Abstract
Introduction
Several reports suggest unexpectedly high rates of late abdominal aortic aneurysm (AAA) rupture occur after endovascular AAA repair (EVAR). However, a population-based study examining causes of late death after EVAR vs open surgical repair has not been performed.
Methods
We performed a retrospective cohort study of patients undergoing infrarenal AAA repair using information from the Medicare inpatient hospital discharge records (MedPAR files), physician claim files (Part B files, 20% sample), and Medicare Denominator Files for the years 2001 to 2004. Using the Social Security Death Index, we identified all “late” deaths, defined as deaths occurring >30 days and after hospital discharge. We used the National Death Index to identify cause of death information; in particular, those deaths that were likely caused by late rupture. We compared causes of late death and survival between EVAR and open repair using Wilcoxon log-rank and rank-sum tests.
Results
Between 2001 and 2004, 13,971 patients underwent AAA repair (6119 EVAR, 7852 open repair). After a mean follow-up of 1.6 years in the EVAR cohort and 1.9 years in the open cohort, mortality rates were similar across repair type (15.4% EVAR, 15.9% open repair), with an adjusted odds ratio for death after open repair of 0.98 (95% confidence interval, 0.90–1.07). Of the 2194 documented deaths, 523 occurred before discharge or ≤30 days, and 1671 occurred >30 days and after hospital discharge. Cause of death information for the 1671 late deaths was available from the National Death Index for 1515 (91%). The 15 most common codes for causes of late death were dominated by cardiac disease (atherosclerotic heart disease, acute myocardial infarction) and pulmonary disease (lung cancer, respiratory failure). Causes of late death with specific mention of aneurysm were identified in 37 patients (2.4% of all deaths), but this event was not more common in EVAR or open repair (15 [0.3%] in the EVAR group, 22 [0.3%], in the open repair group; P=.71).
Conclusions
Late deaths from aneurysm rupture after EVAR or open repair appear to be relatively infrequent and similarly distributed across procedure type. Our results emphasize that the effectiveness of EVAR is comparable to open AAA repair in preventing aneurysm-related death.
Several controlled trials and national cohort studies have demonstrated lower perioperative morbidity and mortality with endovascular abdominal aortic aneurysm (AAA) repair (EVAR) compared with open repair.1–6 However, regardless of the technique of repair, operative treatment of an AAA does not completely ensure that a patient will remain free from aneurysm-related morbidity or mortality for the rest of his or her life.7–9 For example, patients who undergo open repair can experience graft-related complications, such as the development of a pseudoaneurysm or latent graft infection.8
Similarly, along with significant device costs4,10 and the need for extended follow-up and serial imaging,11,12 patients who undergo EVAR often have endoleaks, which occur when the stent graft fails to exclude blood flow from the aneurysm sac.12–14 Although pseudoaneurysm or infection after open repair is relatively uncommon, endoleaks occur in 5% to 10% of all EVARs and have been associated with late rupture of AAAs in several reports.1,15–17 The relative frequency of EVAR-related complications, such as endoleaks, and lack of survival difference between patients undergoing EVAR and open repair have caused many to question if the potential benefits of the less-invasive procedure are outweighed by excess late deaths caused by delayed procedural complications.16–18
To further characterize late deaths occurring after EVAR and open AAA repair, we therefore examined patients undergoing infrarenal AAA repair in the Medicare population and compared short-term (combined in-hospital and 30 day) and long-term mortality rates with EVAR and open repair. Then, using death records obtained from the National Death Index (NDI), we compared causes of late death after EVAR and open repair for the years 2001 to 2004, including deaths due to late aneurysm rupture.
METHODS
The Institutional Review Board at Dartmouth Medical School reviewed and approved our study protocol.
Databases and exclusion criteria
We studied all patients undergoing infrarenal AAA repair in the Medicare population for this analysis. Using the Medicare Part A (MedPAR) files, a 20% random sample of physician claim files (Part B), and the Medicare Denominator Files for the years 2001 to 2004, we examined occurrences of AAA repair codes (Appendix A, online only).19 These records each contained a patient identifier, a Current Procedural Terminology (CPT) procedure code and procedure date, and up to 10 diagnosis codes from the International Classification of Diseases (ICD), 9th Clinical Modification.
Our unit of analysis in this study was the patient. We used CPT codes (Appendices A and B, online only) to identify the procedure as open or endovascular. If a patient had more than one AAA procedure code, we counted only the initial event and assigned the patient to that type of repair (EVAR or open repair). We assumed that the first procedure was endovascular if both an open and an endovascular procedure occurred in the same patient on the same day.
We excluded suprarenal repairs, any event with a procedure code that indicated repair of a ruptured aortic aneurysm, and any event with a procedure code for a thoracic aortic aneurysm repair. We excluded claims that did not contain a diagnosis code for nonruptured AAA and those patients whose repairs had a concurrent diagnosis code of AAA rupture. Lastly, we excluded patients eligible for Medicare for reasons other than age (eg, disability), managed care enrollees, and patients not assigned to Medicare Part A and Part B at the time of the index procedure. We then used the CPT codes, shown in Appendix A (online only), for each event and characterized the procedure as open or endovascular.
