2011;36:E531–7. doi: 10.1097/BRS.0b013e3181dc8426. [DOI] [PubMed] [Google Scholar]
- 14.Klekamp J, Samii M. Surgery of spinal nerve sheath tumors with special reference to neurofibromatosis. Neurosurgery. 1998;42:279–90. doi: 10.1097/00006123-199802000-00042. [DOI] [PubMed] [Google Scholar]
- 15.Klekamp J, Samii M. Surgical results for spinal meningiomas. Surgical neurology. 1999;52:552–62. doi: 10.1016/s0090-3019(99)00153-6. [DOI] [PubMed] [Google Scholar]
- 16.Lot G, George B. Cervical neuromas with extradural components: surgical management in a series of 57 patients. Neurosurgery. 1997;41:813–20. doi: 10.1097/00006123-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. Journal of neurosurgery Spine. 2'11; 14:758–64. doi: 10.3171/2011.1.SPINE09860. [DOI] [PubMed] [Google Scholar]
- 18.Mayfield FH. Autologous fat transplants for the protection and repair of the spinal dura. Clinical neurosurgery. 1980;27:349–61. doi: 10.1093/neurosurgery/27.cn_suppl_1.349. [DOI] [PubMed] [Google Scholar]
- 19.McCormick PC. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery. 1996;38:294–300. doi: 10.1097/00006123-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Mehta AI, Adogwa O, Karikari IO, Thompson P, Verla T, Null UT, Friedman AH, Cheng JS, Bagley CA, Isaacs RE. Anatomical location dictating major surgical complications for intradural extramedullary spinal tumors: a 10-year single-institutional experience. Journal of neurosurgery Spine. 2013;19:701–7. doi: 10.3171/2013.9.SPINE12913. [DOI] [PubMed] [Google Scholar]
- 21.Nambiar M, Kavar B. Clinical presentation and outcome of patients with intradural spinal cord tumours. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2012;19:262–6. doi: 10.1016/j.jocn.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Nanda A, Kukreja S, Ambekar S, Bollam P, Sin AH. Surgical Strategies in the Management of Spinal Nerve Sheath Tumors. World neurosurgery. 2015;83:886–99. doi: 10.1016/j.wneu.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C. Spinal dumbbell tumors: an analysis of a series of 118 cases. Journal of neurosurgery Spine. 2007;7:587–93. doi: 10.3171/SPI-07/12/587. [DOI] [PubMed] [Google Scholar]
- 24.Parsa AT, Lee J, Parney IF, Weinstein P, McCormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. Journal of neuro-oncology. 2004;69:291–318. doi: 10.1023/b:neon.0000041889.71136.62. [DOI] [PubMed] [Google Scholar]
- 25.Sacko O, Haegelen C, Mendes V, Brenner A, Sesay M, Brauge D, Lagarrigue J, Loiseau H, Roux FE. Spinal meningioma surgery in elderly patients with paraplegia or severe paraparesis: a multicenter study. Neurosurgery. 2009;64:503–510. doi: 10.1227/01.NEU.0000338427.44471.1D. [DOI] [PubMed] [Google Scholar]
- 26.Safavi-Abbasi S, Senoglu M, Theodore N, Workman RK, Gharabaghi A, Feiz-Erfan I, Spetzler RF, Sonntag VK. Microsurgical management of spinal schwannomas: evaluation of 128 cases. Journal of neurosurgery Spine. 2008;9:40–7. doi: 10.3171/SPI/2008/9/7/040. [DOI] [PubMed] [Google Scholar]
- 27.Shirzadi A, Drazin D, Gates M, Shirzadi N, Bannykh S, Fan X, Hunt L, Baron EM, King WA, Kim TT, Johnson JP. Surgical management of primary spinal hemangiopericytomas: an institutional case series and review of the literature. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013;22(Suppl 3):S450–9. doi: 10.1007/s00586-012-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C, Pluchino F. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25:153–60. [PubMed] [Google Scholar]
- 29.Song KW, Shin SI, Lee JY, Kim GL, Hyun YS, Park DY. Surgical results of intradural extramedullary tumors. Clinics in orthopedic surgery. 2009;1:74–80. doi: 10.4055/cios.2009.1.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantino R, Donnarumma P, Nigro L, Rullo M, Santoro A, Delfini R. Surgery of intradural extramedullary tumors: retrospective analysis of 107 cases. Neurosurgery. 2014;75:509–14. doi: 10.1227/NEU.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 31.Traul DE, Shaffrey ME, Schiff D, Part I. spinal-cord neoplasms-intradural neoplasms. The Lancet Oncology. 2007;8:35–45. doi: 10.1016/S1470-2045(06)71009-9. [DOI] [PubMed] [Google Scholar]
- 32.Weber C, Piek J, Gunawan D. Health care costs of incidental durotomies and postoperative cerebrospinal fluid leaks after elective spinal surgery. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014 doi: 10.1007/s00586-014-3504-7. [DOI] [PubMed] [Google Scholar]
- 33.Wright NM, Park J, Tew JM, Kim KD, Shaffrey ME, Cheng J, Choudhri H, Krishnaney AA, Graham RS, Mendel E, Simmons N. Spinal sealant system provides better intraoperative watertight closure than standard of care during spinal surgery: a prospective, multicenter, randomized controlled study. Spine. 2015;40:505–13. doi: 10.1097/BRS.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 34.Yasargil MG, Tranmer BI, Adamson TE, Roth P. Unilateral partial hemi-laminectomy for the removal of extra- and intramedullary tumours and AVMs. Advances and technical standards in neurosurgery. 1991;18:113–32. doi: 10.1007/978-3-7091-6697-0_3. [DOI] [PubMed] [Google Scholar]
- 35.Zadnik PL, Gokaslan ZL, Burger PC, Bettegowda C. Spinal cord tumours: advances in genetics and their implications for treatment. Nature reviews Neurology. 2013;9:257–66. doi: 10.1038/nrneurol.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]


