Abstract
By use of microdialysis we assessed the concentrations of telithromycin in muscle and adipose tissue to test its ability to penetrate soft tissues. The ratios of the area under the concentration-versus-time curve from 0 to 24 h to the MIC indicated that free concentrations of telithromycin in tissue and plasma might be effective against Streptococcus pyogenes but not against staphylococci and human and animal bite pathogens.
The spread of resistant pathogens that result in treatment failures is an increasing problem worldwide (16, 19). Therefore, the development of new antimicrobial agents with low potentials to select for resistance is desirable. The ketolides were designed to overcome the resistance of macrolide-resistant gram-positive cocci and have also been shown to possess activities against atypical and anaerobic pathogens (7, 8, 10). The mechanism of action of ketolides is similar to that of macrolides, but due to the presence of a 3-keto group in the ketolides, the development of resistance is unlikely to occur (1). Furthermore, the C-11 and C-12 carbamate side chain of ketolides enables binding to macrolide-streptogramin-lincosamide (MLS)-resistant ribosomes, which may explain the high levels of activity of the ketolides against MLS-resistant organisms (17). Telithromycin is the first member of this new family of antimicrobials and was recently introduced into clinical practice.
Telithromycin reaches high concentrations in inflammatory fluids, bronchopulmonary tissues, tonsillar tissue, and saliva (6, 13). As telithromycin may have some antimicrobial advantages against streptococcal species, it has been speculated that this compound may be an option for the treatment of skin and soft tissue infections (STIs), although the majority of erythromycin-resistant staphylococci are also resistant to telithromycin (10).
Therefore, the present study was carried out to determine the concentration-versus-time profile of telithromycin in the interstitial space fluid of soft tissues after administration of a single dose to young healthy volunteers. The minimally invasive microdialysis (MD) technique was used. This technique has been shown to be an excellent tool for testing the concentrations of antimicrobials at the sites of bacterial infection (11, 14, 15).
MATERIALS AND METHODS
The study was performed in accordance with the local ethics committee; the 1964 Declaration of Helsinki, including current revisions; the Austrian Drug Law (Arzneimittelgesetz); and the Good Clinical Practice guidelines of the European Commission. Approval from the local ethics committee was obtained before initiation of the study. Volunteers received a detailed description of the study before any examination or intervention was undertaken. Written informed consent was obtained prior to the conduct of the study.
Healthy volunteers.
This study included 10 male volunteers ages 18 to 40 years. The health of each volunteer was determined by one of the investigators by obtaining a medical history; performing a physical examination, routine laboratory tests, and a 12-lead electrocardiography; and determining the blood pressure and heart rate.
Study protocol.
The volunteers were admitted to the clinical research ward on the morning of the study day. A plastic cannula was inserted into an antecubital vein to monitor the blood telithromycin concentrations at defined time points. The concentrations in the interstitial space fluid of skeletal muscle and subcutaneous adipose tissue were determined by MD.
The principle of MD has been described in detail previously (11, 15). In brief, a thigh muscle and subcutaneous adipose tissue of the lower extremity were punctured with a steel guidance cannula without anesthesia. The MD probes (CMA10 microdialysis probe; CMA/Microdialysis AB, Stockholm, Sweden) were placed into the tissue by using this guidance cannula. The guidance cannula was removed, leaving the MD probe in the tissue. The MD system was connected and perfused with Ringer's solution at a flow rate of 1.5 μl/min with a precision pump (CMA100; CMA/Microdialysis AB). After a 30-min baseline sampling period in vivo, the probe was calibrated for approximately 30 min. Then, telithromycin was added to the perfusion medium and its rate of disappearance through the membrane was used to determine the in vivo recovery. After a washout period of 40 min, 800 mg of telithromycin (Ketek 800 mg Tabletten; Aventis Pharma, Romainville, France) was administered orally. Sampling of dialysates and venous blood was performed at 20-min intervals for the first 4 h and afterwards at 30-min intervals for up to 8 h. Blood was collected and placed into tubes containing lithium and heparin, and after each sampling the venous catheter was rinsed with physiological saline solution. Blood samples were kept on ice for a maximum of 60 min and were centrifuged at 1,600 × g for 5 min at 4°C, the cells were discharged, and plasma was obtained. Plasma and dialysate samples were stored at −80°C until analysis.
