Abstract
Objective
Medical management with antiplatelet (AP) and statin therapy is recommended for nearly all patients undergoing vascular surgery to reduce cardiovascular events. We assessed the association between preoperative use of AP and statin medications and postoperative in-hospital myocardial infarction (MI) in patients undergoing high-risk open surgery.
Methods
We studied patients who underwent elective suprainguinal (n = 3039) and infrainguinal (n = 8323) bypass and open infrarenal abdominal aortic aneurysm repair (n = 3007) in the Vascular Quality Initiative (VQI, 2005–2014). We assessed the association between AP or statin use and in-hospital postoperative MI and MI/death. Multivariable logistic analyses were performed to identify the patient, procedure, and preoperative medication factors associated with postoperative MI and MI/death across procedures and patient cardiac risk strata. Secondary end points included bleeding, transfusion, and thrombotic complications.
Results
Most patients were taking both AP and statin preoperatively (56% both agents vs 19% AP only, 13% statin only, and 12% neither agent). Use of both agents was more common for patients in the highest cardiac risk stratum (low, 54%; intermediate, 59%; high, 61%; P < .01). Increased cardiac risk was associated with higher MI rates (1.8% vs 3.8% vs 6.5% for low, intermediate, and high risk; P < .01). By univariate analysis, MI rate was paradoxically higher for patients taking both agents (3.7%, vs statin only 2.8%, AP only 2.6%, or neither AP nor statin 2.4%; P =.003). After multivariable adjustment, rates of MI in patients taking preoperative AP only (odds ratio [OR], 0.9; 95% confidence interval [CI], 0.7–1.2) and statin only (OR, 0.8; 95% CI, 0.6–1.2) were not different from those in patients taking either or neither medication (neither agent compared with taking both agents: OR, 1.0; 95% CI, 0.7–1.4; P > .05 for all). Similarly, rates of MI/death were not associated with medication status after multivariable adjustment. Estimated blood loss >1 liter (OR, 2.4; 95% CI, 1.6–3.7; P < .01) and transfusions of 1 or 2 units (OR, 2.5; 95% CI, 2.0–3.3; P < .01) and ≥3 units (OR, 4.0; 95% CI, 3.1–5.3; P < .01) were highly associated with MI, with similar findings related to composite MI/death in multivariable analysis. Rates of blood loss were slightly higher with AP use for all procedures; however, increased transfusions occurred only for infrainguinal bypass with AP use. Rates of reoperation for bleeding, graft thrombosis, or graft revision did not differ by preoperative AP use.
Conclusions
Preoperative AP and statin medications as used in VQI were not associated with the rate of in-hospital MI/death after major open vascular operations. Rather, predicted cardiac risk and operative blood loss were significantly associated with in-hospital MI or MI/death. AP and statin medications appear to be more useful in reducing late mortality than early postoperative MI/death in VQI. However, they were not harmful, so their long-term benefit argues for continued use.
Patients undergoing major vascular surgery have high rates of concomitant cardiovascular disease burden,1,2 and management of these patients includes treatment with antiplatelet (AP) medications (aspirin or P2Y12a antagonists such as clopidogrel) and cholesterol control with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins). In addition, blood pressure control and smoking cessation remain important additional treatments.3 These measures have been shown to reduce long-term risks of myocardial infarction (MI) and stroke.4 Prior work has shown that increasing the use of these medications is associated with a significant reduction in overall 5-year mortality after vascular surgery.5
Despite the known long-term benefits of aspirin for secondary prevention of cardiovascular disease, the benefit of continued AP medication in the perioperative setting for noncardiac, noncarotid vascular surgery is unclear.6 AP medications are thought necessary for carotid interventions and often for surgical bypass; however, aspirin has been shown to have higher rates of bleeding when it is used perioperatively without reducing MI rates in other settings.6 Although statin therapy has been shown to be effective in the preoperative setting for vascular surgery to reduce MI,7 the effect of statins in patients taking other cardioprotective medications, such as AP agents, remains unclear in real-word practice.
The purpose of this study was to describe the perioperative use of AP and statin medications in patients undergoing open infrarenal abdominal aortic aneurysm repair (OAR) and arterial bypass procedures in the Vascular Quality Initiative (VQI). In addition, we also sought to evaluate the association of these cardioprotective medications with postoperative MI and bleeding risks.
