Abstract
Background and Objectives
Breast cancer treatment can cause premature ovarian failure, yet the majority of young cancer patients do not receive adequate education about treatment effects before initiating chemotherapy. We studied the impact of an oncofertility program on access to fertility preservation.
Methods
An oncofertility program was initiated to foster collaboration between oncologists and reproductive endocrinologists, and to help increase access to fertility preservation. Documented conversations about fertility concerns, specialist referrals, appointments, and fertility preservation procedures were compared between breast cancer patients from 2004 to 2006, before oncofertility program initiation, and 2007–2012, after program initiation. The study included women <45, stages 0–III, diagnosed before (n =278) and after (n =515) program initiation.
Results
Demographics for the cohorts were similar. Fertility discussions (P <0.0001), patients interested in maintaining fertility at diagnosis (P =0.0041), referrals to reproductive endocrinologists (P <0.0001), appointments (P <0.0001), and fertility preservation procedures (P <0.0183) increased significantly after programmatic implementation.
Conclusions
An oncofertility program increased discussions about fertility preservation and access to assisted reproductive procedures. This program positively impacted compliance with national guidelines advising reproductive-age cancer patients to be offered fertility preservation counseling as an initial component of the multidisciplinary care plan.
Keywords: oncofertility, breast cancer, reproductive endocrinology, fertility preservation
INTRODUCTION
Among the numerous concerns that a cancer diagnosis imposes on newly diagnosed young patients, the risk of infertility is a critical issue [1,2]. Both the patient’s primary malignancy and cancer treatment, including surgery, chemotherapy, and radiation, can impact fertility temporarily or permanently [3–5]. For young patients seeking fertility preservation, referral to a fertility specialist should occur early in the multidisciplinary care plan [6]. Clinician-driven discussions should be facilitated by providers who are knowledgeable about treatment-related fertility risks and fertility preservation options before potentially gonadotoxic therapies have been initiated. Accordingly, guidelines established by the American Society of Clinical Oncology (ASCO), American Society of Reproductive Medicine (ASRM), and National Comprehensive Cancer Network (NCCN) support the pre-treatment counsel of cancer patients about fertility preservation and referral to reproductive specialists when indicated [7–9].
Despite these guidelines, data show that 30–50% of patients may not receive adequate information about fertility risks and preservation practices before initiating cancer treatment [10,11]. Female patients are at particular disadvantage with regard to receiving information or referrals to fertility specialists, with one survey showing pediatric oncologists routinely referring only 23% of female patients for fertility counseling, as opposed to 66% of male patients [12]. The barriers to these conversations and referrals are multifactorial: limited time for clinical encounters, focus on treatment planning concerns, reluctance to discuss sensitive subjects such as sexuality and fertility, inadequate familiarity with fertility preservation techniques, or a lack of insight or education associated with initiating conversations about fertility [13].
To this end, communication regarding fertility preservation options and appropriate referral to a fertility specialist are both critical to quality-of-life during survivorship, and may also impact disease outcomes. Affected patients have indicated that the feeling of distress associated with the threat to fertility posed by cancer treatment was on par with the distress associated with receiving a cancer diagnosis [14,15]. Furthermore, fertility concerns were found to negatively impact treatment decision-making, with some breast cancer patients opting to alter recommended treatment regimens in attempt to spare fertility [15,16]. Lastly, patients who did not pursue fertility preservation options, and subsequently became infertile after cancer treatment, have expressed regret associated with increased anxiety and depression during survivorship [17,18].
The scope of fertility preservation for cancer patients is broad, and it can be challenging for practitioners to facilitate discussions about fertility amid other critical and life-altering topics. A formal oncofertility program can serve multiple functions to help implement this clinical practice. With the assistance of dedicated oncofertility care coordinators, a standardized narrative for providers can be instituted to initiate the discussion about fertility with patients as part of a pre-treatment dialogue, while also building a contextual framework for the available fertility preservation resources, and a referral network for providers who can offer fertility preservation practices [19,20]. In 2007, Northwestern University implemented an oncofertility program encouraging oncologists to address treatment-related infertility, discuss fertility preservation options, and refer to reproductive specialists prior to starting potentially gonadotoxic cancer treatments. In the current study, we assessed the impact of this program on access to fertility preservation by comparing two cohorts of breast cancer patients diagnosed before and after program implementation. We hypothesized that the oncofertility program would help to increase provider-driven discussions about fertility, referrals to fertility specialists, and use of fertility preservation procedures.
