Abstract
Objectives
Persistent variation in practice surrounds preoperative clopidogrel management at the time of vascular surgery. While some surgeons preferentially discontinue clopidogrel citing a perceived risk of perioperative bleeding, others will proceed with surgery in patients taking clopidogrel for an appropriate indication. The purpose of this study was to determine whether preoperative clopidogrel use was associated with significant bleeding complications during peripheral arterial surgery.
Methods
We reviewed a prospective regional vascular surgery registry recorded by 66 surgeons from 15 centers in New England from 2003 to 2009. Preoperative clopidogrel use within 48 hours of surgery was analyzed among patients undergoing carotid endarterectomy (CEA), lower extremity bypass (LEB), endovascular abdominal aortic aneurysm repair (EVAR), and open abdominal aortic aneurysm repair (oAAA). Ruptured AAAs were excluded. Endpoints included postoperative bleeding requiring reoperation, as well as the incidence and volume of blood transfusion. Statistical analysis was performed using analysis of variance, Fisher exact, χ2, and Wilcoxon rank-sum tests.
Results
Over the study interval, a total of 10,406 patients underwent surgery, including 5264 CEA, 2883 LEB, 1125 EVAR, and 1134 oAAA repair. Antiplatelet use among all patients varied, with 19% (n = 2010) taking no antiplatelet agents, 69% (n = 7132) taking aspirin (ASA) alone, 2.2% (n = 229) taking clopidogrel alone, and 9.7% (n = 1017) taking both ASA and clopidogrel. Clopidogrel alone or as dual antiplatelet therapy was most frequently used prior to CEA and least frequently prior to oAAA group (CEA 16.1%, LEB 9.0%, EVAR 6.5%, oAAA 5%). Reoperation for bleeding was not significantly different among patients based on antiplatelet regimen (none 1.5%, ASA 1.3%, clopidogrel 0.9%, ASA/clopidogrel 1.5%, P = .74). When analyzed by operation type, no difference in reoperation for bleeding was seen across antiplatelet regimens. There was also no difference in the incidence of transfusion among antiplatelet treatment groups (none 18%, ASA 17%, clopidogrel 0%, ASA/clopidogrel 24%, P = .1) and none when analyzed by individual operation type. Among patients who did require transfusion, there was no significant difference in the mean number of units of packed red blood cells required (none 0.7 units, ASA 0.5 units, clopidogrel 0 units, ASA/clopidogrel 0.6 units, P = .1) or when stratified by operation type.
Conclusions
Patients undergoing peripheral arterial surgery in whom clopidogrel was continued either alone or as part of dual antiplatelet therapy did not have significant bleeding complications compared with patients taking no antiplatelet therapy or ASA alone at the time of surgery. These data suggest that clopidogrel can safely be continued preoperatively in patients with appropriate indications for its use, such as symptomatic carotid disease or recent drug-eluting coronary stents.
Since the advent and subsequent approval of clopidogrel (Plavix) by the Food and Drug Administration, its use has become increasingly prevalent in contemporary practice. Indications for clopidogrel therapy now include acute coronary syndrome, myocardial infarction, stroke, and peripheral vascular disease.1,2 Accordingly, clopidogrel now ranks among the most commonly prescribed prescription medications and accounts for more than 5 billion dollars in annual pharmaceutical-derived revenue.3
Because of its common use, vascular surgeons are frequently confronted with decision making around perioperative clopidogrel management. Several studies have documented increased postoperative bleeding potential and transfusion requirements among clopidogrel-treated patients undergoing cardiac surgery.1,4–7 Conversely, clopidogrel cessation within 12 months following drug-eluting coronary stent implantation has been associated with adverse sequelae, including stent thrombosis and ensuing myocardial infarction (MI).8–10 Interestingly, there are few studies documenting the safety profile of clopidogrel therapy at the time of carotid endarterectomy (CEA), even when performed in the context of symptomatic carotid occlusive disease.1 Moreover, such studies are small single-center experiences with limited sample size that are likely underpowered to detect differences in infrequent events, such as reoperation for bleeding.
In this setting, clinical debate persists surrounding the optimal preoperative management of clopidogrel therapy. Indeed, a recent published survey of European surgeons demonstrated widespread variation in preoperative clopidogrel management.11 While some surgeons favored clopidogrel cessation at the time of surgery citing a perceived increased risk of bleeding complications, others favored continued perioperative therapy to prevent thrombosis. The purpose of this study was to determine whether clopidogrel was associated with significant bleeding complications measured by reoperation for bleeding and transfusion requirement at the time of major peripheral arterial surgery.
METHODS
Subjects and database
This study reflects data collected in the prospectively compiled registry of the Vascular Study Group of New England (VSGNE).12 Over the study interval from 2003 to 2009, patients were treated at 15 academic and community centers by 66 participating surgeons. Trained clinical data abstractors, nurses, and surgeons entered data prospectively for over 70 clinical, operative, and demographic variables for each procedure. All analysts were blinded to patient, surgeon, and center identification.
