Abstract
In 2009, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established the Pediatric Terminology Harmonization Initiative to establish a core library of terms to facilitate the acquisition and sharing of knowledge between pediatric clinical research, practice, and safety reporting. A coalition of partners established a Pediatric Terminology Adverse Event Working Group in 2013 to develop a specific terminology relevant to international pediatric adverse event (AE) reporting. Pediatric specialists with backgrounds in clinical care, research, safety reporting, or informatics, supported by biomedical terminology experts from the National Cancer Institute’s Enterprise Vocabulary Services participated. The multinational group developed a working definition of AEs and reviewed concepts (terms, synonyms, and definitions) from 16 pediatric clinical domains. The resulting AE terminology contains >1000 pediatric diseases, disorders, or clinical findings. The terms were tested for proof of concept use in 2 different settings: hospital readmissions and the NICU. The advantages of the AE terminology include ease of adoption due to integration with well-established and internationally accepted biomedical terminologies, a uniquely temporal focus on pediatric health and disease from conception through adolescence, and terms that could be used in both well- and underresourced environments. The AE terminology is available for use without restriction through the National Cancer Institute’s Enterprise Vocabulary Services and is fully compatible with, and represented in, the Medical Dictionary for Regulatory Activities. The terminology is intended to mature with use, user feedback, and optimization.
Pediatric Adverse Events: Global Need for Terminology Development
Broad Interest in Adverse Events
Adverse events (AEs) that have health or medical effects are of interest to many stakeholders for a variety of reasons (for some examples, see Table 1).
TABLE 1.
Stakeholder Interest in AEs
| Potentially Interest From | Possible Emphasis |
|---|---|
| Patients | Safety |
| Epidemiologists | Incidence, prevalence, exposure correlations |
| Pharmacovigilance analysts | Incidence, prevalence, product risk correlations |
| Regulators | Study participant safety, benefit/risk analysis |
| Public health officials | Incidence, prevalence, exposure correlations, policy, response |
| Scientists | Mechanism of action, exposure correlations, benefit/risk analysis |
| Health care administrators | Quality of care, resource allocation, staff training |
| Clinical trial investigators | Decision making and analysis for trial status and planning |
| Research monitoring and oversight bodies | Study participant safety and decision making for trial status |
| Practitioners | Incidence and prevention |
A major challenge to understanding and analyzing patterns of AEs is that most of the AEs that occur are not known to any of the stakeholders. Thus, any analysis deals only with AEs that are reported to a stakeholder that processes and disseminates the data. Another challenge is that each stakeholder uses different resources, different naming conventions, and processes the information in different ways.
Lack of Consistency in General Terminology Systems
Regulatory authorities around the world, such as the US Food and Drug Administration (FDA), World Health Organization (WHO), and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) use different terminologies for AEs, but some common features are:
An AE is an untoward medical occurrence or an unfavorable and unintended sign, symptom, or illness.
The AE occurs in the context of exposure to a product, either in an investigational setting or during use of a marketed product.
There is no need to establish a causality relationship between exposure and the AE.
In addition to regulatory authorities, other organizations have their own definitions of AEs that apply in other settings, such as health care delivery. The lack of consistency across organizations makes it difficult to analyze information across disciplines.
Different stakeholders use different systems for classifying and describing health and medical concepts and terms. Some of the larger and more common terminologies currently used are:
The International Classification of Diseases (ICD), which is used by epidemiologists for population analyses and by health care managers for resource allocation and reimbursement. ICD is formally considered a diagnostic tool, and is endorsed by the WHO.
The International Classification of Functioning, Disability, and Health, which is the WHO framework for measuring health and disability at both individual and population levels.
The Systematized Nomenclature of Medicine-Clinical Terms, which is an extensive clinical terminology that has been designated as a US standard for electronic health information exchange. It is freely distributed by the National Library of Medicine.
The Medical Dictionary for Regulatory Activities (MedDRA) developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), which is specific, standardized medical terminology to facilitate the international sharing of regulatory information for medical products used by humans. MedDRA is intended for use in the registration, documentation, and safety monitoring of medical products, both before and after a product has been authorized for marketing.