Chemical analysis.
The telithromycin concentrations in the microdialysates and the plasma samples were analyzed by a validated high-performance liquid chromatography (HPLC) method (13).
Reagents and standard solutions.
Pure telithromycin was a gift from Aventis Pharma (Romaineville, France). Ringer's solution was purchased from Mayrhofer Pharmazeutica GmbH (Linz, Austria). All other reagents were obtained from Sigma-Aldrich (Steinheim, Germany). Calibration standards for the microdialysates were freshly prepared each day by diluting telithromycin with Ringer's solution at concentrations ranging from 0.005 to 5 mg/liter. Calibration standards for the plasma samples were prepared by spiking plasma with telithromycin at concentrations ranging from 0.01 to 5 mg/liter.
The plasma samples were deproteinized by the addition of 50% trichloroacetic acid and centrifugation at 12,000 × g at 4°C for 5 min. The supernatant was neutralized with sodium hydroxide. The microdialysate samples were analyzed without further preparation. The injection volume was 15 μl. The HPLC system (all instruments were from Shimadzu, Kyoto, Japan) consisted of an LC9A pump, a SIL6B autoinjection port, and an RF-551 spectrofluorometric detector connected to a CR-6A Chromatopac integrator. Isocratic separation was carried out at room temperature with a LiChroCART RP-18e analytic column (250 by 4.0 mm; column particle size, 5 μm; Merck, Darmstadt, Germany). The mobile phase was composed of ammonium acetate (0.03 M), adjusted to pH 5.2 with acetic acid, and acetonitrile at a 56:44 ratio, by volume. The flow rate was 1 ml per min. The spectrofluorometer was set at 263 and 460 nm for the excitation and emission wavelengths, respectively. The limit of quantification was 0.002 mg/liter for both plasma and microdialysate samples. The within- and between-day accuracy and precision were calculated by measuring spiked plasma samples and analyte standards in Ringer's solution at three different concentrations in triplicate on three different days. Coefficients of variations were below 8%.
Protein binding studies.
Aliquots (0.3 ml) of plasma from each volunteer obtained at 60 and 180 min were ultrafiltrated by centrifugation at 12,000 × g for 60 min at room temperature by using centrifugal filter units equipped with a low-binding regenerated cellulose membrane (nominal molecular weight limit, 5,000; Ultrafree-MC; Millipore Corp., Bedford, Mass.). Ultrafiltrates were analyzed as described above by using spiked standards for calibration. For determination of the binding of telithromycin to the ultrafiltration membrane during the filtration process, standards of telithromycin diluted in Ringer's solution (1, 2.5, and 10 mg/liter) were ultrafiltrated and analyzed in the same way as described above for the plasma samples.
PK calculations and statistical analysis.
The telithromycin concentrations in interstitial fluid were calculated by use of the individual recovery values determined in our in vivo experiments. Pharmacokinetic (PK) analysis was carried out with commercially available software (Kinetica, version 3.0; Innaphase Sarl, Paris, France). The areas under the concentration-time curves (AUCs) for plasma and interstitial fluid were calculated from nonfitted data by use of the trapezoidal rule. The volume of drug distribution (V) and total drug clearance (CL) were calculated for plasma by use of standard formulae, as follows: V = dose/(AUC0-∞ · kel) and CL = V · kel, respectively, where AUC0-∞ represents the AUC from zero to infinity and kel represents the elimination rate constant. The dose was corrected for absolute bioavailability (F) of 60%. The half-life for the terminal slope (t1/2β) was calculated by the equation ln(2)/kel. The ratios of the AUC from 0 to 8 h (AUC0-8) for tissues (AUC0-8 muscle and AUC0-8 subcutis) to the AUC0-8 for plasma (AUC0-8 plasma) were calculated as a measure of drug penetration from the central compartment to peripheral sites. Statistical analysis was performed with a commercially available computer program (Statistica; StatSoft Inc., Tulsa, Okla.). All data are presented as means ± standard deviations (SD). Wilcoxon paired tests were used for comparison of parameters between plasma and tissues.