METHODS
Database
This is a retrospective analysis of data collected prospectively by the VQI, a nationwide quality improvement initiative developed originally in 2002 in New England8 to improve outcomes of vascular procedures.9 Registry data are compared with hospital claims in annual audits, and missing cases are retrieved to track all procedures.8
Construction of analytic cohort
All patients undergoing first-time procedures within the VQI data set from 2005 to 2014 for infrainguinal and suprainguinal arterial bypass and OAR were identified. This yielded our initial cohort of 19,388 patients. These patients were selected because multiple guidelines support the use of AP (aspirin or clopidogrel) and statin medications as long-term medications for patients with symptomatic peripheral arterial disease to reduce cardiovascular events.2,3,10–12 Although aneurysmal disease carries no specified societal recommendations regarding medication treatment with AP and statin medications, most of these patients have indications for known coronary risk factors that support their use,13 including a history of coronary artery disease, hypertension, positive stress test result, prior coronary revascularization, prior arterial bypass or peripheral intervention, and prior carotid revascularization (all variables collected in VQI). Overall, 89% of abdominal aortic aneurysm patients in our cohort had at least one of these cardiovascular risk factors to recommend AP and statin use.
All cases were elective; urgent or emergent cases were excluded (1466 emergent and 3398 urgent, 114 missing urgency data). These exclusion criteria were designed to provide a cohort of patients with the potential to be prescribed AP and statin medications before elective surgery. Finally, patients were removed from analysis for missing preoperative medication data (n = 41; 0.28%). This resulted in 14,369 patients with preoperative data available.
Definitions of exposures and outcomes
Our primary exposure was the use of AP or statin medication preoperatively. Statin use was defined as taking any type of statin medication at any dose. Patients were considered to be taking AP medication if they were taking aspirin (any dose) or any P2Y12a antagonist (commonly clopidogrel). Preoperative medication use was defined as taking the medication within 36 hours of surgery, but changes before this are not recorded. Patients classified as being intolerant to AP and statin were considered as not taking these medications (only 62 eligible patients [0.4%] were classified as being intolerant to any AP and 223 patients [1.6%] intolerant to statins). No serologic tests were done on drug efficacy, lipid levels, or other biochemical markers.
Our primary outcome was postoperative, in-hospital MI. Postoperative MI is defined as either an electrocardiographic (ECG) or clinical MI consistent with MI or an isolated troponin elevation without ECG ischemic changes or clinical symptoms. Although troponin-only elevation may have fewer clinical implications, both definitions were included in the primary end point to catch all myocardial ischemic events. Further, even troponin-only elevation events are important as they have been associated with reduced survival.14 Thus, given the importance of troponin elevation MI, they were included in our primary outcome as a measure of postoperative cardiac ischemia. In preliminary analysis, trends in rates for clinical and ECG MI were similar to those that included troponin elevations across cardiac risk strata and medication status (Supplementary Table I, online only). Secondary end points included in-hospital postoperative death and transfusion rates (total units transfused during hospitalization), estimated blood loss (EBL), and reoperation for bleeding based on AP status. For arterial bypasses, we also evaluated reoperation for graft thrombosis and discharge graft patency based on AP status. Definitions of medical comorbidities in the VQI cohort have been previously published.15 Cardiac risk was defined as low, intermediate, and high risk by the scoring system developed by Bertges et al16 using the Vascular Study Group of New England registry (low cardiac risk, −1 to 4 points; intermediate cardiac risk, 5 or 6 points; high cardiac risk, ≥7 points). Factors (and their point values) include age ≥80 years (4), age 70 to 79 years (3), age 60 to 69 years (2), coronary artery disease (2), congestive heart failure (2), chronic obstructive pulmonary disease (2), creatinine level >1.8 mg/dL (2), smoking history (1), insulin-dependent diabetes mellitus (1), long-term use of beta blockers (1), and prior coronary artery bypass or stent/ angioplasty (−1). This scoring system has been shown to be more accurate for cardiac risk prediction than the Revised Cardiac Risk Index.16
Physicians, nurses, or clinical data abstractors entered data prospectively on clinical and demographic variables. Research analysts were blinded to patient, surgeon, and hospital identity. The Institutional Review Board at Mayo Clinic has approved the use of de-identified data from VQI for research purposes. Patient consent was not obtained. The VQI is a federally listed Patient Safety Organization authorized under the Patient Safety and Quality Improvement Act of 2005 to allow collection of patient data without consent for quality improvement purposes.
Statistical analysis
Demographic information of patients was compared across medical regimen groups (neither AP nor statin, AP only, statin only, both medications) to assess for differences in cardiac risk factors that may account for the different medical treatment strategies and are possible confounders. Then, we examined the association of medication use status for our primary outcome of postoperative MI and MI/death (with Bonferroni corrections for six comparisons among the four exposure groups; these comparisons were deemed significant if P < .05/6 = .0083). Explanatory variables for analysis included patient and procedural characteristics as well as medication status to assess for possible confounding between medication use and our outcomes of MI and MI/ death. Univariate comparisons were performed with the t-test or χ2 test for continuous and categorical data, respectively. EBL and transfusions were evaluated with the Wilcoxon rank sum test because of their nonparametric distribution. We chose potentially confounding variables to be included in a multivariable logistic model by assessing the relationship between the exposures of patient characteristics, operative details, and medical regimens, with the outcome MI or MI/death based on their univariate association with the primary outcomes of MI and MI/ death. Variables of clinical significance and those with a P value of < .4 by univariate analysis were included in our final logistic models for MI and MI/death. Continuous variables with a nonlinear relationship with MI were categorized for analysis. Age was categorized by quartiles. Probability values of < .05 were considered significant. Analyses were done with SAS version 9.3 (SAS Institute, Cary, NC) and Stata Release 13 (Stata Corp, College Station, Tex).