In addition to the major terminology initiatives, multiple specialized tools for specifically assessing AEs are in use. The most widely used and significant is the Common Terminology Criteria for Adverse Events (CTCAE) from the National Cancer Institute (NCI). The CTCAE was developed for, and is primarily used in, oncology research, where the tolerance, acceptability, and expectations for AEs differ from most other medical domains.1
Pediatric Specific Needs for AE Terminology
What most terminologies have in common is that they are neither designed for general pediatric use nor do they have the granularity and specificity needed for either pediatric research or for quality and safety monitoring.2
Children are particularly vulnerable to experiencing harm associated with medical intervention because developmental, stage-dependent variability in disease progression and response to therapy are significant risk factors for precipitating AEs.2–4 In addition, rates of potential adverse drug events are higher in pediatric patients than in adult populations for many reasons, including the added complexities of dosing medications based on weight or selecting devices based on size.5,6
Addressing the Need
To address this critical gap and to develop a new paradigm for capturing information about AEs in children, a coalition of partners, led by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), with the collaboration of the NCI’s Enterprise Vocabulary Services (NCI EVS), which included the Global Research in Pediatrics consortium with representation from the FDA, MedDRA, and others, built on existing NICHD and NCI EVS resources. A specific driver for this initiative was the need to develop a comprehensive, generic, pediatric resource for AEs that can be used for all children from birth through early adulthood.
Development of an AE Definition for This Project
Given the range of terminology for AEs and the need to apply the terminology to medical care in the pediatric setting without limiting the context of use to specific products, the Pediatric Adverse Event Terminology Working Group (PAETWG) developed the following definition for AEs:
Any untoward medical occurrence in a patient or clinical investigation subject that occurs in association with medical management and that does not necessarily have a causal relationship with this management.
An AE can therefore be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the medical management, whether known to be related to the medical management or not.
Medical management includes diagnostic, therapeutic, or preventive procedures, as well as the use of medicinal products, medical supplies, or equipment.
An AE may or may not be preventable.
Inclusion criteria: AEs occurring in the perinatal and/or pediatric population, as well as maternal AEs that directly impact the fetus or newborn.
Exclusion criteria: AEs that occur exclusively in adult populations, as well as pregnancy-related AEs that do not directly impact the fetus or newborn.
The following topics were deferred for future discussion: outcome status, relatedness, severity, and toxicity grade.
Project Goals
The major project goal was to provide a common, readily accessible, dynamic resource for describing and cataloging AEs that occur for any reason in children. The uses are multiple and include applications to patient care, research, epidemiology, quality control, pharmacovigilance, decision support, policy development, medical information exchange, and scholarship. Recognizing the need for ongoing updating and revision, anyone can comment or contribute through a structured process.
The Context
The child health AE terminology is designed to be integrated with other resources. Collaboration with regulatory agencies and MedDRA were essential components of the project. The AE concepts and terms are housed and maintained in the NCI Thesaurus (NCIt).7,8 Companion child health–related terminologies focused on perinatology, neonatology, rheumatology, nephrology/urology, endocrinology, infectious diseases, and hematology–oncology are either currently or will be published in the NCIt and are publicly accessible through the NCI Term Browser.9 The NCIt provides access to multiple other resources, thus both enriching the placement of the child health AE terminology and facilitating comprehensive, state-of-the-art information services.
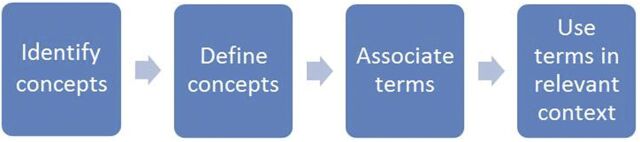
Logical Model
The logical model is to identify concepts, reach consensus on their definition, identify, or, if need be, develop terms to associate with the concepts and apply the terms to the need. The terms can be used as variable names, metadata tags, category names, or whatever is applicable for the information need. The process is summarized in Fig 1 and is iterative; that is, as terms are applied in different settings, the concepts and the terms can be modified to provide both better precision and greater use. The applicability is intended to be broad to address the needs of the stakeholders, as listed in Table 1, as well as additional users. The terminology is intended to have broad applicability, as well as to become a single, unifying resource to describe and catalog AEs related to children, irrespective of the context.