Determination of patient characteristics
Next, we used the MedPAR file (Part A file) and the denominator file to ascertain demographic characteristics of each patient (date of birth, sex, race, and date of death) and patient-level comorbidities. Comorbidities were identified according to the Dartmouth-Manitoba claims-based modification of the Charlson comorbidity index,20,21 Using the comorbidities listed in Table I, we evaluated for the presence of cardiac or cerebrovascular disease, diabetes, pulmonary disease, cancer, renal insufficiency, dementia, and immunologic disease.
Table I.
Patient characteristics of the endovascular and open repair cohorts
| Variable |
EVAR (n = 6119) No. (%) |
Open repair (n = 7852) No. (%) |
P value |
|---|---|---|---|
| Age | |||
| 65–69 | 847 (13.8) | 1378 (17.6) | <.0001 |
| 70–74 | 1556 (25.4) | 2315 (29.5) | |
| 75–79 | 1792 (29.3) | 2361 (30.1) | |
| 80–84 | 1317 (21.5) | 1385 (17.6) | |
| ≥85 | 607 (9.9) | 413 (5.3) | |
| Male | 5110 (83.5) | 6016 (76.6) | <.0001 |
| Race | |||
| White | 5859 (95.8) | 7496 (95.5) | .18 |
| Black | 158 (2.6) | 192 (2.5) | |
| Other/unknown | 102 (1.7) | 2164 (2.1) | |
| Comorbidities | |||
| Vascular disease | 6119 (100.0) | 7852 (100.0) | NA |
| Myocardial infarction | 1002 (16.4) | 980 (12.5) | <.0001 |
| Congestive heart failure | 351 (5.7) | 284 (3.6) | <.0001 |
| Cerebrovascular disease | 217 (3.6) | 308 (3.9) | .25 |
| Paralysis | 8 (0.1) | 18 (0.2) | .18 |
| Mild diabetes | 844 (13.8) | 818 (10.4) | <.0001 |
| Severe diabetes | 75 (1.2) | 59 (0.8) | .004 |
| COPD | 2073 (33.9) | 3040 (38.7) | <.0001 |
| Cancer | 631 (10.3) | 585 (7.5) | <.0001 |
| Metastatic cancer | 50 (0.8) | 54 (0.7) | .38 |
| Mild liver disease | 23 (0.4) | 23 (0.3) | .4 |
| Severe liver disease | 11 (0.2) | 9 (0.1) | .31 |
| Renal disease | 127 (2.1) | 189 (2.4) | .19 |
| Dementia | 62 (1.0) | 55 (0.7) | .04 |
| Ulcer | 56 (0.9) | 54 (0.7) | .13 |
| Rheumatologic disease | 94 (1.5) | 105 (1.3) | .32 |
| AIDS | 1 (0.0) | 1 (0.0) | .86 |
AIDS, Acquired immunodeficiency syndrome; COPD, chronic obstructive pulmonary disease; EVAR, endovascular aneurysm repair; NA, not applicable.
Main outcome measures and analysis
Our study had three main outcome measures: short-term mortality, long-term mortality, and cause of death. Short-term mortality was defined as death occurring within the index hospital stay or ≤30 days of the procedure date. Long-term mortality was defined as death occurring after discharge and >30 days; we termed these events “late” deaths.
To determine cause of death, we used information contained in the NDI, a central computerized index of death record information maintained by the National Center for Health Statistics. Using the patient’s Social Security number, Medicare HIC identifiers, and age and gender identifiers, we matched late deaths identified in the Medicare denominator (eligibility) file and the NDI. This was accurately accomplished in 1515 of 1671 deaths (91%) in our cohort (Fig 1).
Fig 1.
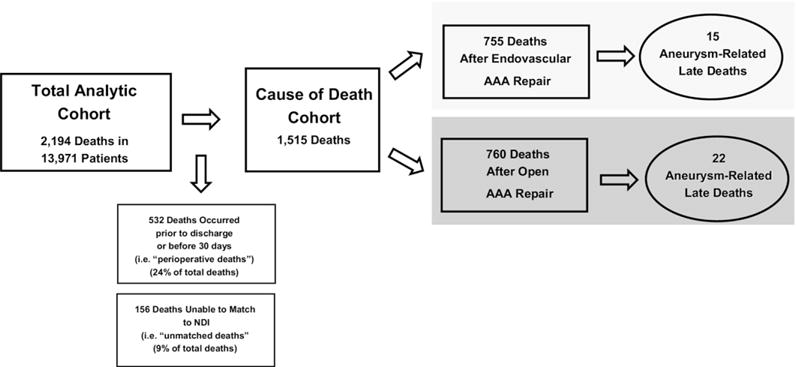
Construction of the open and endovascular cohorts for analysis. AAA, Abdominal aortic aneurysm; NDI, National Death Index.