MATERIALS AND METHODS
Patient Selection
After institutional review board (IRB) approval, we identified female patients age ≤45 years treated at Northwestern Memorial Hospital’s Lynn Sage Comprehensive Breast Center (Chicago, IL) who had been diagnosed with stages 0–III, ER+ and/or PR+ breast cancer between January 1, 2004 and May 31, 2012. The cohorts included premenopausal patients who would be undergoing treatment that would impact fertility, including chemotherapy and tamoxifen anti-hormonal therapy (hormone receptor positive patients).
An oncofertility program was implemented at Northwestern Hospital in 2007. This multifaceted program included outreach and education for medical providers regarding risks of cancer treatment-related infertility. A multidisciplinary oncofertility case conference promoted cross-disciplinary collaboration between oncologists and reproductive endocrinologists to facilitate referrals, establish treatment plans, and expedite procedures for patients undergoing cancer treatment. A patient navigator helped patients as they pursued assisted reproductive techniques, and were on-call to provide resources and answer patient questions. A 24-hr fertility preservation hotline staffed by the patient navigator was available to both patients and providers (Fig. 1). The program website (myoncofertility.org) compiled helpful educational materials and a glossary of terms related to oncofertility and reproductive endocrinology in a disease-specific fashion. Additionally, the electronic medical record was modified requiring oncology providers to document a discussion about treatment-related infertility with patients before the initial intake consultation encounter could be closed.
Fig. 1.
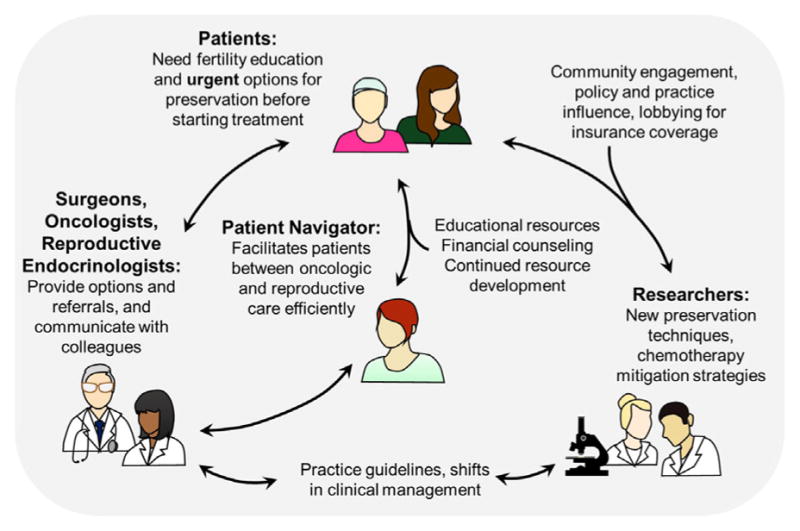
The interaction between patients, providers, patient navigators, and researchers in an oncofertility program. An oncofertility program should foster collaboration to preserve fertility in the setting of cancer care [22].
Identified patients were separated into a pre-oncofertility program cohort, diagnosed between January 1, 2004 and December 31, 2006, and a post-program cohort diagnosed between January 1, 2007 and May 31, 2012. We identified 987 women ages 25–45 (median age 41) treated from 2004 to 2012. Of these patients, 302 were diagnosed from 2004 to 2006, and 685 were diagnosed from 2007 to 2012. After exclusions, including postmenopausal status at diagnosis, recurrent or stage IV disease, no adjuvant therapy information, or adjuvant therapy not recommended, the 2004–2006 cohort consisted of 278 patients (Table I) and the 2007–2012 cohort, 515 (Table II).
TABLE I.