Outcomes and variable definitions
Over the study interval, preoperative clopidogrel or aspirin use defined as being taken within 48 hours of surgery was recorded among all patients undergoing CEA, lower extremity bypass (LEB), endovascular aneurysm repair (EVAR), and open abdominal aortic aneurysm repair (oAAA) repair. Ruptured AAAs were excluded from further analysis. The primary endpoints of this study were postoperative bleeding requiring reoperation (to determine a detrimental effect of clopidogrel) and perioperative transfusion requirements, defined as transfusion at the time of surgery or within the index hospitalization. All endpoints were restricted to the hospitalization for the respective surgery. In addition, total transfusion requirements were assessed among clopidogrel-treated and untreated patients who required transfusion therapy. Perioperative transfusion is not recorded in the VSG registry for CEA, in light of its associated infrequent incidence.
Statistical analysis
Patient demographics were compared using χ2 analysis and t test for categorical and continuous variables, respectively. Reoperations for bleeding and ongoing transfusion requirement were assessed among patients using analysis of variance (ANOVA) and χ2 (Fisher exact correction). Wilcoxon rank-sum test was performed to compare transfusion burden among patients who received perioperative blood transfusion. The use of deidentified data for this analysis was approved by the Institutional Review Board of Dartmouth Medical School.
RESULTS
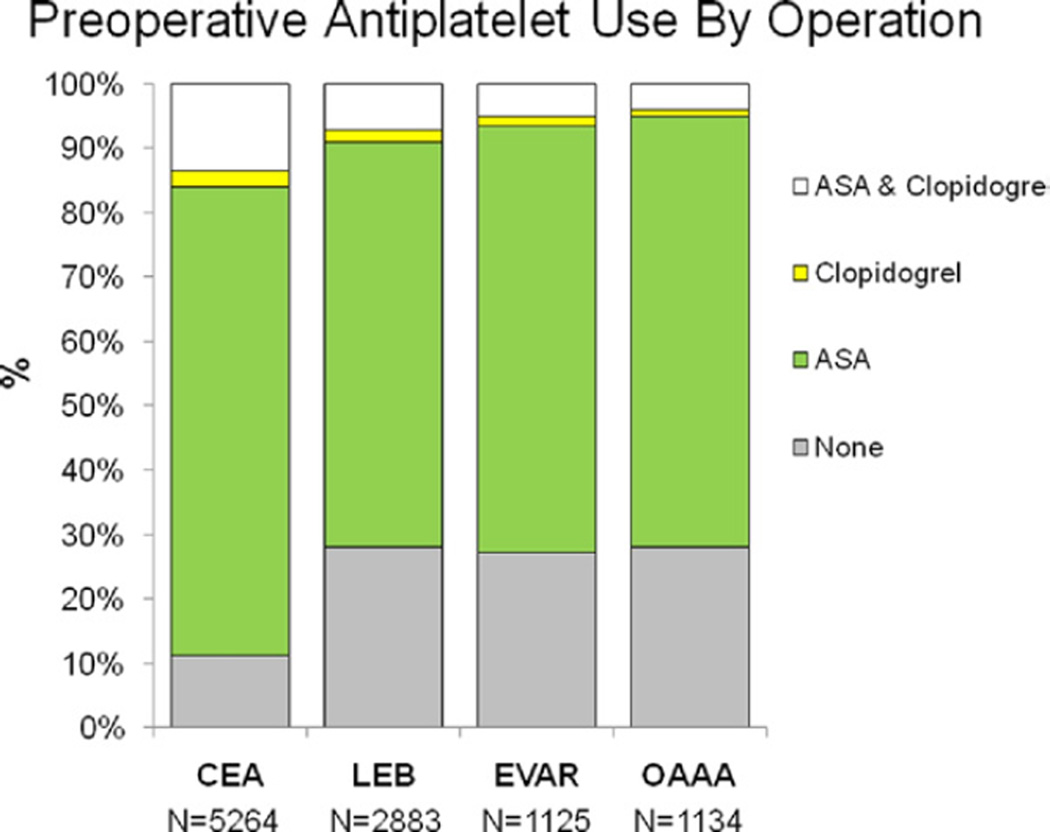
Over the study interval, a total of 10,406 patients were identified who underwent CEA (5264), LEB (2883), EVAR (1125), and oAAA repair (1134). Preoperative antiplatelet agent use among all patients varied with 19.3% (n = 2010) taking no antiplatelet agents, 68.5% (n = 7132) taking aspirin (ASA) alone, 2.2% (n = 229) taking clopidogrel alone, and 9.7% (n = 1017) taking clopidogrel and ASA. Patients most likely to be on preoperative clopidogrel (alone or as dual antiplatelet therapy) were those undergoing CEA, while this was least likely in the oAAA group (CEA 16.1%, LEB 9.0%, EVAR 6.5%, oAAA 5%) (Fig 1). Indications for clopidogrel use were unknown. Demographic details and comorbidities of all patients stratified by antiplatelet regimen are depicted in the Table.