RESULTS
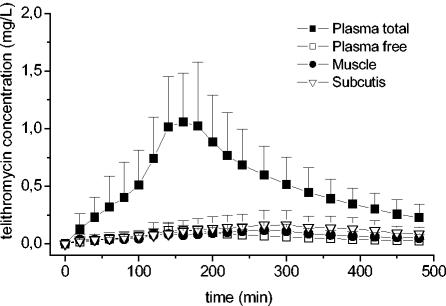
The present study evaluated the PK profiles of telithromycin in the tissue and plasma of healthy volunteers. Figure 1 shows the mean concentration-versus-time profiles of telithromycin in plasma, muscle, and subcutaneous adipose tissue after the administration of a single oral dose of 800 mg to healthy volunteers (n = 10).
FIG. 1.
Mean time-versus-concentration profiles of total and free telithromycin in plasma, muscle, and subcutis after administration of a single oral dose of 800 mg.
The values of the PK parameters for telithromycin in tissue and plasma are shown in Table 1. The mean AUC0-8 of telithromycin was 4.1 ± 1.5 mg · h/liter for total plasma (AUC0-8 total plasma) and 0.5 ± 0.2 mg · h/liter for free telithromycin (AUC0-8 free plasma). Mean AUC0-8s of 0.6 ± 0.3 and 0.9 ± 0.6 mg · h/liter were calculated for muscle tissue and subcutaneous adipose tissue, respectively. The AUC0-24s for total plasma, free plasma, subcutaneous adipose tissue, and muscle were 6.1 ± 2.4, 0.7 ± 0.3, 1.6 ± 1.0, and 0.8 ± 0.5 mg · h/liter, respectively. The AUC0-24s of free telithromycin for plasma and tissues were not significantly different (P > 0.05).
TABLE 1.
Mean values of pharmacokinetic parameters for telithromycin following administration of a single oral dose of 800 mga
| Compartment | AUC0-8 (mg · h/liter) | AUC0-24 (mg · h/liter) | Cmax (mg/L) | Tmax (min) | AUC0-8 tissue/ AUC0-8 total plasma | AUC0-8 tissue/ AUC0-8 free plasma | t1/2β (min) | V/F (liters) | CL/F (liters/min) |
|---|---|---|---|---|---|---|---|---|---|
| Plasma (total) | 4.1 ± 1.5 (1.9-6.3) | 6.1 ± 2.4 (3.9-9.2) | 1.2 ± 0.5 (0.6-2.3) | 164 ± 35 (120-240) | ND | ND | 185 ± 75 (105-284) | 634 ± 274 (311-1,029) | 1.5 ± 0.8 (0.8-3.1) |
| Plasma (free) | 0.5 ± 0.2 (0.3-0.9) | 0.7 ± 0.3 (0.3-1.3) | 0.1 ± 0.1 (0.08-0.25) | 164 ± 35 (120-200) | ND | ND | 160 ± 25 (55-346) | ND | ND |
| Subcutis | 0.9 ± 0.6 (0.3-1.8) | 1.6 ± 1.0 (0.5-3.4) | 0.2 ± 0.1 (0.05-0.4) | 306 ± 36 (240-360) | 0.2 ± 0.2 (0.1-0.7) | 2.1 ± 1.6 (0.4-5.5) | 169 ± 77 (104-353) | ND | ND |
| Muscle | 0.6 ± 0.3 (0.1-1) | 0.8 ± 0.5 (0.1-1.7) | 0.2 ± 0.1 (0.03-0.4) | 243 ± 63 (140-360) | 0.2 ± 0.1 (0.1-0.3) | 1.5 ± 0.9 (0.4-3.2) | 176 ± 104 (68-395) | ND | ND |
Values represent mean ± SDs (ranges). Abbreviations: Cmax, maximum concentration of drug in plasma; Tmax, time to maximum concentration of drug in plasma; ND, not determined.