Baseline Characteristics of Premenopausal Patients Diagnosed With Stages I–III ER+ and/or PR+ Breast Cancer Between 2004 and 2006, Advised to Take Adjuvant Tamoxifen (n =278)
| Demographics for patients in the pre-oncofertility program cohort from 2004 to 2006
| ||
|---|---|---|
| Characteristic | No. of patients | % of total cohort |
| Age at diagnosis (years) | ||
| <37 | 70 | 25.2 |
| >37 | 208 | 74.8 |
| Median age | 41 | |
| Race/ethnicity | ||
| White | 201 | 72.3 |
| Black | 30 | 10.8 |
| Hispanic | 24 | 8.6 |
| Asian | 11 | 4.0 |
| Other | 4 | 1.4 |
| Missing | 8 | 2.9 |
| Marital status | ||
| Unmarried | 86 | 30.9 |
| Married, partnered, domestic partner | 191 | 68.7 |
| Missing | 1 | 0.4 |
| Smoking status | ||
| Current smoker | 22 | 7.9 |
| Never smoker | 209 | 75.2 |
| Former smoker | 44 | 15.8 |
| Missing | 3 | 1.1 |
| Alcohol use | ||
| None or <1 drink per week | 130 | 46.8 |
| 1–4 drinks per week | 101 | 36.3 |
| 5–9 drinks per week | 34 | 12.2 |
| 10–19 drinks per week | 7 | 2.5 |
| >19 drinks per week | 2 | 0.7 |
| Missing | 4 | 1.4 |
| Parity | ||
| Nulliparous | 94 | 33.8 |
| Parity >1 | 175 | 62.9 |
| Missing | 9 | 3.2 |
| Insurance status | ||
| Private insurance | 232 | 83.5 |
| Public insurance/uninsured | 46 | 16.5 |
| Fertility concerns | ||
| Desires future fertility at diagnosis | 37 | 13.3 |
| Does not express fertility concerns at diagnosis | 241 | 86.7 |
| Surgery | ||
| Mastectomy | 122 | 43.9 |
| Lumpectomy | 155 | 55.8 |
| Axillary dissection only | 1 | 0.3 |
| Chemotherapy (among patients with stage >1) | ||
| No | 52 | 21.6. |
| Yes | 188 | 78.0 |
| Missing | 1 | 0.4 |
| Radiation | ||
| Patient declined | 12 | 4.3 |
| Yes | 194 | 69.8 |
| XRT not indicated | 70 | 25.2 |
| Missing | 2 | 0.7 |
| Stage | ||
| 0 | 37 | 13.3 |
| I | 96 | 34.5 |
| II | 102 | 36.7 |
| III | 43 | 15.5 |
TABLE II.
Baseline Characteristics of Premenopausal Patients Diagnosed With Stages I–III ER+ and/or PR+ Breast Cancer Between 2007 and 2012, Advised to Take Adjuvant Tamoxifen (n =515)
| Demographics for patients in the post-oncofertility program cohort from 2007 to 2012
| ||
|---|---|---|
| Characteristic | No. of patients | % of total cohort |
| Age at diagnosis (years) | ||
| <37 | 127 | 24.7 |
| >37 | 388 | 75.3 |
| Median age | ||
| Race/ethnicity | ||
| White | 363 | 70.5 |
| Black | 63 | 12.2 |
| Hispanic | 39 | 7.6 |
| Asian | 38 | 7.4 |
| Other | 10 | 1.9 |
| Missing | 2 | 0.4 |
| Marital status | ||
| Unmarried | 182 | 35.3 |
| Married, partnered, domestic partner | 333 | 64.7 |
| Missing | 0 | 0.0 |
| Smoking status | ||
| Current smoker | 38 | 7.4 |
| Never smoker | 365 | 70.9 |
| Former smoker | 108 | 21.0 |
| Missing | 4 | 0.8 |
| Alcohol use | ||
| None or <1 drink per week | 242 | 47.0 |
| 1–4 drinks per week | 188 | 36.5 |
| 5–9 drinks per week | 59 | 11.5 |
| 10–19 drinks per week | 16 | 3.1 |
| >19 drinks per week | 4 | 0.8 |
| Missing | 6 | 1.2 |
| Parity | ||
| Nulliparous | 196 | 38.1 |
| Parity >1 | 312 | 60.6 |
| Missing | 7 | 1.4 |
| Insurance status | ||
| Private insurance | 445 | 86.4 |
| Public insurance/uninsured | 70 | 13.6 |
| Fertility concerns | ||
| Desires future fertility at diagnosis | 112 | 21.7 |
| Does not express fertility concerns at diagnosis | 403 | 78.3 |
| Surgery | ||
| Mastectomy | 272 | 52.8 |
| Lumpectomy | 240 | 46.6 |
| Axillary dissection only | 1 | 0.2 |
| No surgery | 2 | 0.4 |
| Chemotherapy (among patients w/stage >1) | ||
| No | 121 | 29.1 |
| Yes | 295 | 70.9 |
| Radiation | ||
| Patient declined | 36 | 7.0 |
| Yes | 355 | 68.9 |
| XRT not indicated | 124 | 24.1 |
| Stage | ||
| 0 | 99 | 19.2 |
| I | 156 | 30.3 |
| II | 183 | 35.5 |
| III | 77 | 15.0 |