Fig 1.
Antiplatelet use among patients undergoing carotid endarterectomy (CEA), lower extremity bypass (LEB), endovascular abdominal aortic aneurysm repair (EVAR), and open AAA repair (oAAA). As depicted, clopidogrel use, either alone or as part of dual antiplatelet therapy was most common among patients undergoing CEA and least for oAAA. Transfusion data were not recorded for patients undergoing CEA given its clinical infrequent incidence.
Table.
Comparison of patient demographics, comorbidities, and preoperative risk factors among antiplatelet treatment groups
| No antiplatelet agent n/total (%) |
ASA only n/total (%) | Clopidogrel only n/total (%) |
ASA and clopidogrel n/total (%) |
P value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Male gender | 1353/2013 (67) | 4720/7137 (66) | 141/229 (62) | 681/1016 (67) | .338 |
| White race | 1988/2011 (99) | 7053/7128 (99) | 224/228 (98) | 1004/1014 (99) | .757 |
| Age | |||||
| Less than 40 years | 9/2013 (0.5) | 10/7139 (0.1) | 0/229 (0) | 1/1017 (0.1) | <.001 |
| Age 40–49 | 84/2013 (4) | 180/7139 (3) | 12/229 (5) | 30/1017 (3) | |
| Age 50–59 | 282/2013 (14) | 947/7139 (13) | 27/229 (12) | 163/1017 (16) | |
| Age 60–69 | 535/2013 (27) | 2205/7139 (31) | 59/229 (26) | 309/1017 (30) | |
| Age 70–79 | 733/2013 (36) | 2682/7139 (38) | 89/229 (39) | 370/1017 (36) | |
| Age 80–89 | 349/2013 (17) | 1069/7139 (15) | 40/229 (17) | 139/1017 (14) | |
| Age 90 or greater | 21/2013 (1) | 46/7139 (0.6) | 2/229 (0.9) | 5/1017 (0.5) | |
| Smoking (prior or current) |
1666/2010 (83) | 5954/7130 (84) | 187/229 (82) | 829/1012 (82) | .532 |
| Hypertension | 1622/2013 (81) | 6170/7134 (86) | 199/229 (87) | 921/1017 (91) | <.001 |
| Diabetes | 654/2013 (32) | 2341/7138 (33) | 89/229 (39) | 378/1017 (37) | .010 |
| Beta blockers | 1534/2011 (76) | 6060/7135 (85) | 181/229 (79) | 896/1017 (88) | <.001 |
| Coronary disease | 528/2012 (26) | 2528/7135 (35) | 84/229 (37) | 509/1015 (50) | <.001 |
| CABG/PTCA | 417/2012 (21) | 2305/7136 (32) | 76/228 (33) | 485/1017 (48) | <.001 |
| Congestive heart failure |
209/2012 (10) | 721/7137 (10) | 22/229 (10) | 130/1017 (13) | .071 |
| COPD | 640/2013 (32) | 1953/7137 (27) | 66/229 (29) | 305/1016 (30) | <.001 |
| Dialysis | 89/2013 (4) | 161/7138 (2) | 6/229 (3) | 22/1017 (2) | <.001 |
| Creatinine ≥1.8% | 201/1941 (10) | 585/6959 (8) | 25/223 (11) | 75/988 (8) | .014 |
| Stress test | |||||
| Not done | 1188/1994 (60) | 3959/7088 (56) | 135/228 (59) | 627/1008 (62) | <.001 |
| Normal result | 607/1994 (30) | 2171/7088 (31) | 72/228 (32) | 225/1008 (22) | |
| Abnormal result | 199/1994 (10) | 958/7088 (14) | 21/228 (9) | 156/1008 (15) | |
| Statin | 979/2013 (49) | 5087/7136 (71) | 153/229 (67) | 810/1016 (79) | <.001 |
ASA, Aspirin; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PTCA, percutaneous transluminal coronary angioplasty.