RESULTS
Patient characteristics
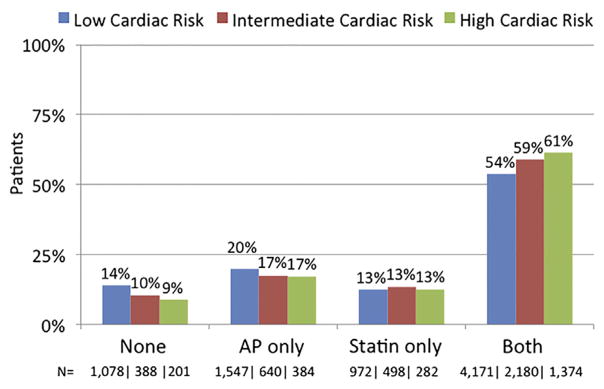
From 2005 to 2014, 14,369 patients underwent first-time operative procedures in the VQI for OAR repair (n = 3007), suprainguinal bypass (n = 3039), and infrainguinal bypass (n = 8323). Overall, a majority of patients were taking both AP and statin medication (56% taking both vs 12% taking neither medication, 19% taking AP only, and 13% taking statin only). Patients taking both medications had higher rates of diabetes, current tobacco use, coronary artery disease, congestive heart failure, and positive stress test results and were more likely to be male (Table I). Medication status was not associated with a meaningful difference in age or statistical difference in race or the presence of chronic obstructive pulmonary disease (Table I). When stratified by cardiac risk, patients taking neither medication or AP only were more likely to be at low cardiac risk (P < .01; Fig). Alternatively, patients taking both AP and statin agents were more likely to be at high cardiac risk (61% at high risk vs 54% at low risk taking both medications; P < .01; Fig).
Table I.
Patient characteristics
| None, % (n = 1740) | AP only, % (N = 2708) | Statin only, % (n = 1819) | Both, % (n = 8102) | Pa | |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 65 (13) | 66 (11.7) | 67.5 (10.5) | 66.9 (10.1) | <.01 |
| Sex | .002 | ||||
| Male | 12 | 18 | 13 | 57 | |
| Female | 12 | 20 | 13 | 54 | |
| Hispanic | 13 | 20 | 13 | 53 | .58 |
| Race | .34 | ||||
| White | 12 | 19 | 13 | 60 | |
| American Indian | 9 | 15 | 21 | 55 | |
| Asian | 15 | 18 | 15 | 52 | |
| Black | 15 | 20 | 12 | 54 | |
| Native Hawaiian | 0 | 25 | 0 | 75 | |
| >1 | 20 | 0 | 20 | 60 | |
| Unknown | 16 | 20 | 14 | 54 | |
| Smoking status | <.01 | ||||
| Never | 15 | 22 | 13 | 50 | |
| Prior (>12 months ago) | 10 | 17 | 13 | 60 | |
| Current | 13 | 20 | 12 | 54 | |
| Hypertension | 10 | 18 | 13 | 59 | <.01 |
| Diabetes mellitus | <.01 | ||||
| None | 14 | 20 | 12 | 53 | |
| Diet controlled | 11 | 17 | 12 | 59 | |
| Oral medication only | 8 | 15 | 14 | 64 | |
| Insulin dependent | 9 | 17 | 13 | 62 | |
| Coronary artery disease | <.01 | ||||
| None | 14 | 20 | 14 | 51 | |
| History of MI | 6 | 15 | 10 | 68 | |
| Stable angina | 6 | 14 | 10 | 70 | |
| Unstable angina or recent MI | 5 | 17 | 8 | 72 | |
| Prior coronary revascularization | 5 | 14 | 11 | 70 | <.01 |
| Congestive heart failure | <.01 | ||||
| None | 13 | 19 | 13 | 56 | |
| Asymptomatic | 9 | 17 | 13 | 60 | |
| Mild | 9 | 18 | 11 | 62 | |
| Moderate | 7 | 18 | 12 | 62 | |
| Severe | 8 | 13 | 4 | 75 | |
| COPD | .24 | ||||
| None | 12 | 19 | 12 | 56 | |
| Not treated | 13 | 19 | 12 | 57 | |
| On medication | 11 | 18 | 14 | 57 | |
| On oxygen | 12 | 20 | 15 | 53 | |
| Dialysis | .09 | ||||
| No | 12 | 19 | 13 | 56 | |
| Transplant | 8 | 23 | 8 | 60 | |
| On dialysis | 10 | 23 | 10 | 56 | |
| Creatinine >1.8 mg/dL | 9.5 | 18 | 16 | 57 | .02 |
| Stress test | <.01 | ||||
| Not done | 13 | 20 | 12 | 54 | |
| Normal | 12 | 18 | 14 | 56 | |
| Abnormal | 7 | 14 | 11 | 67 | |
| Beta blockers | <.01 | ||||
| None | 18 | 23 | 14 | 45 | |
| Perioperative (30 days) | 10 | 22 | 10 | 58 | |
| Chronic | 8 | 16 | 13 | 64 | |
| Operative day only | 21 | 17 | 17 | 45 | |
| Preoperative ACE inhibitors | 1 | 16 | 13 | 64 | <.01 |
ACE, Angiotensin-converting enzyme; AP, antiplatelet agent (aspirin or P2Y12a antagonist); COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; SD, standard deviation.