Chart Review
The patient charts for both study cohorts were reviewed for demographics, disease characteristics, and fertility concerns, as documented from new patient intake questionnaires, progress notes, operative reports, and pathology reports (Tables I and II). To evaluate the impact of the oncofertility program, findings from the 2004 to 2006 pre-program, and 2007 to 2012 post-program cohorts were reviewed and compared with regard to number of documented discussions with a provider about treatment-related infertility, patients expressing an interest in fertility preservation at the time of diagnosis, referrals to a fertility preservation provider, appointments with a fertility specialist, and fertility preservation procedures.
Information about fertility concerns at diagnosis for patients in the pre-program cohort was available when documented in provider notes. After implementation of the formal oncofertility program in 2007, prior to closing the initial new patient encounter, a prompt was placed into the medical record requiring oncology providers to document a discussion with premenopausal patients regarding potential cancer treatment effects on fertility, patient interest in fertility preservation, and, if requested, referral for a fertility preservation consultation. Information regarding the number of fertility preservation appointments and assisted reproductive procedures, including oocyte and embryo cryopreservation, was obtained from the patient records.
Statistical Analyses
Two-tailed Fisher’s exact test was used to compare the number of discussions about treatment effects on fertility, rates of referral to fertility specialists, fertility appointments, and assisted reproductive procedures before and after the institution of the oncofertility program. Analyses were conducted using Stata (StataCorp. 2013. Stata Statistical Software: Release 13).
RESULTS
Demographic characteristics of the pre-program (Table I) and post-program (Table II) cohorts were compared. Median age was 41 for both cohorts, with a similar number of patients diagnosed before the age of 37 in the pre-program cohort, 25.2%, and the post-program group, 24.7%. For race and ethnicity, the pre-program cohort was 72.3% white, 10.1% black, 8.6% Hispanic, and 4% Asian, and the post-program cohort was 70.5% white, 12.2 black, 7.6% Hispanic, and 7.4% Asian, showing the demographic stability of the patient population at the study cancer center over time. The majority of patients in both cohorts were married or had a domestic partner, 68.7% pre-program, as compared to 64.7% in the post-program cohort. Comparisons between the two cohorts regarding disease stage, treatment related issues, smoking history, alcohol use, and insurance coverage were also very similar. Regarding parity at the time of diagnosis, 33.8% of patients in the preprogram cohort, versus 38.1% of patients in the post-program cohort, were nulliparous and, correspondingly, a similar proportion of patients in each group had one or more children.
Patient-provider discussions about treatment effects on fertility increased significantly after initiation of the oncofertility program, from 9% to 38%, (P <0.001). Significantly more patients in the post-program cohort were documented as having an interest in maintaining fertility at the time of diagnosis, increasing from 13.3% to 21.7% (P <0.0041). Furthermore, providers made more referrals to reproductive specialists, (4% vs. 28%, P <0.001), and more patients had fertility preservation appointments, (5% vs. 18%, P <0.001) and procedures (4% vs. 8%, P <0.0183) in the post-program cohort when compared to the pre-program cohort (Fig. 2).