FIGURE 1.
Logical model for pediatric AE terminology development.
Specific Approach
The PAETWG consisted of 62 subject matter experts (SMEs) from Australia, Canada, Italy, the United Kingdom, and the United States with backgrounds in clinical care, research, safety reporting, regulatory activities, epidemiology, or informatics. SMEs participated in either subdomain-specific review or review of the entire terminology set, depending on their area of expertise and availability. The working group met during 2013 and 2014.
The starter set of terms for the PAETWG was derived from multiple sources: the non–pediatric-specific NCI CTCAE events (version 4.0); NCIt NICHD terminology; the FDA Adverse Event Reporting System Year 2012 Quarter 2-Year 2013 Quarter 1 and Vaccine Adverse Event Reporting System components of MedWatch; Systematized Nomenclature of Medicine-Clinical Terms and MedDRA components of the November 2013 release of the Unified Language System Metathesaurus; and the ICD (Ninth and Tenth Revisions) and the International Classification of Primary Care, Version 2 Plus. Additionally, PAETWG participants provided material for discussion from their clinical research, from the Vermont Oxford Network, the Pediatric Heart Network, and Global Research in Pediatrics. The final terminology comprises pediatric diseases, disorders, and clinical findings that meet the definitional criteria of an AE as adapted from ICH document E6, which comprises investigational as well as approved products and has the most comprehensive criteria for an AE.
AEs occurring in the perinatal or pediatric population, as well as maternal AEs that directly affect the fetus or newborn, were included in the terminology subset. AEs occurring exclusively in adult populations, as well as pregnancy-related AEs that were not considered to directly affect the fetus or newborn, were excluded. Concept modifiers related to AE outcome status, relatedness, severity, and toxicity grade were also considered out of scope due to the absence of consistent, objective criteria that were broadly applicable.
Before the first PAETWG meeting, a draft list of terms and associated synonyms related to the clinical domain of interest were compiled by the NCI EVS terminology experts using data mining of primary sources (eg, case report forms, clinical trial data collection instruments, proceedings from standards organizations, published scientific literature, and existing biomedical terminologies), and draft definitions were developed. The working group members (see Supplemental Material) and additional SMEs were asked to review the terminology set, draft definitions, and identify and submit any additional, relevant primary resource materials that could be used to eliminate gaps in the terminology.
PAETWG meetings were conducted via web conference, generally on a weekly basis, to review the draft terms, synonyms, and definitions, all of which were accepted, deleted, or modified as necessary. The NCI EVS terminology experts also participated in the PAETWG meetings to ensure adherence to best practices for terminology development. As a result, the working group developed highly specific definitions, which are flexible enough to be used internationally and in both high- and low-resource environments. The PAETWG leader was responsible for managing both the workflow and the governance committee interactions to adjudicate unresolved issues.
Initial Outcomes
A total of 1223 terms and definitions were adopted after ∼18 months of review by the PAETWG members and SMEs. These terms were classified into clinical subdomains for the purpose of organizing SME consultation. Those subdomains include cardiology, constitutional, dermatology, endocrinology, gastroenterology, hematology/oncology, immunology/rheumatology, infectious disease, metabolic disease, miscellaneous, nephrology/urology, neurology, ophthalmology, psychiatry, pulmonology, and surgery/orthopedics. The domain information was not kept as part of the resulting terminology structure. Terms that fit multiple clinical domains were reviewed by SMEs from all relevant domains and were modified as necessary.
At the completion of the PAETWG term review, the newly developed terminology underwent a rigorous quality control process, and the terms were integrated into an overarching NICHD Pediatric Terminology Harmonization Initiative hierarchical structure curated within the NCIt.