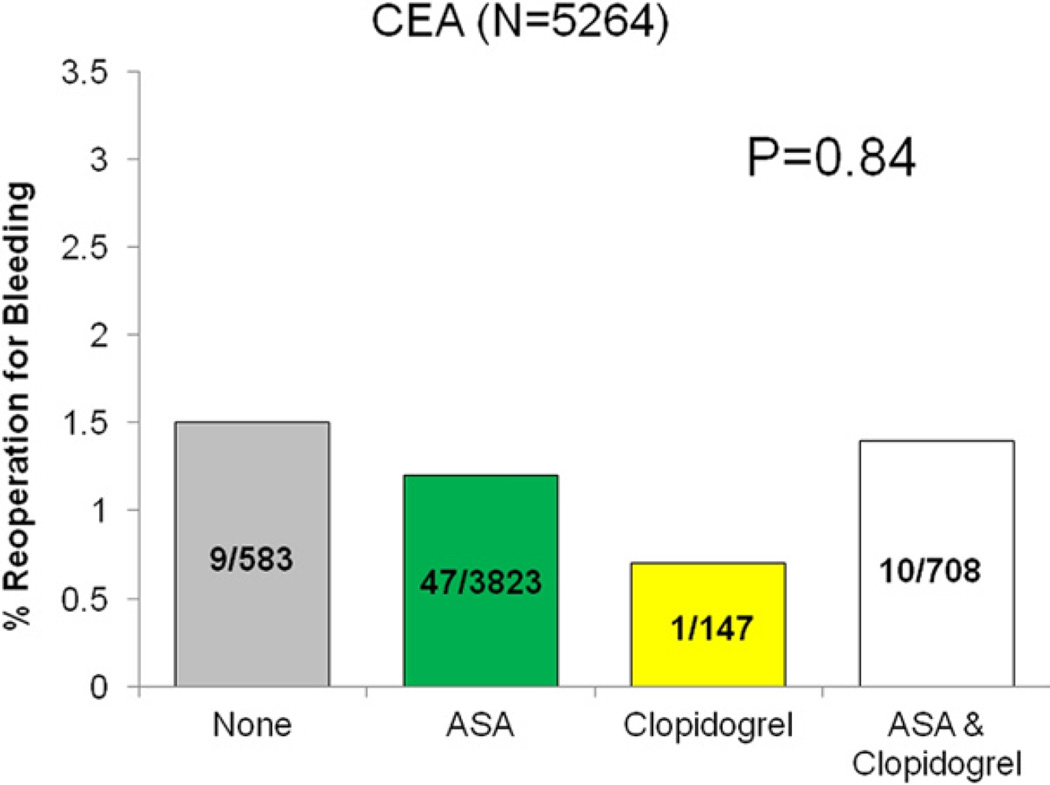
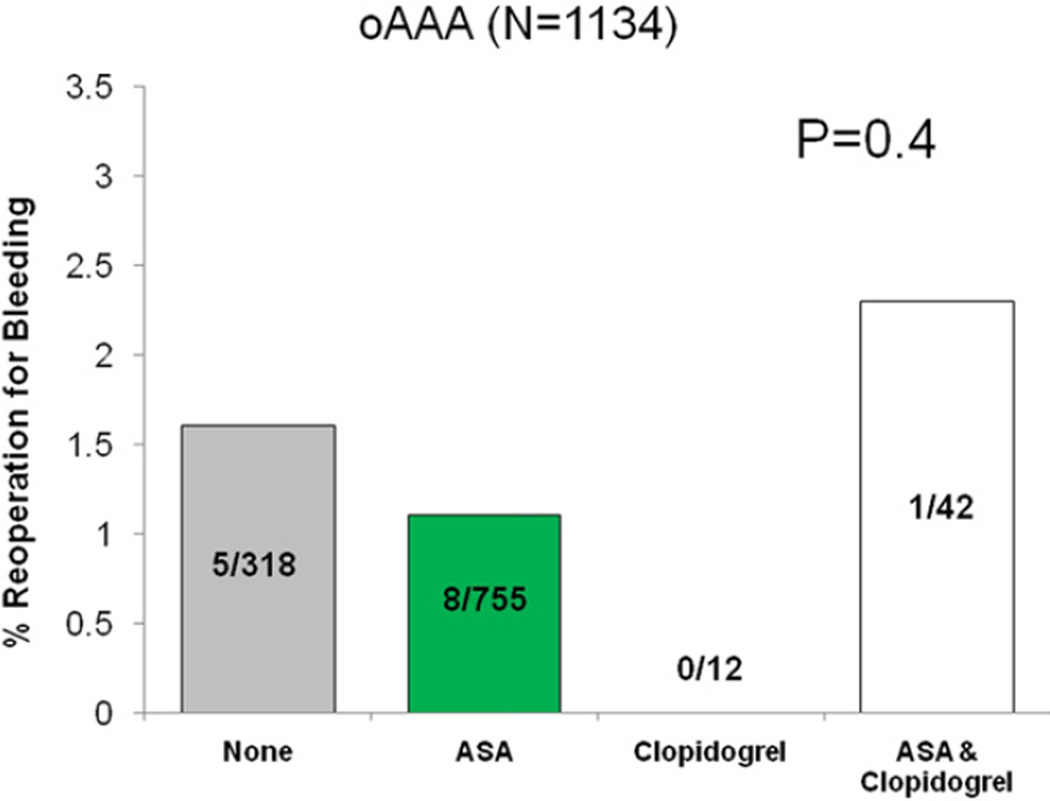
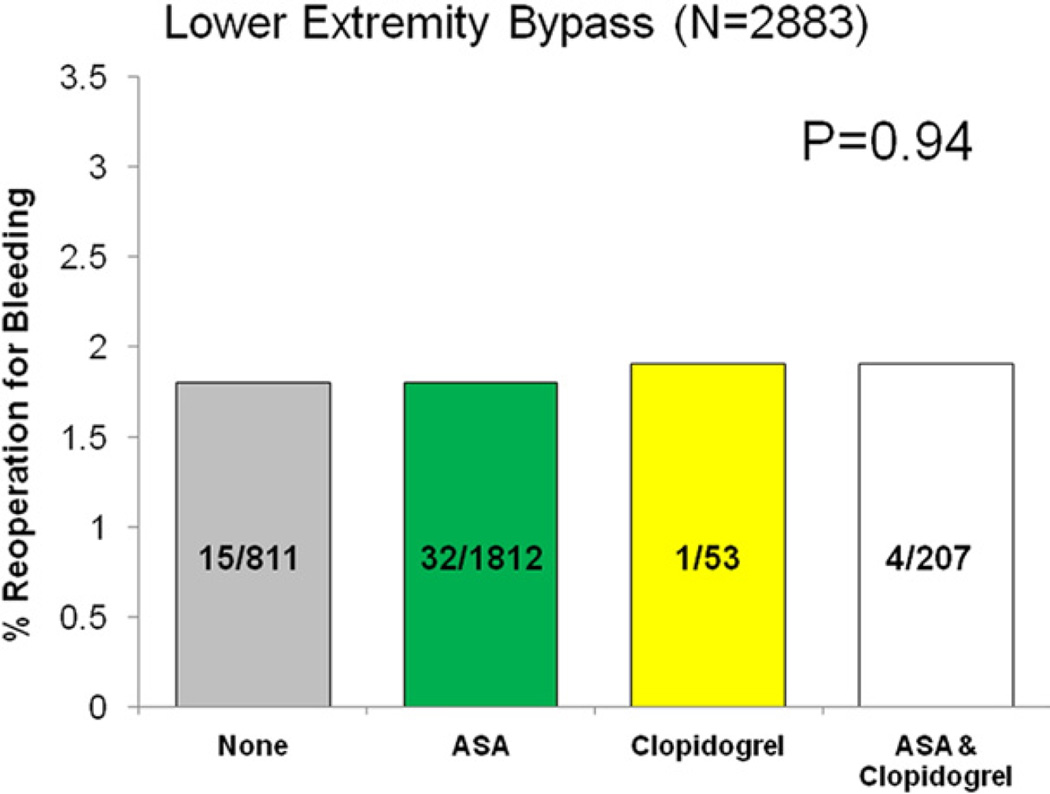
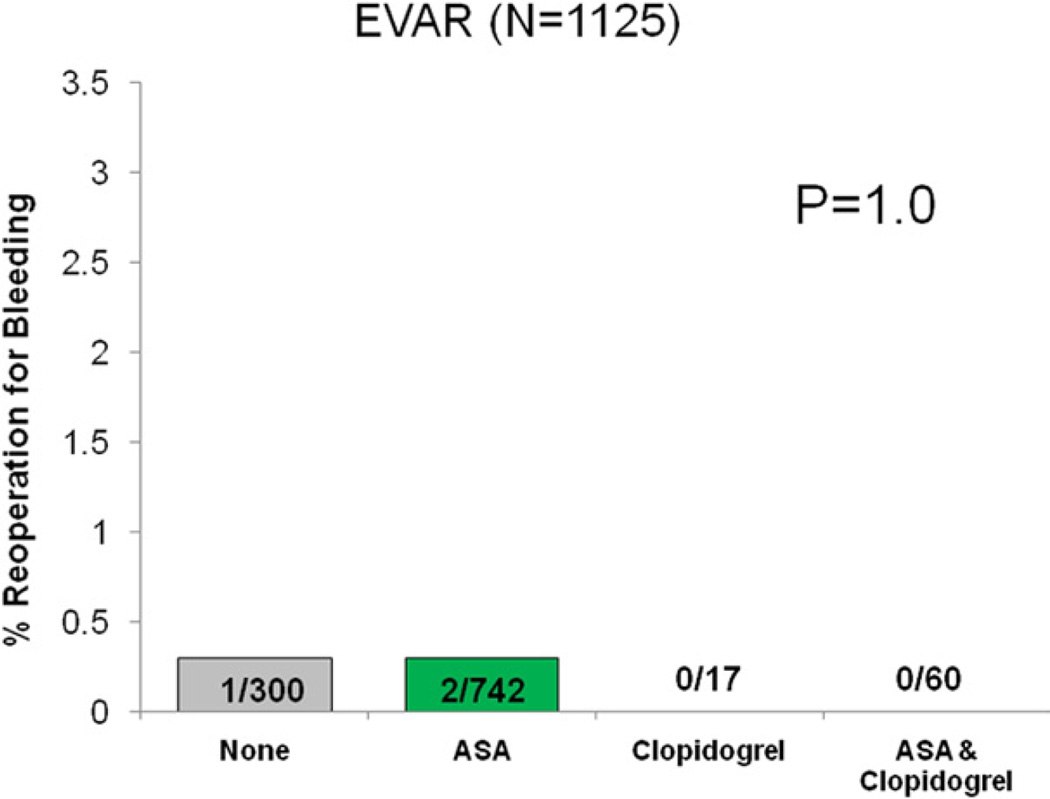
Reoperation for bleeding was not significantly different among patients based on antiplatelet regimen (none 1.5%, ASA 1.3%, clopidogrel 0.9%, ASA/clopidogrel 1.5%, P = .74). When analyzed by operation type, no difference in reoperation for bleeding was seen across each respective antiplatelet regimen (Figs 2–5).