Fig. 2.
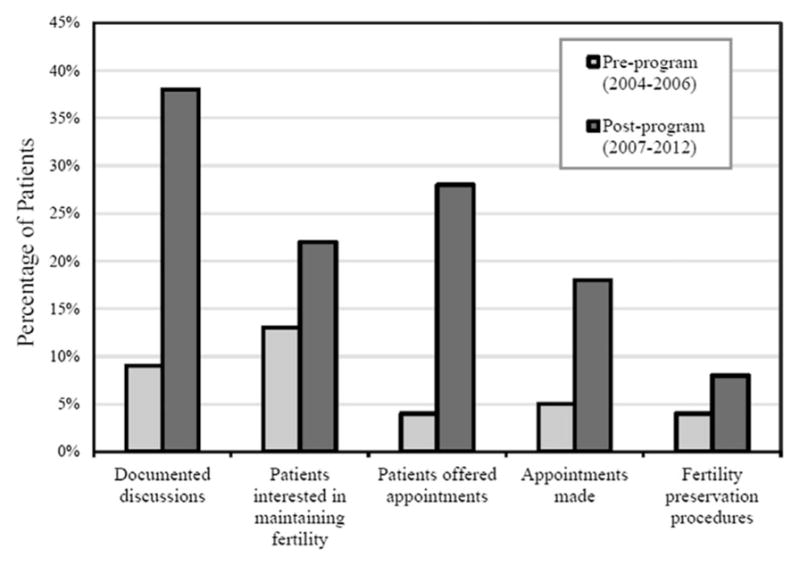
Impact of an oncofertility program on fertility discussions, referrals, and use of fertility preservation options. Pre-program 2004–2006 cohort, n =278. Post-program 2007–2012 cohort, n =515. Discussions about treatment-related infertility increased from 9% to 38%, (P <0.001). Documented interest in maintaining fertility at diagnosis increased from 13.3% to 21.7% (P <0.0041). Referrals to reproductive specialists (4% vs. 28%, P <0.001), appointments (5% vs. 18%, P <0.001), and procedures (4% vs. 8%, P <0.0183) all increased in the post-program cohorts.
DISCUSSION
In this study, we found that in a relatively stable patient population over time, an oncofertility program promoting discussion about fertility concerns significantly increased the rate of documented patient-provider conversations about treatment-related infertility. The number of fertility referrals, consultations, and procedures also increased during this timeframe (Fig. 2). Additionally, after program implementation, which included the initiation of a fertility preservation discussion prompt in the electronic medical record, the number of patients who expressed interest in preserving their future fertility rose as well. This finding emphasizes that although patients value fertility in the context of their cancer treatment, they may not voice these concerns unless prompted to do so, secondary to a number of factors including the intense nature of a cancer diagnosis, a focus on survival, or a lack of awareness about the risks to fertility posed by cancer therapy.
As cancer therapies become more effective, an increasing number of young patients are surviving to look forward to a healthy future, which may include starting a family. Oncofertility is an evolving interdisciplinary field seeking to link reproductive medicine with oncology for young cancer patients threatened with fertility loss [21]. Often, newly diagnosed young patients must make decisions about fertility preservation during a time of great stress and limited time for assessment and reflection. Patients benefit from an approach that provides information about reproductive options in the context of their cancer treatment, balancing cancer care and survivorship with the possibility for fertility preservation [14]. A formal oncofertility program can provide a mechanism to educate oncology caregivers about fertility preservation, while also increasing familiarity with evolving reproductive techniques.