P value represents analysis of variance for age comparison and χ2 remaining comparison of demographics across medication groups.
Fig.
Preoperative medication status within each cardiac risk stratum. AP, Antiplatelet agent (aspirin or P2Y12a antagonist).
Postoperative in-hospital MI
Overall, there were 463 postoperative in-hospital MIs (3.2%), and this varied by procedure (4.7% OAR, 3.3% suprainguinal bypass, 2.7% infrainguinal bypass; P < .01). In addition, MI rates increased with increasing cardiac risk (low risk, 1.8%; intermediate risk, 3.8%; high risk, 6.5%; P < .01). In evaluating the association of medication use with MI rates and adjusting for multiple comparisons, there was a higher MI rate among patients taking both medications preoperatively compared with those taking neither medication (3.7% both vs 2.4% neither medication; P = .008) or taking AP-only regimens (3.7% both vs 2.6% AP only; P = .008). MI rates for those taking statin only were not different from the rates of those taking both medications (3.7% both vs 2.8% statin only; P = .06; Table II, A). Stratified by cardiac risk, there was a nonsignificant trend of increased MI rates for patients taking both medications (Table II, A). The rate of MI or death was not different by medication status (Table II, B). Similarly, the rates of MI by medication use were not significantly different when each procedure was evaluated individually and stratified by cardiac risk (Supplementary Table II, online only).
Table II.
| A, Myocardial infarction (MI) rates
| |||||
|---|---|---|---|---|---|
| None, % | AP only, % | Statin only, % | Both | Pa | |
| Overall | 2.4 | 2.6 | 2.8 | 3.7 | .003b |
| Cardiac risk | |||||
| Low | 1.4 | 1.5 | 1.9 | 2.1 | .3 |
| Medium | 3.1 | 2.5 | 3.0 | 4.6 | .054 |
| High | 5.0 | 6.0 | 5.3 | 7.1 | .48 |
| B, Myocardial infarction (MI) or death rates
| |||||
|---|---|---|---|---|---|
| None, % | AP only, % | Statin only, % | Both, % | Pa | |
| Overall | 3 | 4 | 4 | 5 | .06 |
| Cardiac risk | |||||
| Low | 1.9 | 1.8 | 2.2 | 2.4 | .41 |
| Medium | 4.6 | 4.2 | 4.4 | 5.3 | .62 |
| High | 7.0 | 8.1 | 9.2 | 8.9 | .78 |
AP, Antiplatelet agent (aspirin or P2Y12a antagonist).
χ2 comparison.
P value listed for overall comparison across groups. After correction for multiple comparisons, MI rate for patients taking both medications was significantly higher than for patients taking neither medication (P = .008) and AP only (P = .008) but not compared with those taking statin only (P = .06).
Because of differences in medical management by cardiac risk, multivariable adjustment was performed on postoperative MI rates to adjust for confounders. The odds of MI were increased with age, positive stress test result, increasing severity of diabetes, coronary artery disease, and Vascular Study Group of New England cardiac risk (Table III). Notably, transfusions and increasing EBL were highly associated with MI. After adjustment for these patient factors, preoperative medication status had no significant association with postoperative MI in patients taking AP only (odds ratio [OR], 0.9; 95% confidence interval [CI], 0.7–1.2; P = .31), statin only (OR, 0.8; 95% CI, 0.6–1.2; P = .28), or neither agent (OR, 1.0; 95% CI, 0.7–1.4; P = .94 compared with taking both agents; Table III). Similarly, the odds of MI or death was not different by medication status in patients taking AP only (OR, 1.0; 95% CI, 0.8–1.2; P = .75), statin only (OR, 1.0; 95% CI, 0.8–1.3; P = .93), or neither agent (OR, 1.0; 95% CI, 0.8–1.5; P = .67 compared with taking both agents; Supplementary Table III, online only). Neither beta blocker nor angiotensin-converting enzyme inhibitor use preoperatively was associated with MI.