Fig 2.
Reoperation for bleeding among patients undergoing carotid endarterectomy (CEA), stratified by antiplatelet medication.
Fig 5.
Reoperation for bleeding among patients undergoing open abdominal aortic aneurysm repair (oAAA), stratified by antiplatelet medication.
There was also no difference in the incidence of transfusion among antiplatelet treatment groups (none 18%, ASA 17%, clopidogrel 0%, ASA/clopidogrel 24%, P = .1). This finding was also observed when patients were stratified by operation for each antiplatelet regimen (LEB: none 18.8%, ASA 17.3%, clopidogrel 0%, ASA/clopidogrel 25.3%, P = .12, EVAR: none 9.6%, ASA 9.6%, clopidogrel 0%, ASA/clopidogrel 16%, P = .66, oAAA: none 37.8%, ASA 27.9%, clopidogrel 0%, ASA/clopidogrel 42.8%, P = .37).
Among patients who did require transfusion, there was no significant difference in the mean number of units of packed red blood cells required among the total study cohort (none 0.7 units, ASA 0.5 units, clopidogrel 0 units, ASA/clopidogrel 0.6 units, P = .1). When further analyzed by operation type, there was no statistical difference in the mean number of units required among antiplatelet regimens for each operation type (LEB: none 0.6 units, ASA 0.5 units, clopidogrel 0 units, ASA/clopidogrel 0.6 units, P = .14, EVAR: none 0.3 units, ASA 0.2 units, clopidogrel 0 units, ASA/clopidogrel 0.2 units, P = .7, oAAA: none 1.4 units, ASA 0.8 units, clopidogrel NA, ASA/clopidogrel 2.0 units, P = .18).
Estimated blood loss was recorded for patients undergoing EVAR and oAAA repair, and this showed no significant differences among antiplatelet treatment regimens for these procedure types (EVAR: none 317 mL, ASA 322 mL, clopidogrel 585 mL, ASA/clopidogrel 282 mL, P = .66; oAAA: none 1394 mL, ASA 1456 mL, clopidogrel 1955 mL, ASA/clopidogrel 1817 mL, P = .62.
DISCUSSION
This multi-institutional regional registry provides evidence that preoperative clopidogrel, when taken alone or as part of dual antiplatelet therapy, is not associated with increased serious bleeding complications in a diverse group of peripheral vascular operations. These findings have significant implications for contemporary practice because surgeons are now confronted with a growing percentage of patients with extensive comorbidities such as symptomatic carotid occlusive disease and coronary disease treated with drug-eluting stents, who have an appropriate indication for clopidogrel therapy.1,13,14
Based often on anecdotal experience, some surgeons favor preoperative clopidogrel cessation, perceiving an increased risk of bleeding complications, while others continue clopidogrel to avoid perioperative thrombotic complications, and this may vary by the type of planned operation. A recent survey of 650 European vascular surgeons by Hamish et al depicts this variation in clinical practice among patients undergoing CEA.11 Specifically, the authors report 43% and 55% of surgeons, respectively, would stop clopidogrel among symptomatic and asymptomatic patients undergoing CEA. Moreover, 49% and 57% of surveyed surgeons would favor clopidogrel cessation more than 7 days prior to CEA, for both symptomatic and asymptomatic patients.11 This demonstrates a clear lack of consensus among practicing surgeons regarding optimal perioperative clopidogrel management even for patients with a strong indication, such as those with symptomatic carotid disease undergoing CEA. Presumably for operations with larger potential blood loss, such as oAAA repair or LEB, the percentage of surgeons who would stop clopidogrel treatment would be even higher.