In 2007, the Northwestern oncofertility program initiated a prompt in the electronic medical record for oncologists to document fundamental information regarding patient education, interest, and referrals for fertility preservation. This intervention provided a reminder for clinicians to routinely introduce fertility concerns and options for preservation when discussing the trajectory of a new patient’s oncologic care. To further aid providers and patients in deciphering this information, a collection of informative oncofertility educational materials were generated to translate society guidelines and treatment options into more basic language, and these materials were disseminated to caregivers [21]. Some tools used linear, decision tree-based models to show options facilitating fertility preservation in different scenarios for male and female patients, adolescents, or patients at high risk for the development of breast or ovarian cancer, serving to aid clinicians in explaining the various options to their patients [19,21]. Additionally, a multidisciplinary fertility preservation case conference was instituted to discuss treatment planning for newly diagnosed young oncology patients.
Other critical components of success for the fertility preservation program included the use of a dedicated patient navigator to help address fertility preservation-related questions and concerns, arrange appropriate referrals and specialist appointments, and to act as a liaison between referring providers, reproductive specialists, and the patient. A fertility preservation hotline was also available to patients and providers and afforded information and access to the patient navigator, typically within 24 hr. Educational materials were also made available on a patient-facing website (myoncofertility.org) that defined various oncofertility-related terms and provided disease-specific fertility preservation information in multiple languages [19]. The components of this oncofertility program are highlighted in Figure 1 [22].
At its inception, the oncofertility program was primarily composed of breast cancer patients, as this patient group made up the largest referral population to reproductive specialists. As stated, premenopausal breast cancer patients may incur a significant fertility risk associated with exposure to chemotherapy and lengthy tamoxifen treatment. As the field of fertility preservation has grown, it now includes larger numbers of pediatric patients, adolescents, and grown men and women. Furthermore, young patients treated for rheumatologic issues are also being referred for fertility preservation. Data are currently being gathered and published to address the impact of programmatic implementation for these different patient populations. To this end, similar improvements in access to fertility preservation have been observed among male cancer patients after the launch of an oncofertility program [23].
Opportunities for providers to increase their awareness and understanding of assisted reproduction techniques must be routinely facilitated, and several fertility preservation options are currently in practice. For female patients, oocyte and embryo cryopreservation are components of reproductive medical practice and the standard of therapy for female cancer patients pursuing fertility preservation [8,24]. Patients can typically initiate chemotherapy within a day or two of oocyte retrieval. The finding that hormonal stimulation can be initiated at any point in the menstrual cycle without compromising oocyte yield, a “random-start” protocol, has further decreased the time to chemotherapy initiation [25,26]. Additionally, for certain patients, increasing flexibility within cancer treatment schedules can help to facilitate fertility preservation while balancing appropriate oncologic therapy goals [27]. For breast cancer patients, sequencing tamoxifen treatment and fertility interventions, including delaying initiation to attempt pregnancy, or a tamoxifien hiatus to pursue pregnancy after initiating therapy, may retain significant therapeutic benefit [1,16,28,29]. The prospective IBCSG POSITIVE trial to examine the impact of a tamoxifen treatment hiatus on pregnancy and disease specific outcomes is ongoing [30]. Additional research efforts are examining methods to protect gonadal reserve from damage by chemotherapy or radiation. As an example, gonadotropin-releasing-hormone analogues may help to protect ovarian function from the effects of chemotherapy by inducing cellular quiescence [31–33]. For young oncology patients who do not undergo gamete preservation, additional options include implementation of donor oocytes/sperm, surrogacy, and adoption [1].
CONCLUSIONS
It is critical that oncology practitioners prioritize discussions about fertility preservation during the initial consultation with newly diagnosed young oncology patients. This study demonstrated the favorable impact of a formal oncofertility program on physician–patient communication regarding cancer treatment related effects on fertility. Additionally, comparing two cohorts of demographically similar young breast cancer patients over eight years, more patients who were treated after oncofertility program implementation expressed an interest in maintaining fertility and were then referred to a fertility preservation specialist. The positive findings during the study period may have been influenced by the majority of study population being married, the growing momentum to incorporate fertility preservation into clinical practice, as well as the acceptance of oocyte cryopreservation as standard therapy.