Once deaths were matched, causes of death were obtained from the NDI22 and compared between EVAR and open repair. The NDI determines the underlying cause of death by using data obtained from death certificates. Death certificates require the provider to describe the immediate cause of death and subsequently list up to four conditions that contributed toward the underlying cause of death. Other significant conditions that contributed to death (but did not result in the underlying cause of death) are also recorded. For this analysis, we used the ICD-10 code for the underlying cause of death.
We selected ICD-10 codes that explicitly stated the word “aneurysm” in the cause of death and then compared the incidence of these causes of death in the cohorts undergoing EVAR vs open repair. Because “aneurysm” appears in a variety of ICD-10 codes, we categorized the relationship of the aneurysm to the mode of death. These descriptions were graded by level of suspicion (high, moderate, or low), with highest suspicion indicating that the cause of death was most likely to represent a late rupture of an infrarenal aortic aneurysm after repair. The comparison of interest in this study was the cause of late mortality after endovascular vs open repair. We described and compared causes of death in the two groups using proportions. Survival analyses of patients whose causes of death were deemed likely to be aneurysm-related were conducted using Wilcoxon log-rank and rank-sum tests, censoring those who were lost to follow-up or died of causes that could not be attributed directly to an AAA.
RESULTS
Patient characteristics
We studied 6119 Medicare beneficiaries who underwent EVAR and 7852 patients who underwent open repair between 2001 and 2004 (Table I). Patients undergoing EVAR were slightly older (31% aged >80 years for EVAR vs 23% for open repair, P < .0001) and more likely to be male (85% EVAR; 77% open repair; P < .0001). Patients undergoing EVAR had higher rates of preoperative myocardial infarction (16% EVAR, 13% open repair; P < .0001) and congestive heart failure (6% EVAR, 4% open repair; P < .0001). However, 34% of patients undergoing EVAR carried a diagnosis of chronic obstructive pulmonary disease vs 39% in open patients (P < .0001). Although other differences may have been statistically significant because of our large sample size, few other clinically relevant differences were evident between the two cohorts.
Short-term and long-term mortality
Short-term mortality, a combined measure of in-hospital or 30-day mortality, was 1.8% in patients undergoing EVAR and 5.3% in open repair (crude odds ratio [OR], 0.33; 95% confidence interval [CI], 0.27–0.41, P < .0001). Adjustment for patient characteristics had little effect on the risk of death for patients undergoing EVAR (risk-adjusted OR, 0.31; 95% CI, 0.25–0.38; P < .0001).
Overall long-term mortality was similar in both cohorts—15.3% for EVAR and 15.9% for open repair—at a median follow-up of 1.6 and 1.9 years, respectively, with an adjusted hazard ratio for mortality for patients undergoing endovascular repair of 0.98 (95% CI, 0.90–1.07). EVAR conferred no significant survival advantage compared with open repair (Fig 2 and Table II). As expected, Fig 2 demonstrates the likelihood of early mortality to occur in patients undergoing open repair. Deaths in patients undergoing EVAR occurred later, with the two curves crossing at approximately 1.6 years of follow-up. The differences in long-term mortality between EVAR and open repair were not statistically significant, either unadjusted (OR for EVAR, 1.07; 95% CI, 0.99–1.17; P > .05) or when adjusted for patient comorbidities (adjusted OR for EVAR, 0.98; 95% CI, 0.90–1.07; P > .05).
Fig 2.
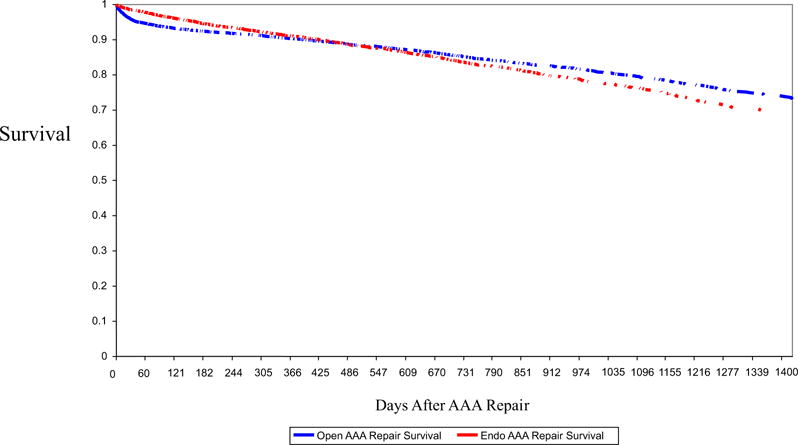
Survival after abdominal aortic aneurysm (AAA) repair by procedure type.
Table II.
Mortality after endovascular and open repair
| Variable |
AAA repair
|
|
|---|---|---|
| Open | Endovascular | |
| Cases, No. | 7852 | 6119 |
| Follow-up, y | 1.9 | 1.6 |
| Total deaths, No. | 1250 | 944 |
| Death rate,a % | 15.9 | 15.4 |
AAA, Abdominal aortic aneurysm.