AUC0-8s for muscle and subcutaneous adipose tissue were significantly lower (P < 0.005) than those for total plasma, and the ratios of AUCtissue/AUCtotal plasma at 8 h were 0.2 ± 0.1 for muscle and 0.2 ± 0.2 for subcutis. No significant difference (P > 0.34) between the AUC0-8s for unbound telithromycin in plasma and the AUC0-8s for telithromycin in soft tissues was observed, with AUCtissue/AUCplasma ratios of 1.5 ± 0.9 and 2.1 ± 1.6 for muscle and subcutis, respectively.
The mean plasma protein binding (PPB) of telithromycin was 88.5% ± 1.0%, which is in accordance with the findings in the literature (4). The mean recovery rates from the microdialysis experiments were 62.9% ± 2.9% and 61.0% ± 2.8% for muscle and subcutis, respectively.
DISCUSSION
Telithromycin was initially designed for the treatment of upper and lower respiratory tract infections, but telithromycin has also been shown to have good in vitro activity against pathogens frequently isolated from skin infections and STIs in humans (10). As a consequence, Goldstein et al. (10) speculated that telithromycin qualifies as an alternative to macrolides for the treatment of STIs. Therefore, in the present study we set out to determine the ability of telithromycin to penetrate soft tissues.
The central finding of our study was that the concentrations in total plasma were significantly higher (P < 0.005) than the corresponding concentrations in the interstitial fluid of soft tissues. The mean ratios of AUC0-8 tissue/AUC0-8 total plasma were 0.15 and 0.20 for muscle and subcutis, respectively. The incomplete tissue penetration was most likely related to the high PPB of telithromycin, which is reported to be about 70% (4, 17). Other studies indicate that the rates of PPB of an antibiotic may vary highly among individuals (12). We therefore measured the rates of PPB of telithromycin for each of the 10 participating volunteers and found that they ranged from 85 to 93%.
The measurement of the non-protein-bound fraction of an antibiotic is particularly important, because only the unbound drug fraction penetrates soft tissues (11). For the description of the tissue penetration of telithromycin, we related the free fraction of the drug in plasma to the corresponding concentration in soft tissues. The mean ratios of the AUCtissue/AUCplasma for free telithromycin were 1.5 and 2.1 for muscle and subcutis, respectively. These ratios indicate complete equilibration of unbound telithromycin in plasma and tissue after approximately 150 min of drug intake. After drug equilibration, the concentrations of telithromycin in the interstitial space fluid of subcutaneous adipose tissue tended to be descriptively higher than those in skeletal muscle tissue. This phenomenon can be explained incompletely by a moderate accumulation of telithromycin in fat cells, white blood cells, and other cells, like fibroblasts (9, 21), from which telithromycin is then slowly released (Fig. 1). The half-lives of telithromycin in tissues and plasma were identical (P > 0.05), although blood perfusion of subcutaneous adipose tissue might be lower than that of skeletal muscle (Fig. 1). Differences in the pH gradient in the interstitial space fluid between muscle and plasma and between the subcutis and plasma have been discussed (20) as a mechanism to account for the ion-trapping phenomenon, but it appears to be unlikely that they are responsible for the differences in tissue telithromycin concentrations in the present study. This is because subjects were in the resting position throughout the study procedures, and pHs may be expected to be similar in muscle and subcutis. However, in our study, the pHs in the interstitial space fluid and plasma were not determined.