Hierarchical Structure
Descending levels of the hierarchy represent narrower or more specific subconcepts. Unlike ontologies without definitions, which rely on large polyhierarchies, parent terms were assigned sparingly, because definitions provide a clearer picture of the concept’s intent. After the development of the hierarchy, the working group lead asked select stakeholders to perform a review of the terminology subset. An example of the general hierarchy structure for all terms is shown in Fig 2. The AE terms were integrated into this general hierarchy.
FIGURE 2.
Example of terminology hierarchy.
Integration With MedDRA
Finally, the participation of MedDRA throughout the process facilitated rapid incorporation of the terminology into MedDRA.
Microsoft Access queries were used to identify identical terms between the MedDRA and the pediatric AE terminologies. The remainder of the terms comprising the pediatric AE terminology was manually mapped to MedDRA by physicians of the MedDRA Maintenance and Support Services Organization. The selection of MedDRA terms follows the ICH MedDRA coding guide document, “MedDRA Term Selection: Points to Consider.” In some cases, new concepts were added to MedDRA as the result of the mapping. The mapping was also reviewed by NCI EVS staff to ensure compliance with the rules of the NCIt.
To accurately represent the MedDRA terminology in the NCIt, NCI EVS added a feature to the NCIt to represent mappings between terminologies at the concept level. This feature permits the addition of a relationship type between the terminologies (eg, “maps to”), because not all ontologies are developed with the same objective or possess a common level of granularity. This new property in the NCIt will provide specific target terminology data and metadata, while also providing a relationship that indicates synonymy, the direction of granularity, or a concept note indicating that there is no appropriate match between identified concepts.
Proof-of-Concept Pilot Testing
After completion of the initial draft AE terminology set, testing of the set was conducted using 2 existing, de-identified data sets. Data sets that are appropriate and relevant for testing are challenging to obtain and require both permission and resources. These examples should be viewed as illustrative and not as definitive.
Data sets were provided from both a single hospital readmission file and from a single randomized clinical trial. Terms and concepts from these data files were mapped to the AE terminology set. Gaps in the terminology set were identified, and new terms were developed and added accordingly.
The AE terminology underwent its first test case with a health care quality data set from Nationwide Children’s Hospital in Columbus, Ohio. The hospital participates in a quality control initiative to examine readmissions, excluding oncology patients, within 7 days of hospital inpatient discharge. In the readmission electronic medical record, signs and symptoms leading to a patient’s readmission were abstracted. After receiving institutional clearance, the PAETWG team received a de-identified data set based on 1889 unique admissions, comprising 3335 terms, 811 of which were unique. The terms were compared with the AE terminology database. On first pass, there was a 66% match.
To fill the identified gaps in the term set, new terms were generated for the AE terminology database, which added 150 new concepts for a total of 1373 concepts in AE version 2, plus 44 new synonyms.
Additionally, 3 new code lists from Clinical Data Interchange Standards Consortium were added to the pediatric terminology: anatomic locations, directionality, and laterality. The 811 unique terms were then compared with the revised AE terminology database (AE version 2), producing a 94% match. Six percent of the quality data set terms were excluded because they were either too vague (eg, “abnormal lab”) or anatomically inaccurate. Thus, with 1 review cycle and revision, the mapping was able to cover most circumstances.
The second test data set was provided by study NCT01941745, “Efficacy of Recombinant Human Clara Cell 10 Protein (rhCC10) Administered to Premature Neonates with Respiratory Distress Syndrome.” This was submitted by the Floating Hospital for Children at Tufts Medical Center. There were 226 abstracted terms in total, 68 of which were deemed unique. Eighty-one percent of the trial AEs were matched to the pediatric AE terminology, version 1. The AE terminology was then expanded to add 21 new concepts, for a total of 1394 concepts, which were included in AE version 2.