Crude hazard ratio, 1.07 (95% confidence interval, 0.99–1.17); adjusted hazard ratio, 0.98 (95% confidence interval, 0.90–1.07).
Causes of late death
We identified 2194 deaths, comprising 944 deaths among 6119 EVAR patients and 1250 deaths among 7852 open repair patients. Of these deaths, 523 (111 EVAR, 412 open repair) occurred before discharge or ≤30 days. The remaining 1671 deaths occurred >30 days and were termed “late deaths.” Using the NDI, we were able to identify the underlying cause of death in 1515 patients (91%).
We limited the following analyses to the cohort of patients who survived the perioperative period and were successfully matched with the NDI. In the EVAR cohort of 5509 patients, we studied 755 deaths that occurred within a mean of 1.7 years (range, 0–3.6 years) of follow-up per patient. Within the open surgery cohort of 6746 patients, we studied 760 deaths that occurred within a mean of 2.0 years (range 0–3.6 years) of follow-up per patient.
Across these 1515 late deaths, there were 250 distinct ICD-10 codes indicating the cause of death. The 15 most common codes for causes of late death in this cohort are listed in Table III. These deaths represent 58% of all deaths in the cohort and are dominated by ischemic heart disease (18%), lung cancer (13%), and pulmonary disease (6%).
Table III.
Fifteen most common ICD-10 codes indicating cause of death in the cohort of Medicare enrollees who underwent aortic aneurysm repair
| ICD-10 code | Freq (% of total) | Category of death | Detail |
|---|---|---|---|
| C349 | 198 (13.0) | Neoplasm | Bronchus or lung, unspecified |
| I251 | 152 (10.0) | Ischemic heart disease | Atherosclerotic heart disease |
| I219 | 114 (7.5) | Ischemic heart disease | Acute myocardial infarction |
| J449 | 94 (6.2) | Respiratory system | |
| I64 | 41 (2.7) | Cerebrovascular disease | Stroke, unspecified |
| J189 | 36 (2.4) | Respiratory system | |
| I250 | 32 (2.1) | Ischemic heart disease | Atherosclerotic cardiovascular disease |
| C61 | 30 (2.0) | Neoplasm | Prostate |
| I500 | 26 (1.7) | Heart disease | Congestive heart failure |
| A419 | 24 (1.6) | Infection | Septicemia unspecified |
| C679 | 26 (1.7) | Neoplasm | Bladder, unspecified |
| C80 | 26 (1.7) | Neoplasm | Malignancy with no specified site |
| I619 | 25 (1.7) | Cerebrovascular disease | Intracerebral hemorrhage |
| I714 | 24 (1.6) | Vascular disease | AAA, without mention of rupture |
| J439 | 24 (1.6) | Respiratory system |
AAA, Abdominal aortic aneurysm; ICD, International Classification of Diseases.
Aneurysm-related late deaths
AAA without mention of rupture (ICD-10 I714) was the 14th most common cause of death (24 patients, 1.6% of all deaths studied, Table III). However, aortic aneurysm appears in a variety of other cause-of-death descriptions. These descriptions were graded by level of suspicion (high, moderate, or low), with highest suspicion indicating that the cause of death was most likely to represent a late rupture of an infrarenal aortic aneurysm after repair. For example, two patients (0.1% of all deaths) died with cause of death listed as ruptured AAA (ICD-10 I713); this was listed in the “highest level of suspicion” category (Table IV). In contrast, in the “lowest level of suspicion” category, one patient died with a cause of death listed as rupture of a thoracic aortic aneurysm (ICD-10 I711). In all, we identified 40 aneurysm-related late deaths (2.6% of all deaths); of which 26 were high suspicion, 11 were moderate suspicion, and 3 were low suspicion.
Table IV.
Eight ICD-10 codes indicating cause of death related to an aortic aneurysm in the cohort of Medicare enrollees who underwent aortic aneurysm repair
| ICD-10 code | Freq. (% of total) | Category of death | Detail |
|---|---|---|---|
| High suspicion | |||
| I713 | 2 (0.1) | Vascular disease | AAA ruptured |
| I714 | 24 (1.6) | Vascular disease | AAA, without mention of rupture |
| Moderate suspicion | |||
| I718 | 2 (0.1) | Vascular disease | AA of unspecified site, ruptured |
| I719 | 5 (0.3) | Vascular disease | AA of unspecified site, no mention of rupture |
| I729 | 4 (0.3) | Vascular disease | aneurysm of unspecified site |
| Low suspicion | |||
| I711 | 1 (0.1) | Vascular disease | TAA rupture |
| I712 | 1 (0.1) | Vascular disease | TAA without mention of rupture |
| I716 | 1 (0.1) | Vascular disease | TAA, without mention of rupture |
AAA, Abdominal aortic aneurysm; ICD, International Classification of Diseases; TAA, thoracic aortic aneurysm.