Table III.
Multivariable factors associated with postoperative myocardial infarction (MI)
| Variable | OR | Overall | ||
|---|---|---|---|---|
| 95% CI | Pa | Pb | ||
| Cardiac risk | .08 | |||
| Low | 1.0 Reference | |||
| Intermediate | 1.3 | 0.9–1.9 | .06 | |
| High | 1.8 | 1.2–2.9 | <.01 | |
| Medication status | .59 | |||
| Both AP and statin | 1.0 Reference | |||
| AP only | 0.9 | 0.7–1.2 | .31 | |
| Statin only | 0.8 | 0.6–1.2 | .28 | |
| Neither agent | 1.0 | 0.7–1.4 | .94 | |
| Age quartiles | .06 | |||
| <60 | 1.0 Reference | |||
| 60–67 | 1.4 | 1.0–2.0 | .07 | |
| 68–74 | 1.5 | 1.0–2.2 | .04 | |
| >75 | 1.8 | 1.2–2.6 | <.01 | |
| Male | 1.1 | 0.9–1.3 | .48 | |
| Hispanic | 0.5 | 0.2–0.9 | .04 | |
| Race | <.01 | |||
| White | 1.0 Reference | |||
| American Indian | 1.5 | 0.4–6.6 | .6 | |
| Asian | 33 | 1.5–7.3 | <.01 | |
| Black | 0.5 | 0.3–0.8 | <.01 | |
| Otherc | 1.0 | 0.5–2.0 | .94 | |
| Smoking | .05 | |||
| Never | 1.0 Reference | |||
| Current | 0.8 | 0.6–1.2 | .32 | |
| Prior (>12 months ago) | 1.1 | 0.8–1.5 | .42 | |
| Hypertension | 1.2 | 0.8–1.7 | .31 | |
| Diabetes mellitus | .003 | |||
| None | 1.0 Reference | |||
| Diet | 1.2 | 0.8–1.8 | .48 | |
| Oral medication | 1.5 | 1.1–1.9 | <.01 | |
| Insulin | 1.6 | 1.2–2.1 | <.01 | |
| Coronary artery disease | .04 | |||
| None | 1.0 Reference | |||
| Prior MI | 1.1 | 0.8–1.5 | .60 | |
| Stable angina | 1.6 | 1.1–2.2 | .<01 | |
| Unstable angina or MI | 1.5 | 0.8–2.6 | .21 | |
| CABG or PCI within 5 years | 1.2 | 0.9–1.5 | .12 | |
| Congestive heart failure | 1.2 | 0.9–1.6 | .2 | |
| Chronic obstructive pulmonary disease | .76 | |||
| None | 1.0 Reference | |||
| History, but not treated | 0.9 | 0.6–1.3 | .56 | |
| On medication | 1.0 | 0.7–1.3 | .77 | |
| On oxygen | 0.7 | 0.4–1.3 | .31 | |
| On dialysis | 0.8 | 0.2–3.0 | .80 | |
| Preoperative stress test | <.01 | |||
| Normal | 1.0 Reference | 1.3–2.4 | ||
| (+) Ischemia | 1.7 | 0.9–2.0 | <.01 | |
| (+) MI | 1.3 | 0.6–2.3 | .22 | |
| (+) Both | 1.2 | 0.6–0.9 | .55 | |
| Not done | 0.7 | <.01 | ||
| Procedure | .19 | |||
| Infrainguinal bypass | 1.0 Reference | |||
| Suprainguinal bypass | 1.3 | 0.9–1.7 | .12 | |
| OAR | 1 | 0.7–1.4 | .92 | |
| Blood transfusion | <.01 | |||
| None | 1.0 Reference | |||
| 1 or 2 | 2.5 | 2.0–3.3 | <.01 | |
| ≥ 3 | 4.0 | 3.1–5.3 | <.01 | |
| Unknown/missing | 5.5 | 3.7–8.4 | <.01 | |
| EBL | <.01 | |||
| <150 mL | 1.0 Reference | |||
| 150–350 mL | 1.3 | 0.9–1.9 | .15 | |
| 351–1000 mL | 1.6 | 1.1–2.3 | .02 | |
| >1000 mL | 2.4 | 1.6–3.7 | <.01 | |
| Unknown/missing | 1.3 | 0.8–2.1 | .38 | |
| Transfer from home or rehabilitation facility vs hospital | 1.5 | 0.9–2.5 | .15 | |
| Preoperative living at home (vs nursing home) | 1.0 | 0.6–1.8 | .98 | |
| Prior bypass | 0.9 | 0.7–1.2 | .42 | |
| Prior CEA or CAS | 1.1 | 0.8–1.5 | .72 | |
| Prior PTA/stent | 0.9 | 0.7–1.1 | .28 | |
| Prior major amputation | 1.5 | 0.9–2.5 | .13 | |
| Beta blocker use | .75 | |||
| None | 1.0 Reference | |||
| Preoperative within | 1.2 | 0.9–1.9 | .22 | |
| 30 days only | ||||
| Chronic use (>30 days) | 1.1 | 0.9–1.5 | .35 | |
| Intolerant | 1.2 | 0.5–3.0 | .74 | |
| Operative day only | 1.4 | 0.6–2.9 | .43 | |
AP, Antiplatelet; CABG, coronary artery bypass; CAS, carotid artery stent; CEA, carotid endarterectomy; CI, confidence interval; EBL, estimated blood loss; OAR, open abdominal aortic aneurysm repair; OR, odds ratio; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty.