Overall, the initial pilot testing demonstrated that, of the concepts determined to be of sufficient specificity to be included in a terminology set, 76% were included in version 1 of the draft terminology set. Approximately 19% were considered to be gaps in this initial terminology set, and new terms were developed and added. Thus, ongoing review with use cases can improve the utility of the terminology set. A term suggestion page has been established to accommodate feedback and facilitate review.10
Value Proposition
Terminology harmonization enhances communication between researchers and practitioners, ensuring that data meet established standards. This terminology harmonization is a fundamental step toward enabling interoperability, which, based on the mapping and linkage between concepts, terms, and variables, allows for accurate information exchange between systems.11–13 It is this accurate exchange of information that facilitates data re-use, increases the value of the initial investment, and allows for new analyses, including meta-analyses, epidemiologic analyses, safety surveillance, quality improvement, and assessment of secular effects of general advances in healthcare. Additionally, data mapping to established, widely used, existing terminologies can lead to additional increases in research scope.
Advantages of the pediatric AE terminology include ease of adoption due to integration with well-established and internationally accepted biomedical terminologies, a uniquely temporal focus on pediatric health and disease from conception through adolescence, and terms that are intended for use in both well- and underresourced environments.
Where to Find and How to Use
The pediatric AE terminology is available for use without restriction through NICHD and NCI EVS and is compatible with, and represented in, MedDRA.14
The terminology is currently available for public access through the NCI term browser and the NCI ftp site.9,14 The pediatric AE terminology is provided in both Microsoft Excel and text formats. The entire NCIt is available in Web ontology language format, which can be loaded into Protégé, a public source ontology editor and framework product of Stanford University.15 Documentation, including a summary of the contents of the terminology, information about the terminology development, information about terminology application in support of research studies, and details regarding the process for updating the terminology, is provided on the NCI EVS homepage.16 Information specific to the pediatric AE terminology can be found on the pediatric terminology “About” page.14 An example of a term listing with subsets and definitions is provided in Table 2.
TABLE 2.
Example of a Term Listing From the NCI Thesaurus With Definitions and Codes
| NCIt Code | NCIt Preferred Term | Subset Preferred Term | Subset Synonym | NCIt Definition | Subset Definition | NCIt Code of Subset | PT of NICHD Subset |
|---|---|---|---|---|---|---|---|
| C99140 | Intraventricular hemorrhage of the newborn with ventricular dilatation | Intraventricular hemorrhage of the newborn with ventricular dilatation | Grade 3 intraventricular (nontraumatic) hemorrhage of fetus and newborn|grade 3 intraventricular hemorrhage of the newborn|intraventricular (nontraumatic) hemorrhage, grade 3, of fetus and newborn|intraventricular hemorrhage of newborn grade 3 | Bleeding into the lateral cerebral ventricles of a newborn infant with ventricular dilatation directly attributable to the acute bleeding. | Bleeding into the lateral cerebral ventricles of a newborn infant with ventricular dilatation directly attributable to the acute bleeding. | C98996 | Pediatric Adverse Events Terminology |
The pediatric AE terminology is dynamic and updates are expected as additional use cases are identified and scientific knowledge increases. A framework for maintaining and tracking change requests, and for labeling successive versions of the terminology, has been established. A listserv supported by the National Institutes of Health to notify members to both newly published material and to changes to existing terminology is available for public subscription.17 The NICHD listserv is titled: evs-nichd-terms-l. The pediatric AE terminology will continue to mature and improve with use, user feedback, and optimization based on application to additional use cases.
Supplementary Material
Acknowledgments
We thank R. McClead, R. Brilli, R. Comer, and C. Jones of Nationwide Children’s Hospital and J. M. Davis of Tufts University School of Medicine for providing de-identified use case data.