Aneurysm-related late deaths by type of aneurysm repair
We studied the effect of the type of aneurysm repair (EVAR vs open repair) on the incidence of aneurysm-related late deaths. As reported in Table V, the causes of aneurysm-related late deaths were similar across EVAR and open repair. Overall, the number of deaths related to an aortic aneurysm was low in both cohorts, and no statistical differences existed across categories of high, moderate, and low suspicion of cause of death. In summary, late death due to AAA rupture was rare and was not more common in EVAR vs open repair, occurring in 15 patients (0.3%) in the EVAR group and in 22 (0.3%), in the open repair group (P = .71).
Table V.
Causes of late death by level of suspicion for aneurysm-related cause of death and procedure type
| Level of suspicion |
EVAR (n = 755 deaths) No. (% of all deaths) |
Open repair (n = 760 deaths) No. (% of all deaths) |
|---|---|---|
| None | 739 (97.9) | 736 (96.8) |
| Low | 1 (0.1) | 2 (0.3) |
| Moderate | 4 (0.5) | 7 (0.9) |
| High | 11 (1.5) | 15 (2.0) |
Last, we compared the timing of late deaths among patients whose late deaths were aneurysm-related. In this analysis, we defined “aneurysm-related” as any death coded with a “high suspicion” or “moderate suspicion” ICD-10 code and censored individuals who died of other causes or were lost to follow-up. Of the 37 deaths that were high or moderate suspicion, survival of patients experiencing late aneurysm-related deaths was similar between those undergoing EVAR and open repair (log-rank P = .86). The distribution of times of death is reported in Table VI. These differences are small in magnitude and are not statistically significant.
Table VI.
Distribution of times of death among abdominal aortic aneurysm repair patients who died of a high or moderate suspicion cause, by procedure type
| Time to death, d |
Procedure type
|
|
|---|---|---|
|
EVAR (n = 15) |
Open repair (n = 22) |
|
| 31–60 | 0 | 5 |
| 61–90 | 3 | 2 |
| 91–120 | 1 | 3 |
| 121–180 | 3 | 3 |
| 181–365 | 4 | 1 |
| 366–730 | 1 | 3 |
| >730 | 3 | 5 |
DISCUSSION
The goal in the treatment of AAA is to provide protection from aneurysm rupture, because untreated rupture is almost always fatal, and repair in the setting of rupture poses a significantly higher risk for open than for elective repair.8 Before 1996, open repair was the only surgical option for prevention of rupture. Although it is invasive and morbid, open AAA repair is effective in preventing aneurysm rupture because late rupture rarely occurs after open repair.7 After 1996, EVAR emerged as an alternative treatment for infrarenal AAA.13 However, the need for repeat intervention after EVAR is common, and there are numerous reports of late aneurysm rupture after EVAR.14,23,24
These two facts have led many to express concerns about the long-term efficacy of EVAR in preventing aneurysm rupture and aneurysm-related death.4,18 However, our national analysis of death certificate information from >12,000 patients who underwent AAA repair reveals that late deaths from aneurysm rupture after AAA repair are uncommon and occur at similar rates for open repair and EVAR. These findings do not support evidence, anecdotal or otherwise, for higher risks of late aneurysm rupture-related mortality after EVAR.
Several investigators have studied late rupture in long-term follow-up studies of EVAR. For example, in the Lifeline Registry of Endovascular Aneurysm Repair, 18 events categorized as “late ruptures” occurred within a 6-year follow-up period in 2664 EVAR patients.1 In this registry designed to study the safety and efficacy of EVAR, no late ruptures occurred among 334 matched controls that underwent open repair. However, this difference in late rupture rates did not result in a significant difference in aneurysm-related deaths. In terms of aneurysm-related death (defined as death from any cause ≤30 days of the primary procedure, death ≤30 days of a secondary procedure or surgical conversion, or death due to aneurysm rupture or graft complication), no significant differences were found at 1 year between EVAR and open repair patients. The authors concluded that this established EVAR as a safe, effective, and durable treatment for infra-renal AAA.
Critics of this report note that 34% of the EVAR group died during follow-up, with limited information on the cause of death.18 In addition, they note a nearly 20% repeat intervention rate was necessary to ensure efficacy of the endografts in preventing rupture. In contrast, in a study of long-term outcomes of a multicenter randomized trial of EVAR vs open repair in the Netherlands, Blankensteijn et al25 reported no late ruptures in either group at the 2-year follow-up. Further, although their study demonstrated a higher aneurysm-related death rate in those undergoing open repair (5.7% vs 2.1%, P = .05), this difference in aneurysm-related mortality was based entirely on the difference in in-hospital (perioperative) mortality; only one late death occurred in each group.