Hence, our observation of descriptively lower concentrations of telithromycin in muscle compared to those in the subcutis is not fully understood at present. Further clinical studies that will test the time-concentration profiles of telithromycin in the interstitium of soft tissues and plasma at steady state are necessary. In addition, the redistribution phenomenon needs to be clarified by using simultaneously with the MD method other techniques, such as positron emission tomography, which allow description of the time-concentration profile of the total drug in whole tissues. The use of these techniques combined will help to reinterpret actual knowledge on the tissue penetration characteristics of the drug (2).
The antimicrobial efficacy of ketolides was reported to be best related to the AUC0-24/MIC ratio (6, 18). Given the MICs at which 90% of isolates are inhibited (MIC90s) for pathogens that frequently cause STIs (3, 10), the AUC0-24/MIC90 ratios indicate that telithromycin will be sufficient to eradicate highly susceptible bacteria such as Streptococcus pyogenes from tissues and plasma (5, 22). However, the AUC0-24/MIC90 ratios for telithromycin appeared to be too low for the treatment of infections caused by Staphylococcus aureus or bite pathogens, such as Prevotella canis. Nevertheless, it should be kept in mind that our calculations for tissues were performed with the unbound concentrations of telithromycin, for which AUC/MIC breakpoints have not yet been defined.
In conclusion, on the basis of our PK and pharmacodynamic calculations, the present data show that the AUC0-24/MIC ratio achieved with free telithromycin in plasma and soft tissue might be sufficient to kill S. pyogenes. However, it seems unlikely that telithromycin can reach concentrations adequate to achieve activity against staphylococci, and thus, its role in the treatment of STIs and skin infections appears to be limited.
REFERENCES
- 1.Barman Balfour, J. A., and D. P. Figgitt. 2001. Telithromycin. Drugs 61:815-829. [DOI] [PubMed] [Google Scholar]
- 2.Brunner, M., O. Langer, G. Dobrozensky, U. Müller, M. Zeitlinger, W. Wadsak, R. Dudczak, K. Kletter, and M. Müller. 2004. [18F]ciprofloxacin, a new positron emission tomography tracer for noninvasive assessment of the tissue distribution and pharmacokinetics of ciprofloxacin in humans. Antimicrob. Agents Chemother. 48:3850-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxbaum, A., S. Forsthuber, W. Graninger, and A. Georgopoulos. 2003. Comparative activity of telithromycin against typical community-acquired respiratory pathogens. J. Antimicrob. Chemother. 52:371-374. [DOI] [PubMed] [Google Scholar]
- 4.Cantalloube, C., V. Bhargava, E. Sultan, F. Vacheron, I. Batista, and G. Montay. 2003. Pharmacokinetics of the ketolide telithromycin after single and repeated doses in patients with hepatic impairment. Int. J. Antimicrob. Agents 22:112-121. [DOI] [PubMed] [Google Scholar]
- 5.Critchely, I. A., D. F. Sahm, C. Thornsberry, R. S. Blosser-Middleton, M. E. Jones, and J. A. Karlowsky. 2002. Antimicrobial susceptibilities of Streptococcus pyogenes isolated from respiratory and skin and soft tissue infections: United States LIBRA surveillance data from 1999. Diagn. Microbiol. Infect. Dis. 42:129-135. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. 2001. Pharmacodynamic and pharmacokinetic considerations in antimicrobial selection: focus on telithromycin. Clin. Microbiol. Infect. 7(Suppl.3):24-29. [PubMed] [Google Scholar]