This being said, while the number of conversations about fertility preservation and subsequent actions resulting from these conversations increased for the post-program cohort, overall, participation remained modest. The median age of the study population was 41% and 75% of the cohort was >37, an age when female fertility rates drop significantly. Advanced maternal age, which is associated with an increased risk of adverse maternal and fetal events, may have independently biased the study findings, reflecting the overall low number of patients undergoing fertility preservation procedures. The age distribution of the study cohort reflects both the disease epidemiology and our referral population. Simultaneously, pediatric and adolescent cancer patient studies also demonstrate the need for increased provider-driven education about infertility risk and fertility preservation options [34,35].
While there is clearly a continued need to improve upon physician-patient engagement in oncofertility, factors such as financial issues to cover oncofertility services may also be a concern. Currently, in the United States, most insurance carriers do not routinely cover fertility preservation costs for cancer patients. This issue is being addressed through agencies including the American Medical Association, and on an institutional level through efforts to bundle payments for affected patients. Until this financial issue is resolved, future studies will also focus on the impact of household income as a marker of financial stability the association with family planning and utilization of fertility preservation options.
Patients who are rendered infertile post-treatment have expressed considerable anxiety and remorse over a lack of information or referral for fertility preservation counseling before the initiation of therapy, when fertility preservation options would have been possible [11,17]. To this end, providers have a responsibility for educating patients about their treatment options and facilitating referrals for patients interested in pursuing fertility preservation techniques [17]. A comprehensive, formal oncofertility program including physician collaboration, patient care coordination, and consistent documentation provides a structured means of incorporating fertility preservation into the multidisciplinary process of oncologic treatment. As studies continue to show the beneficial impact of fertility preservation programs on survivorship, and increasing numbers of institutions develop formal programs, it is expected that the oncofertility guidelines delineated by ASCO, ASRM, and NCCN will be more widely applied in the clinical setting.
SYNOPSIS.
An oncofertility program, available to newly diagnosed breast cancer patients, facilitated discussions about fertility preservation options and increased fertility specialist referrals, appointments, and assisted reproduction procedures.
Acknowledgments
This work was supported by the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health (NIH)/NICHD National Center for Translational Research in Reproduction and Infertility (NCTRI).
Grant sponsor: National Institutes of Health (NIH)/NICHD National Center for Translational Research in Reproduction and Infertility (NCTRI); Grant number: P50HD076188.
Footnotes
Institution where study was performed: Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611.
DISCLOSURE
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or author-affiliated institutions.
References
- 1.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 3.Gadducci A, Cosio S, Genazzani AR. Ovarian function and childbearing issues in breast cancer survivors. Gynecol Endocrinol. 2007;23:625–631. doi: 10.1080/09513590701582406. [DOI] [PubMed] [Google Scholar]
- 4.Meirow DD. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 5.Salama M, Winkler K, Murach KF, et al. Female fertility loss and preservation: Threats and opportunities. Ann Oncol. 2013;24:598–608. doi: 10.1093/annonc/mds514. [DOI] [PubMed] [Google Scholar]
- 6.Partridge AH, Ruddy KJ. Fertility and adjuvant treatment in young women with breast cancer. Breast. 2007;16:S175–S181. doi: 10.1016/j.breast.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Ethics Committee of the American Society for Reproductive M. Fertility preservation and reproduction in patients facing gonadotoxic therapies: A committee opinion. Fertil Steril. 2013;100:1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology. J Natl Compr Cancer Netw. 2012;10:1112–1150. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]