Despite these reassurances from randomized trials in high-volume centers of excellence in EVAR, many have reported significant concerns about late rupture and aneurysm-related death from EVAR.2,16,26 Stent graft migration, loss of fixation, endoleaks, and stent fracture have all been reported after EVAR, and each has been linked to late rupture.27,28 In addition, late rupture has occurred even in the absence of endoleak, likely related to endotension, which is caused when porosity of the stent-graft material transmits pressure into the aneurysm sac.29–31 Many wondered, as EVAR has become common in clinical practice outside of randomized trials,5 if a rising incidence of graft-related complications, coupled with less-than-ideal follow-up, might result in a higher rate of late rupture and aneurysm-related death in those patients undergoing EVAR.32 Further, a recent national analysis of Medicare patients undergoing AAA repair concluded that late aneurysm rupture was more common in patients who underwent EVAR than open repair (1.8% vs 0.5%, P < .001), although no long-term survival differences were noted between groups.5
The differing conclusions about the likelihood of late aneurysm rupture found between our study and that report may lie in the methodologic differences across the studies. For example, Schermerhorn et al5 used a propensity score to match similar patients who had undergone open repair and EVAR and monitored the cohort of matched patients for >3 years.
First, our study did not use propensity scoring to account for selection bias in the assignment of patients to EVAR or open repair. Propensity matching has been well described, but one of the weaknesses of this technique is that propensity scores tend to overestimate treatment effects because of survivor bias—only those patients contributing to the risk prediction model are those that survived to undergo treatment.33,34 Rather than propensity score matching, we used logistic regression models using the Dartmouth-Manitoba claims-based modification of the Charlson comorbidity index. This methodology, although limited in the extent of clinical precision, has been validated broadly in surgical and nonsurgical settings.20,21,35 Further, it is important to note that regardless of risk-adjustment techniques, little published evidence suggests that patients differ dramatically across treatment groups, especially in a large, national analysis.36,37
Second, in the prior studies of late aneurysm rupture in Medicare patients, late aneurysm rupture was defined using ICD-9 codes rather than ICD-10 death certificate information, which may have contributed further to differences in our findings. We did not have access to the incidence of autopsy confirmation of the cause of death in our study, and therefore it is possible—and some may argue likely— that our study produced a low estimate of late deaths related to AAA repair.
Could our study have missed a higher rate of late rupture or aneurysm-related death in patients who have undergone EVAR? Although this is possible for several reasons, we believe this is unlikely:
First, the limitations of death certificates in determining the cause of death have been well described22,38 when used in combination with administrative identifiers such as Social Security records and ICD-9 codes, but the NDI has been found to be 97% sensitive and 99% specific in identifying known deaths.22
Second, studies comparing known causes of death from a direct information source with causes of death registered in the NDI have found a discrepancy of less than 4% between the two records.22,39
Finally, because late aneurysm-related death or rupture can occur in patients undergoing EVAR or open repair, there is little reason to believe that errors in assignment of cause of death would vary systematically by the type of aneurysm repair. However, we recognize that deaths due to aneurysm rupture often present clinically as sudden death and may be attributed to other causes (eg, myocardial infarction). This may result in undercoding of aneurysm-related death, regardless of repair type,22,40,41 given that a patient may not undergo autopsy to rule out aneurysm-related causes of death.
Our study has several other limitations. The patient characteristics in our groups were similar; however, certain key variables important in determining outcome after AAA repair, such as aneurysm diameter and precise location, are not available in administrative data,2,42 and it is therefore possible that significant selection bias may have confounded our outcome measures. However, any bias introduced by differences in aneurysm size or complexity would tend to favor EVAR because large aneurysm size has been directly associated with late rupture,2 and patients with more complex anatomy are more likely to have received open repair.43
Second, coding errors other than those that have been described have occurred. However, the use of both Medicare Part A and Part B, as well as Social Security and NDI information, make systematic administrative errors in either EVAR or open repair unlikely.
Third, our definition of aneurysm-related death was more stringent than what was used in the Lifeline registry. Their end point of aneurysm-related death included not only deaths after aneurysm rupture but also all perioperative deaths that occurred within the first 30 days after surgery as well as within the first 30 days after any secondary intervention. Given that our interest was in examining the effectiveness of EVAR in preventing aneurysm-related death, we studied that end point specifically rather than a broader outcome that would capture perioperative events from secondary procedures occurring in the early postoperative period.
Further, our study, as well as others,5,25 found that most aneurysm-related deaths not caused by rupture involve perioperative mortality from the initial AAA repair, regardless of procedure type. Therefore, it is unlikely our findings would change significantly by including perioperative events after secondary procedures because of the lower incidence of morbidity and mortality after secondary procedures relative to the incident AAA repair.
Finally, our data set was expansive but was limited by a relative short mean follow-up of just less than 2 years. Although traditional end points of aneurysm-related death classify late deaths as those occurring >30 days,7,9 these events may be better termed “midterm” death after AAA repair. Future postmarket surveillance efforts aimed at combining clinical registries and administrative data sets will attempt to establish longer follow-up of these large cohorts of patients to discern if differences emerge in the underlying cause of death over time.