In addition, the recent Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial, demonstrated that patients undergoing below-knee bypass showed no significant difference in severe bleeding complications between clopidogrel vs placebo-treated groups.15 Interestingly, however, clopidogrel therapy was initiated following a postoperative lag period of 2 days to permit “complete” hemostasis, again, presumably reflecting a perceived increased risk of bleeding associated with preoperative clopidogrel use. It should be highlighted that the number of severe bleeding events were small in this trial, (n = 9/426 [2.1%] clopidogrel vs n = 5/422 [1.2%] placebo, P = NS) potentially limiting definitive conclusions. Severe bleeding was defined as per the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) classification, which included intracranial hemorrhage, and hemodynamic compromise.16 In contrast, both mild (no transfusion) (n = 46/426 [10.8%] clopidogrel vs n = 21/422 [5.0%] placebo, P = .002) and moderate bleeding complications (transfusion but no hemodynamic compromise) (n = 16/426 [3.8%] clopidogrel vs n = 4/422 [0.9%] placebo, P = .007) were observed more commonly in the clopidogrel-treated cohort compared with placebo, still leaving some degree of ambiguity pertaining to this important question.15
Studies of bleeding complications with clopidogrel at the time of surgery have largely been single-center retrospective analyses with relatively small sample size focusing on coronary artery bypass grafting (CABG) rather than peripheral vascular surgery.4,5,17 In this regard, these studies may be underpowered to detect a relatively low frequency complication, and accordingly, remain limited in their ability to reach definitive conclusions.
Some opinion favoring preoperative clopidogrel cessation in vascular surgery appears to be extrapolated from studies documenting the impact of clopidogrel therapy at the time of CABG. Indeed, Filsoufi et al reported both increased transfusion requirements and all-cause mortality among 72 clopidogrel-treated patients undergoing CABG.5 In concordance with these findings, Chu et al demonstrated chest tube blood loss was significantly greater in 312 clopidogrel-treated patients undergoing urgent or emergent CABG. Moreover, mean total blood product transfusions were also significantly higher among clopidogrel-treated patients, as was the incidence of reoperation for bleeding.4 In contrast, Karabulut et al found no significant differences in the incidence of reoperation for bleeding, blood product transfusion, chest tube output, or intensive care length of stay among 1628 patients undergoing CABG.17 Despite this larger overall sample size, only 48 patients constituted the treatment group on clopidogrel, which may have impacted their conclusions.
Although there have been few studies of bleeding complications associated with clopidogrel use in peripheral arterial surgery, Fleming et al reported that clopidogrel-treated patients could safely undergo CEA with no significant difference in hematoma formation or blood loss between treated and untreated patients. However, the authors’ conclusions reflect a sample size of 100 patients, in whom only 19 had received preoperative clopidogrel treatment.1
Our study, by comparison, demonstrates that preoperative clopidogrel therapy is not associated with increased bleeding complications measured by reoperation for bleeding and blood transfusion across a diverse spectrum of vascular surgical operations, including CEA, for which it is most robustly powered, LEB, EVAR, and open aneurysm repair (oAAA). Moreover, we demonstrate that among patients who do require blood transfusion, there was no difference in transfusion volume. These findings suggest that surgeons can achieve sufficient hemostasis in clopidogrel-treated patients during CEA, LEB, EVAR, and oAAA. Moreover, these data would support the conclusion that clopidogrel cessation prior to surgery is unnecessary when managing patients with important indications for clopidogrel use such as symptomatic carotid occlusive disease or a previously placed drug-eluting coronary stent.
This observational study has several intrinsic limitations. First, it is not a randomized comparison of clopidogrel-treated vs untreated patients. Patients were treated and managed at the discretion of the operating surgeon. In this regard, we are unable to comment on the number of patients who may have had prior clopidogrel withheld prior to surgery. It is also possible that a subset of patients had their antiplatelet medication discontinued within the half-life of the medication, yet >48 hours prior to surgery leading to unaccounted-for persistent platelet dysfunction. However, one can speculate that those surgeons, who would favor discontinuation of perioperative clopidogrel prior to surgery, would do so with an adequate window to minimize this effect. Finally, although concomitant use of perioperative anticoagulation use among patients undergoing these types of operations is not common, this is not captured in the VSGNE registry, which could impact the primary endpoint. Despite not being a randomized trial, this study is more robustly powered to detect differences in low-frequency complications in a “real world” clinical experience reflecting both community and academic surgeons.
A second limitation is that the percentage of patients taking clopidogrel either alone or in combination therapy was low, especially in the bypass, EVAR, and oAAA groups. While this may limit our ability to reach definitive conclusions, it still represents more than a fourfold larger experience of perioperative clopidogrel use than previously reported. It should also be noted that the VSGNE registry does not record hematoma formation or surgical drain use, which may have disclosed differences in outcomes. Finally, only 4.7% (n = 54) of patients undergoing oAAA received preoperative clopidogrel treatment at the time of surgery. Consequently, we do not believe that conclusions regarding clopidogrel use at the time of oAAA should be drawn based on our experience. Accordingly, based on sample size, results reported herein are more robust for CEA, LEB, and EVAR, and least for oAAA repair, given the potential for type II error in the latter group.