Glossary
- AE
adverse event
- CTCAE
Common Terminology Criteria for Adverse Events
- ICD
International Classification of Diseases
- ICH
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- MedDRA
Medical Dictionary for Regulatory Activities
- NCI
National Cancer Institute
- NCI EVS
National Cancer Institute’s Enterprise Vocabulary Services
- NCIt
National Cancer Institute Thesaurus
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- PAETWG
Pediatric Adverse Event Terminology Working Group
- SME
subject matter expert
- WHO
World Health Organization
Footnotes
Dr Gipson made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data, and she wrote the manuscript; Ms Herreshoff and Ms Quinn made substantial contributions to the acquisition of data and the analysis and interpretation of data, and they drafted the manuscript; Dr Hirschfeld made substantial contributions to conception and design and analysis and interpretation of data, and he co-wrote the manuscript; Drs Kirkendall, Gumbs-Petty, Steen, Hicks, McMahon, Nicholas, Zhao-Wong, Taylor-Zapata, Turner, Jones, Davis, and Ms Haber made substantial contributions to the acquisition of data and they critically reviewed and edited the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: This work was supported by National Children’s Study interagency agreement NC013003-001.
References
- 1.National Cancer Institute . Common terminology criteria for adverse events. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed August 11, 2016 [DOI] [PMC free article] [PubMed]
- 2.Kahn MG, Bailey LC, Forrest CB, Padula MA, Hirschfeld S. Building a common pediatric research terminology for accelerating child health research. Pediatrics. 2014;133(3):516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matlow AG, Baker GR, Flintoft V, et al. Adverse events among children in Canadian hospitals: the Canadian Paediatric Adverse Events Study. CMAJ. 2012;184(13):E709–E718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tönshoff B. Review of: Ethical conduct of clinical research involving children. Marilyn J. Field and Richard E. Behrman for the Committee on Clinical Research Involving Children of the Institute of Medicine, The National Academies Press, Washington, DC, USA, 2004, 425 pp., USD 47.95, ISBN 0-309-09181-0. Qual Assur J. 2005;9(2):146. [PubMed] [Google Scholar]
- 5.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120 [DOI] [PubMed] [Google Scholar]
- 6.McPhillips HA, Stille CJ, Smith D, et al. Potential medication dosing errors in outpatient pediatrics. J Pediatr. 2005;147(6):761–767 [DOI] [PubMed] [Google Scholar]
- 7.Sioutos N, de Coronado S, Haber MW, Hartel FW, Shaiu WL, Wright LW. NCI Thesaurus: a semantic model integrating cancer-related clinical and molecular information. J Biomed Inform. 2007;40(1):30–43 [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute . NCI thesaurus. Available at: https://ncit.nci.nih.gov/ncitbrowser/. Accessed March 31, 2016
- 9.National Cancer Institute Enterprise Vocabulary Services . NCI term browser. Available at: https://nciterms.nci.nih.gov/. Accessed March 31, 2016
- 10.National Cancer Institute Enterprise Vocabulary Services . Term suggestion page. Available at: https://ncitermform.nci.nih.gov/ncitermform/?dictionary=NCI%20Thesaurus. Accessed March 31, 2016
- 11.Mead CN. Data interchange standards in healthcare IT--computable semantic interoperability: now possible but still difficult, do we really need a better mousetrap? J Healthc Inf Manag. 2006;20(1):71–78 [PubMed] [Google Scholar]
- 12.Ohmann C, Kuchinke W. Future developments of medical informatics from the viewpoint of networked clinical research. Interoperability and integration. Methods Inf Med. 2009;48(1):45–54 [PubMed] [Google Scholar]
- 13.Richesson RL, Nadkarni P. Data standards for clinical research data collection forms: current status and challenges. J Am Med Inform Assoc. 2011;18(3):341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute Enterprise Vocabulary Services . Pediatric terminology files. Available at: https://evs.nci.nih.gov/ftp1/NICHD/About.html Accessed March 31, 2016
- 15.Stanford Center for Biomedical Informatics Research . Protege. Available at: http://protege.stanford.edu/. Accessed March 31, 2016
- 16.National Cancer Institute Enterprise Vocabulary Services . Welcome to EVS. Available at: https://evs.nci.nih.gov/. Accessed March 31, 2016
- 17.National Cancer Institute Enterprise Vocabulary Services . Welcome to NIH listserv. Available at: https://list.nih.gov. Accessed March 31, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.