CONCLUSIONS
Our analysis of outcomes in the real world of Medicare patients suggests that despite a relatively common need for secondary interventions, the midterm (and arguably long-term) effectiveness of EVAR in preventing aneurysm-related death is similar to open repair. Patients and surgeons should expect similar protection from aneurysm-related death from both open and endovascular AAA repair as long as patients are selected appropriately preoperatively and close surveillance is used postoperatively.
Supplementary Material
Footnotes
Competition of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Presented at the Peripheral Vascular Surgery Society’s Annual Winter Meeting, January 31st, 2010, Vail, Colo.
AUTHOR CONTRIBUTIONS
Conception and design: SF, EF, PG
Analysis and interpretation: PG, DT, FL, TG, SF
Data collection: PG, FL, SF
Writing the article: PG, SF
Critical revision of the article: PG, DT, FL, TG, EF, SF
Final approval of the article: PG, DT, FL, TG, EF, SF
Statistical analysis: PG, FL, SF
Obtained funding: SF
Overall responsibility: PG
References
- 1.Lifeline Registry of Endovascular Aneurysm Repair Steering C. Lifeline registry of endovascular aneurysm repair: registry data report. J Vasc Surg. 2002;35:616–20. doi: 10.1067/mva.2002.122232. [DOI] [PubMed] [Google Scholar]
- 2.Lifeline Registry of EPC. Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures [see comment] J Vasc Surg. 2005;42:1–10. doi: 10.1016/j.jvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Parra JR, Lee C, Hodgson KJ, Perler B. Endograft infection leading to rupture of aortic aneurysm. J Vasc Surg. 2004;39:676–8. doi: 10.1016/j.jvs.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 4.Participants ET. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial [see comment] Lancet. 2005;365:2187–92. doi: 10.1016/S0140-6736(05)66628-7. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population [see comment] N Engl J Med. 2008;358:464–74. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 6.Dimick JB, Upchurch GR., Jr Endovascular technology, hospital volume, and mortality with abdominal aortic aneurysm surgery. J Vasc Surg. 2008;47:1150–4. doi: 10.1016/j.jvs.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 7.Batt M, Staccini P, Pittaluga P, Ferrari E, Hassen-Khodja R, Declemy S. Late survival after abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 1999;17:338–42. doi: 10.1053/ejvs.1998.0779. [DOI] [PubMed] [Google Scholar]
- 8.Crawford ES, Saleh SA, Babb JW, 3rd, Glaeser DH, Vaccaro PS, Silvers A. Infrarenal abdominal aortic aneurysm: factors influencing survival after operation performed over a 25-year period. Ann Surg. 1981;193:699–709. doi: 10.1097/00000658-198106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston KW. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20:163–70. doi: 10.1016/0741-5214(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 10.Steinmetz E, Rubin B, Sanchez L, Choi E, Geraghty P, Baty J, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg. 2004;39:306–13. doi: 10.1016/j.jvs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Fillinger M, Excluder Bifurcated Endoprosthesis Clinical I Three-dimensional analysis of enlarging aneurysms after endovascular abdominal aortic aneurysm repair in the Gore Excluder Pivotal clinical trial. J Vasc Surg. 2006;43:888–95. doi: 10.1016/j.jvs.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 12.Fillinger MF. Postoperative imaging after endovascular AAA repair. Semin Vasc Surg. 1999;12:327–38. [PubMed] [Google Scholar]
- 13.Brewster DC, Geller SC, Kaufman JA, Cambria RP, Gertler JP, LaMuraglia GM, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcome of conventional open repair. J Vasc Surg. 1998;27:992–1003. doi: 10.1016/s0741-5214(98)70002-3. discussion 1004–5. [DOI] [PubMed] [Google Scholar]
- 14.Dattilo J, Brewster D, Fan C, Geller S, Cambria R, Lamuraglia G, et al. Clinical failures of endovascular abdominal aortic aneurysm repair: incidence, causes, and management. J Vasc Surg. 2002;35:1137–44. doi: 10.1067/mva.2002.124627. [DOI] [PubMed] [Google Scholar]
- 15.Rosen NA, Cayne NS, Macari M, Jacobowitz GR. “Unpredictable” late rupture of an abdominal aortic aneurysm after bifurcated Ancure endograft repair. Vasc Endovasc Surg. 2008;42:69–73. doi: 10.1177/1538574407308206. [DOI] [PubMed] [Google Scholar]
- 16.Fransen GAJ, Vallabhaneni SR, Sr, van Marrewijk CJ, Laheij RJF, Harris PL, Buth J. EUROSTAR: rupture of infra-renal aortic aneurysm after endovascular repair: a series from EUROSTAR registry. Eur J Vasc Endovasc Surg. 2003;26:487–93. doi: 10.1016/s1078-5884(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 17.Bernhard VM, Mitchell RS, Matsumura JS, Brewster DC, Decker M, Lamparello P, et al. Ruptured abdominal aortic aneurysm after endovascular repair [see comment] J Vasc Surg. 2002;35:1155–62. doi: 10.1067/mva.2002.123758. [DOI] [PubMed] [Google Scholar]
- 18.Manning BJ. Regarding “Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures” [comment] J Vasc Surg. 2006;43:199–200. doi: 10.1016/j.jvs.2005.09.040. discussion 200. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare and Medicaid Services. 2008 www.cms.gov.