- 10.Gwede CK, Vadaparampil ST, Hoffe S, et al. The role of radiation oncologists and discussion of fertility preservation in young cancer patients. Pract Radiat Oncol. 2011;2:242–247. doi: 10.1016/j.prro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Stein DM, Victorson DE, Choy JT, et al. Fertility preservation preferences and perspectives among adult male survivors of pediatric cancer and their parents. J Adolesc Young Adult Oncol. 2014;3:75–82. doi: 10.1089/jayao.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler T, Kondapalli L, Shah A, et al. Results from the survey for preservation of adolescent reproduction (SPARE) study: Gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28:269–277. doi: 10.1007/s10815-010-9504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn GP, Vadaparampil ST, Lee J-H, et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Oncol. 2009;27:5952–5957. doi: 10.1200/JCO.2009.23.0250. [DOI] [PubMed] [Google Scholar]
- 14.Redig AJ, Brannigan R, Stryker SJ, et al. Incorporating fertility preservation into the care of young oncology patients. Cancer. 2011;117:4–10. doi: 10.1002/cncr.25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: A systematic review. Cancer. 2015;121:3938–3947. doi: 10.1002/cncr.29637. [DOI] [PubMed] [Google Scholar]
- 16.Llarena NC, Estevez SL, Tucker SL, et al. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107:1–9. doi: 10.1093/jnci/djv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeruss JS. Discussing fertility preservation with Breast cancer patients. Cancer Treat Res. 2010;156:461–466. doi: 10.1007/978-1-4419-6518-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulvat MC, Jeruss JS. Fertility preservation options for young women with breast cancer. Curr Opin Obstet Gynecol. 2011;23:174–182. doi: 10.1097/GCO.0b013e328345525a. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff TK. Oncofertility: A grand collaboration between reproductive medicine and oncology. Reproduction. 2015;150:S1–10. doi: 10.1530/REP-15-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vos M, Smitz J, Woodruff TK. Fertility preservation 2: Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardino S, Jeruss J, Woodruff T. Using decision trees to enhance interdisciplinary team work: The case of oncofertility. J Assist Reprod Genet. 2010;27:227–231. doi: 10.1007/s10815-010-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodruff TK. The oncofertility consortium–addressing fertility in young people with cancer. Net Rev Clin Oncol. 2010;7:466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth KR, Sharma V, Helfand BT, et al. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol. 2012;187:979–986. doi: 10.1016/j.juro.2011.10.154. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro BS, Daneshmand ST, Garner FC, et al. Similar ongoing pregnancy rates after blastocyst transfer in fresh donor cycles and autologous cycles using cryopreserved bipronuclear oocytes suggest similar viability of transferred blastocysts. Fertility and Sterility. 2009;93:319–321. doi: 10.1016/j.fertnstert.2009.07.966. [DOI] [PubMed] [Google Scholar]
- 25.Sonmezer M, Turkcuoglu I, Coskun U, et al. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95:e2129–e2111. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Cakmak H, Katz A, Cedars MI, et al. Effective method for emergency fertility preservation: Random-start controlled ovarian stimulation. Fertil Steril. 2013;100:1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 27.Turan V, Bedoschi G, Moy F, et al. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril. 2013;100:1681–1685. doi: 10.1016/j.fertnstert.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West ER, Zelinski MB, Kondapalli LA, et al. Preserving female fertility following cancer treatment: Current options and future possibilities. Pediatr Blood Cancer. 2009;53:289–295. doi: 10.1002/pbc.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasi-Cont N, Lambertini M, Hulsbosch S, et al. Strategies for fertility preservation in young early breast cancer patients. Breast. 2014;23:503–510. doi: 10.1016/j.breast.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Pagani O, Ruggeri M, Manunta S, et al. Pregnancy after breast cancer: Are young patients willing to participate in clinical studies? Breast. 2015;24:201–207. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 32.Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aras D, Cinar O, Cakar Z, et al. Can dicoumarol be used as a gonad-safe anticancer agent: An in vitro and in vivo experimental study. Mol Hum Reprod. 2016;22:57–67. doi: 10.1093/molehr/gav065. [DOI] [PubMed] [Google Scholar]
- 34.Font-Gonzalez A, Mulder RL, Loeffen EAH, et al. Fertility preservation in children, adolescents, and young adults with cancer: Quality of clinical practice guidelines and variations in recommendations. Cancer. 2016;122:2216–2223. doi: 10.1002/cncr.30047. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JF, Ott MA. Fertility preservation after a cancer diagnosis: A systematic review of adolescents’, parents’, and providers’ perspectives, experiences, and preferences. J Pediatr Adolesc Gynecol. 2016;29:585–598. doi: 10.1016/j.jpag.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