Overall P value for given categorical variable.
P value for indicator variable within each group compared with reference.
Native Hawaiian/Pacific Islander or >1 race.
Bleeding complications, return to operating room, and graft patency
To assess for potential bleeding risks associated with AP use, we examined both EBL and total transfusions by AP use (aspirin or P2Y12a antagonist) at the time of operation. Overall, there was no significant difference in EBL by AP status, but stratified by procedure, there was a small but statistically significant increase in EBL with AP use in unadjusted analysis (Table IV, A). Overall, transfusion rates were slightly higher with AP use, and this was due to a higher transfusion rate for patients undergoing infrainguinal bypasses on AP agents (Table IV, B). Reoperation was uncommon (8.4% of all cases, 7.2% for OAR, 7.3% for suprainguinal bypass, and 9.3% for infrainguinal bypass). However, of all patients taken back to the operating room, rate of bleeding, graft thrombosis, or graft revision was similar among patients regardless of AP use (Table V). Finally, discharge from the hospital with a patent graft was not affected by preoperative AP use (Table V).
Table IV.
| A, Estimated blood loss (EBL) by procedure and antiplatelet (AP) status preoperatively
| |||
|---|---|---|---|
| No AP | AP | Pa | |
| Overall EBL, mL | 300 (150–800) | 300 (150–750) | .287 |
| OAR, mL | 1180 (750–1850) | 1200 (750–2000) | .02 |
| Suprainguinal bypass, mL | 350 (150–600) | 350 (200–700) | .015 |
| Infrainguinal bypass, mL | 200 (100–300) | 200 (100–350) | <.01 |
| B, Total hospital transfusions by procedure and antiplatelet (AP) status preoperatively
| |||
|---|---|---|---|
| No AP | AP | Pa | |
| Overall transfusions | 0 (0–1) | 0 (0–2) | .01 |
| OAR, units | 0 (0–2) | 0 (0–2) | .422 |
| Suprainguinal bypass, units | 0 (0–1) | 0 (0–2) | .065 |
| Infrainguinal bypass, units | 0 (0-0) | 0 (0–1) | <.01 |
OAR, Open abdominal aortic aneurysm repair.
All values are presented as medians with interquartile ranges.
Rank sum comparison.
Table V.
Causes for return to operating room for complications and discharge graft patency by antiplatelet (AP) use (N = 1208 returns)
| Complication | No AP, % | AP, % | Pa |
|---|---|---|---|
| Return for bleeding | |||
| Total | 14 | 17 | .33 |
| OAR | 22 | 33 | .12 |
| Infrainguinal bypass | 13 | 15 | .66 |
| Suprainguinal bypass | 86 | 80 | .89 |
| Return for graft thrombosis | |||
| Total | 27 | 24 | .30 |
| Infrainguinal bypass | 27 | 24 | .35 |
| Suprainguinal bypass | 28 | 25 | .66 |
| Return for graft revision | |||
| Overall | 22 | 18 | .27 |
| Infrainguinal bypass | 24 | 19 | .10 |
| Suprainguinal bypass | 12 | 17 | .37 |
| Discharge with patent graft | |||
| Overall | 95 | 95 | .12 |
| Infrainguinal bypass | 94 | 95 | .57 |
| Suprainguinal bypass | 96 | 96 | .09 |
OAR, Open abdominal aortic aneurysm repair.
χ2 comparison.