- 20.Needham DM, Scales DC, Laupacis A, Pronovost PJ. Asystematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12–9. doi: 10.1016/j.jcrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Ghali WA, Hall RE, Rosen AK, Ash AS, Moskowitz MA. Searching for an improved clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol. 1996;49:273–8. doi: 10.1016/0895-4356(95)00564-1. [DOI] [PubMed] [Google Scholar]
- 22.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 23.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–17. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura JS, Brewster DC, Makaroun MS, Naftel DC. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37:262–71. doi: 10.1067/mva.2003.120. [DOI] [PubMed] [Google Scholar]
- 25.Blankensteijn JD, de Jong SECA, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SMM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms [see comment] N Engl J Med. 2005;352:2398–405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 26.Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739–49. doi: 10.1067/mva.2000.109990. [DOI] [PubMed] [Google Scholar]
- 27.Chuter TAM. The choice of stent-graft for endovascular repair of abdominal aortic aneurysm. J Cardiovasc Surg. 2003;44:519–25. [PubMed] [Google Scholar]
- 28.Zarins CK, Bloch DA, Crabtree T, Matsumoto AH, White RA, Fogarty TJ. Stent graft migration after endovascular aneurysm repair: importance of proximal fixation. J Vasc Surg. 2003;38:1264–72. doi: 10.1016/s0741-5214(03)00946-7. discussion 1272. [DOI] [PubMed] [Google Scholar]
- 29.Dubenec S, White G, Pasenau J, Tzilalis V, Choy E, Erdelez L. Endotension. A review of current views on pathophysiology and treatment. J Cardiovasc Surg (Torino) 2003;44:553–7. [PubMed] [Google Scholar]
- 30.Goodney PP, Fillinger MF. The effect of endograft relining on sac expansion after endovascular aneurysm repair with the original-permeability Gore Excluder abdominal aortic aneurysm endoprosthesis. J Vasc Surg. 2007;45:686–93. doi: 10.1016/j.jvs.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Thoo C, Bourke B, May J. Symptomatic sac enlargement and rupture due to seroma after open abdominal aortic aneurysm repair with polytetrafluoroethylene graft: implications for endovascular repair and endotension. J Vasc Surg. 2004;40:1089–94. doi: 10.1016/j.jvs.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 32.Medtronic releases five-year clinical data for AneuRx®. http://wwwp.medtronic.com/Newsroom/NewsReleaseDetails.do?itemId=1191329830213&lang=enUSAccessed Dec 12, 2009.
- 33.Martinez-Ramos D, Escrig-Sos J, Miralles-Tena JM, Rivadulla-Serrano MI, Daroca-Jose JM, Salvador Sanchis JL. Influence of surgeon specialization upon the results of colon cancer surgery. Usefulness of propensity scores. Rev Esp Enferm Dig. 2008;100:387–92. doi: 10.4321/s1130-01082008000700002. [DOI] [PubMed] [Google Scholar]
- 34.Adamina M, Guller U, Weber WP, Oertli D. Propensity scores and the surgeon. Br J Surg. 2006;93:389–94. doi: 10.1002/bjs.5265. [DOI] [PubMed] [Google Scholar]
- 35.Nuttall M, van der Meulen J, Emberton M. Charlson scores based on ICD-10 administrative data were valid in assessing comorbidity in patients undergoing urological cancer surgery. J Clin Epidemiol. 2006;59:265–73. doi: 10.1016/j.jclinepi.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Cohen ME, Dimick JB, Bilimoria KY, Ko CY, Richards K, Hall BL. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. J Am Coll Surg. 2009;209:687–93. doi: 10.1016/j.jamcollsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Dimick JB, Birkmeyer JD. Ranking hospitals on surgical quality: does risk-adjustment always matter? J Am Coll Surg. 2008;207:347–51. doi: 10.1016/j.jamcollsurg.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Sesso HD, Paffenbarger RS, Lee IM. Comparison of National Death Index and World Wide Web death searches [see comment] Am J Epidemiol. 2000;152:107–11. doi: 10.1093/aje/152.2.107. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, Office of Analysis and Epidemiology. Health, United States. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 40.Dominitz JA, Maynard C, Boyko EJ. Assessment of vital status in Department of Veterans Affairs national databases. comparison with state death certificates. Ann Epidemiol. 2001;11:286–91. doi: 10.1016/s1047-2797(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 41.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med. 1998;40:808–13. doi: 10.1097/00043764-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Crawford ES, Beckett WC, Greer MS. Juxtarenal infrarenal abdominal aortic aneurysm. Special diagnostic and therapeutic considerations. Ann Surg. 1986;203:661–70. doi: 10.1097/00000658-198606000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL, et al. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49:838–43. doi: 10.1016/j.jvs.2008.10.067. discussion 843–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