- 7.Edelstein, P. A., and M. A. Edelstein. 1999. In vitro activity of the ketolide HMR 3647 (RU 66647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob. Agents Chemother. 43:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felmingham, D., G. Zhanel, and D. Hoban. 2001. Activity of the ketolide antibacterial telithromycin against typical community-acquired respiratory pathogens. J. Antimicrob. Chemother. 48(Suppl. T1):33-42. [DOI] [PubMed] [Google Scholar]
- 9.Gladue, R. P., and M. E. Snider. 1990. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob. Agents Chemother. 34:1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, E. J., D. M. Citron, S. H. Gerardo, M. Hudspeth, and C. V. Merriam. 1998. Activities of HMR 3004 (RU 64004) and HMR 3647 (RU 66647) compared to those of erythromycin, azithromycin, clarithromycin, roxithromycin, and eight other antimicrobial agents against unusual aerobic and anerobic human and animal bite pathogens isolated from skin and soft tissue infections in humans. Antimicrob. Agents Chemother. 5:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joukhadar, C., H. Derendorf, and M. Müller. 2001. Microdialysis, a novel tool for the measurement of antibiotics in interstitium of humans. Eur. J. Clin. Pharmacol. 57:211-219. [DOI] [PubMed] [Google Scholar]
- 12.Joynt, G. M., J. Lipman, C. D. Gomersall, R. J. Young, E. L. Wong, and T. Gin. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47:421-429. [DOI] [PubMed] [Google Scholar]
- 13.Khair, O. A., J. M. Andrews, D. Honeybourne, G. Jevons, F. Vacheron, and R. Wise. 2001. Lung concentrations of telithromycin after oral dosing. J. Antimicrob. Chemother. 47:837-840. [DOI] [PubMed] [Google Scholar]
- 14.Lönnroth, P., P. A. Jansson, and U. A. Smith. 1987. Microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol. 253:228-231. [DOI] [PubMed] [Google Scholar]
- 15.Müller, M., R. Schmid, A. Georgopoulos, A. Buxbaum, C. Wasicek, and H. G. Eichler. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin. Pharmacol. Ther. 57:371-380. [DOI] [PubMed] [Google Scholar]
- 16.Munoz, R., T. J. Coffey, M. Daniels, C. G. Dowson, G. Laible, J. Casal, R. Hakenbeck, M. Jacobs, J. M. Musser, B. G. Spratt, et al. 1991. Intercontinental spread of a multiresistant clone of serotype 23F S. pneumoniae. J. Infect. Dis. 164:302-306. [DOI] [PubMed] [Google Scholar]
- 17.Namour, F., D. H. Wessels, M. H. Pascual, D. Reynolds, E. Sultan, and B. Lenfant. 2001. Pharmacokinetics of the new ketolide telithromycin (HMR 3647) administered in ascending single and multiple doses. Antimicrob. Agents Chemother. 45:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolau, D. P. 2003. Optimizing outcomes with antimicrobial therapy through pharmacodynamic profiling. J. Infect. Chemother. 9:292-296. [DOI] [PubMed] [Google Scholar]
- 19.Sader, H. S., R. N. Jones, J. B. Silva, and Sentry Participants Group (Latin America). 2002. Skin and soft tissue infections in Latin American medical centers: four-year assessment of the pathogen frequency and antimicrobial susceptibility patterns. Diagn. Microbiol. Infect. Dis. 44:281-288. [DOI] [PubMed] [Google Scholar]
- 20.Sörgel, F., J. Bulitta, and M. Kinzing-Schippers. 2002. Pharmacokinetics of quinolones. Chemother. J. 11:25-33. [Google Scholar]
- 21.Vazifeh, D., H. Abdelghaffar, and M. T. Labro. 2002. Effect of telithromycin (HMR 3647) on polymorphonuclear neutrophil killing of Staphylococcus aureus in comparison with roxithromycin. Antimicrob. Agents Chemother. 46:1364-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yassin, H. M., and L. L. Dever. 2001. Telithromycin: a new ketolide antimicrobial for treatment of respiratory tract infections. Expert Opin. Investig. Drugs 10:353-367. [DOI] [PubMed] [Google Scholar]