CONCLUSIONS
Based on our analysis of more than 10,000 patients undergoing a spectrum of major peripheral arterial operations, clopidogrel, either alone or as part of a dual antiplatelet regimen, does not appear to be associated with serious adverse bleeding complications, measured by reoperation for bleeding or incidence of blood transfusion. Moreover, there was no difference in transfusion volume among those patients who did require transfusion. These data suggest that clopidogrel can be safely continued preoperatively in patients with important indications for its use, such as symptomatic carotid disease or recent drug-eluting cardiac stents.
Fig 3.
Reoperation for bleeding among patients undergoing carotid endarterectomy (LEB), stratified by antiplatelet medication.
Fig 4.
Reoperation for bleeding among patients undergoing endovascular abdominal aortic aneurysm repair (EVAR), stratified by antiplatelet medication.
Footnotes
Competition of interest: none.
Presented at the Twenty-fourth Annual Meeting of the Eastern Vascular Society, New York, NY, September 2010.
AUTHOR CONTRIBUTIONS
Conception and design: DS, JC, PG
Analysis and interpretation: DS, PG, JC
Data collection: DS, PG
Writing the article: DS, DW, JC
Critical revision of the article: DS, PG, AS, BN, DL, WT, JA, DW, JC
Final approval of the article: DS, JC
Statistical analysis: PG, BN
Obtained funding: JC
Overall responsibility: DS
REFERENCES
- 1.Fleming MD, Stone WM, Scott P, Chapital AB, Fowl RJ, Money SR. Safety of carotid endarterectomy in patients concurrently on clopidogrel. Ann Vasc Surg. 2009;23:612–615. doi: 10.1016/j.avsg.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Plavix® [Package insert] Bridgewater, NJ: Bristol-Myers Squibb; 2007. [Google Scholar]
- 3.Berenson A. New York Times. Business; 2007. [11/5/07]. Mixed results for would-be Rival to Plavix. [Google Scholar]
- 4.Chu MW, Wilson SR, Novick RJ, Stitt LW, Quantz MA. Does clopidogrel increase blood loss following coronary artery bypass surgery? Ann Thorac Surg. 2004;78:1536–1541. doi: 10.1016/j.athoracsur.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Filsoufi F, Rahmanian PB, Castillo JG, Kahn RA, Fischer G, Adams DH. Clopidogrel treatment before coronary artery bypass graft surgery increases postoperative morbidity and blood product requirements. J Cadiothorac Vasc Anesth. 2008;22:60–66. doi: 10.1053/j.jvca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Hongo RH, Ley J, Dick SE, Yee RR. The effect of clopidogrel in combination with aspirin when given before coronary artery bypass grafting. J Am Col Cardiol. 2002;40:231–237. doi: 10.1016/s0735-1097(02)01954-x. [DOI] [PubMed] [Google Scholar]
- 7.Yende S, Wunderink RG. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Crit Care Med. 2001;29:2271–2275. doi: 10.1097/00003246-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. Guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/non-ST-Elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Col Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Payne DA, Jones CI, Hayes PD, Thompson MM, London NJ, Bell PR, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation. 2004;109:1476–1481. doi: 10.1161/01.CIR.0000121739.05643.E6. [DOI] [PubMed] [Google Scholar]
- 10.Ho PM, Peterson ED, Wang L, Magid DJ, Fihn SD, Larsen GC, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. 2008;299:532–539. doi: 10.1001/jama.299.5.532. [DOI] [PubMed] [Google Scholar]
- 11.Hamish M, Gohel MS, Shepherd A, Howes NJ, Davies AH. Variations in the pharmacological management of patients treated with carotid endarterectomy: a survey of European vascular surgeons. Eur J Vasc Endovasc Surg. 2009;38:402–407. doi: 10.1016/j.ejvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–1101. doi: 10.1016/j.jvs.2007.08.012. discussion 1101-092. [DOI] [PubMed] [Google Scholar]
- 13.King A, Bath PM, Markus HS. Clopidogrel versus dipyridamole in addition to aspirin in reducing embolization detected with ambulatory transcranial Doppler: a randomized trial. Stroke. 2011;42:650–655. doi: 10.1161/STROKEAHA.110.601807. [DOI] [PubMed] [Google Scholar]
- 14.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 15.Belch JJ, Dormandy J, Biasi BM, Cairols M, Diehm C, Eikelboom B, et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CAS-PAR) trial. J Vasc Surg. 2010;52:825–833. 833.e1–833.e2. doi: 10.1016/j.jvs.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 16.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 17.Karabulut H, Toraman F, Evrenkaya S, Goksel O, Tarcan S, Alhan C, et al. Clopidogrel does not increase bleeding and allogenic blood transfusion in coronary artery surgery. Eur J Cardiothorac Surg. 2004;25:419–423. doi: 10.1016/j.ejcts.2003.11.037. [DOI] [PubMed] [Google Scholar]