DISCUSSION
Patients undergoing major vascular surgery have a significant burden of cardiovascular risk factors putting them at risk for cardiovascular events. Many authors have published data regarding the long-term benefit of AP and statin medications in preventing these events.5,17,18 However, the immediate perioperative cardiovascular benefit of these medications appears to be less important than patient-related factors, such as their estimated cardiac risk strata, and procedural factors, such as blood loss. Our data suggest that most patients undergoing major vascular surgery are receiving both AP and statin therapy at the time of surgery. In our analysis, lack of AP or statin therapy was not associated with increased MI/death rates, suggesting that the majority of patients are appropriately prescribed these medications preoperatively on the basis of their perceived cardiac risk. However, these findings do not offset the importance of long-term treatment of patients with peripheral vascular disease to reduce their risk of cardiovascular morbidity and mortality.5
The implications of perioperative AP agents on MI and bleeding risks are interesting, and this link has been suggested in previous publications. The Perioperative Ischemic Evaluation 2 (POISE-2) trial randomized >10,000 patients to aspirin therapy or placebo before noncardiac surgery.6 Although aspirin treatment did not reduce death or nonfatal MI rates (7% vs 7.1%), major bleeding occurred more frequently in patients taking aspirin (4.6% vs 3.8%; P = .04). This finding was consistent among the subset of 600 patients undergoing noncarotid vascular surgery. This challenges the concept that AP agents are important for preventing perioperative MI rates. It may be that acute thrombotic coronary events are not the primary cause of perioperative MI, or bleeding may cause more myocardial supply and demand mismatch, negating the potential benefits of aspirin therapy. In our analysis, we did not see lower MI rates in patients treated with AP agents. In our multivariable model to adjust for patient factors, AP use was not associated with lower MI rates. However, our data are not in the setting of a randomized trial and carry wide CIs (95% CI, 0.7–1.3; Table III).
Balancing the risks of bleeding and thrombotic events with AP use is important in the perioperative setting. Interestingly, our data suggest that transfusions and blood loss were strongly associated with the risk of a postoperative in-hospital MI. Transfusion has previously been shown to be associated with cardiovascular events in vascular surgical patients.19,20 Valentijn et al19 demonstrated that transfusion after vascular surgery was associated with increased odds of cardiovascular events (OR, 6.4; 95% CI, 4.3–9.4). However, the relationship between MI and transfusion may be confounded by the indication for the transfusion (anemia vs myocardial ischemia). VQI does not collect the indications for transfusion, so our data cannot address this limitation. However, the VQI now tracks the lowest hemoglobin level, enabling future study of this type of clinical question. Our data did suggest a small but significant increase in unadjusted bleeding complications for those taking AP agents. However, these differences were small and may not be clinically important, suggesting that these agents appear safe to use perioperatively. A more detailed analysis of these outcomes is necessary to verify these findings because they may be confounded by the perioperative use of other anticoagulants (such as heparin), use of dual AP agents, institutional guidelines for transfusion thresholds, and other factors we cannot account for in the VQI database. The findings must also be placed in the context of the type of procedure. Finally, our analysis did not see increased rates of reoperation for graft revision or graft thrombosis with lack of AP use.
Our data suggest that nearly 70% of patients are taking a statin medication at the time of surgery. The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) III study randomized nearly 500 patients to fluvastatin vs placebo before carotid, lower extremity, or abdominal aortic procedures who were not already taking a statin. Fluvastatin treatment was associated with a reduction in myocardial ischemia (10.8% vs 19%) and reduction in death or nonfatal MI (4.8% vs 10.1%).7 Yet, a similar benefit to statins was not seen in the DECREASE IV study that randomized patients to fluvastatin undergoing noncardiovascular surgery.21 This may suggest differences in populations of patients and in cardiac risk or divergent findings. In a meta-analysis of statin use for only vascular surgery patients, statin use appeared to lower perioperative cardiac events. However, this analysis was mostly from observational studies.22 Two thirds of the patients in our analysis were taking a statin at the time of surgery and would not be eligible for inclusion in the DECREASE III trial. Unlike with AP agents, there are few risks to perioperative statin use, and we would expect either a beneficial effect of statin on MI or a null effect. If a protective effect of MI is present, we may be underpowered to identify this (type II error).
Our study has important limitations. Notably, our findings are observational, and we presume that patients were selected for AP and statin medication on the basis of their perceived cardiac risks. Further, the recording of medication use is likely more accurate than cardiac history, so our multivariable models may not be able to completely correct for the confounding effect of higher risk patients more likely receiving medical management with AP agents and statins. This bias would tend to minimize potential beneficial impact of medical management in the multivariable model. Second, patients did not undergo routine ECG and laboratory testing, so asymptomatic myocardial ischemia was likely underdiagnosed. However, this bias would likely occur across all medication usage patterns and should not affect our overall conclusions. In addition, we included troponin-only ischemia, which has not been used in all studies; however, it has been associated with poor long-term survival. Supplementary Table I (online only) demonstrated that the trends for clinical MI were not different from our analysis including troponin-only events and should not change our findings. Third, although our study is sufficiently powered to detect a 50% reduction in MI rate with AP and statin use compared with neither medication (3% overall to 1%, power 97% with α 5%), not all patients were of similar cardiac risk. We are underpowered to detect a 50% reduction in MI rates for high cardiac risk patients (power 60% with α 5%). Our results that AP and statin medications did not reduce MI may be due to the inclusion of these low-risk patients. Finally, our MI rate is lower than in recent randomized trials as noted before, and this may reflect improved care, less routine ECG assessment, or different groups of patients.6,7,21 Because of these limitations, we cannot make any inferences about the causal relationship of AP agents and statins to MI in the perioperative period. However, this offers evidence to inform future study design to understand the implications of these medications in the perioperative period for this group of patients.
CONCLUSIONS
Most patients undergoing vascular surgery are treated with AP agents and statins, particularly those at high cardiac risk. Although our prior work identified a key role for these medications in long-term survival,5 our work herein failed to identify an association between MI rates with AP or statin use in the short term, especially in the context of other important factors, such as cardiac risk, type of operation, bleeding, and transfusions. Reducing perioperative cardiac events is likely not as simple as routine treatment with AP agents and statins. A clearer understanding of the patient and procedural factors associated with optimal results remains necessary.
Supplementary Material
Acknowledgments
The authors would like to acknowledge and thank Kristine M. Thomsen, Elizabeth B. Habermann, PhD, MPH, and the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery at Mayo Clinic for their assistance with statistical analysis of data for this manuscript.
Footnotes
Author conflict of interest: none.
Presented at the Thirty-ninth Annual Meeting of the Southern Association for Vascular Surgery, Scottsdale, Ariz, January 14–17, 2015.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RD, AB, JC, PG
Analysis and interpretation: RD, AB, AH, SA, JC, PG
Data collection: RD, AB, AH, JH, SA, GU, JC, PG
Writing the article: RD, AB, AH, JC
Critical revision of the article: RD, AB, AH, JH, SA, GU, JC, PG
Final approval of the article: RD, AB, AH, JH, SA, GU, JC, PG
Statistical analysis: RD
Obtained funding: RD
Overall responsibility: RD
References
- 1.Hertzer NR, Beven EG, Young JR, O’Hara PJ, Ruschhaupt WF, 3rd, Graor RA, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223–33. doi: 10.1097/00000658-198402000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Martino RR, Eldrup-Jorgensen J, Nolan BW, Stone DH, Adams J, Bertges DJ, et al. Perioperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. J Vasc Surg. 2014;59:1615–21, 1621.e1. doi: 10.1016/j.jvs.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 7.Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980–9. doi: 10.1056/NEJMoa0808207. [DOI] [PubMed] [Google Scholar]
- 8.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 9.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–37. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Neuro-Interventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 11.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Vasc Surg. 2011;54:e32–58. doi: 10.1016/j.jvs.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(Suppl):815S–43S. doi: 10.1378/chest.08-0686. [DOI] [PubMed] [Google Scholar]
- 13.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 14.Simons JP, Baril DT, Goodney PP, Bertges DJ, Robinson WP, Cronenwett JL, et al. The effect of postoperative myocardial ischemia on long-term survival after vascular surgery. J Vasc Surg. 2013;58:1600–8. doi: 10.1016/j.jvs.2013.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Martino RR, Nolan BW, Goodney PP, Chang CK, Schanzer A, Cambria R, et al. Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg. 2010;52:5–12. e1. doi: 10.1016/j.jvs.2010.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52:674–83–683.e1–3. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 18.Antithrombotic Trialists Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valentijn TM, Hoeks SE, Bakker EJ, van de Luijtgaarden KM, Verhagen HJ, Stolker RJ, et al. The impact of perioperative red blood cell transfusions on postoperative outcomes in vascular surgery patients. Ann Vasc Surg. 2015;29:511–9. doi: 10.1016/j.avsg.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Bursi F, Barbieri A, Politi L, Di Girolamo A, Malagoli A, Grimaldi T, et al. Perioperative red blood cell transfusion and outcome in stable patients after elective major vascular surgery. Eur J Vasc Endovasc Surg. 2009;37:311–8. doi: 10.1016/j.ejvs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Dunkelgrun M, Boersma E, Schouten O, Koopman-van Gemert AW, van Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE-IV) Ann Surg. 2009;249:921–6. doi: 10.1097/SLA.0b013e3181a77d00. [DOI] [PubMed] [Google Scholar]
- 22.Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61:519–32. e1. doi: 10.1016/j.jvs.2014.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.