Abstract
BACKGROUND
Packed RBC transfusion has been postulated to increase morbidity and mortality after cardiac/general surgical operations, but its effects after lower extremity bypass (LEB) have not been studied extensively.
STUDY DESIGN
Using the Vascular Study Group of New England’s database (2003–2010), we examined 1,880 consecutive infrainguinal LEB performed for critical limb ischemia. Perioperative transfusion was categorized as 0 U, 1 to 2 U, and ≥3 U. Cohort frequency group matching was used to compare groups of patients receiving 1 to 2 U and 0 U with patients receiving ≥3 U using age, coronary artery disease, diabetes, urgency, and indication of revascularization. Primary end points were perioperative mortality, wound infection, and loss of primary graft patency at discharge, as well as 1-year mortality and loss of primary graft patency.
RESULTS
In the study cohort, 1,532 LEBs (81.5%) received 0 U, 248 LEBs (13.2%) received 1 to 2 U, and 100 LEBs (5.3%) received ≥3 U transfusion. In the study cohort and group frequency matched cohort, transfusion was associated with significantly higher perioperative wound infection (0 U:4.8% vs 1 to 2 U: 6.5% vs ≥3 U: 14.0%; p = 0.0004) and graft thrombosis at discharge (4.5% vs 7.7% vs 15.3%; p < 0.0001). At 1 year, there were no differences in infection or graft patency. In multivariate analysis, transfusion was independently associated with increased perioperative wound infection in the study cohort and group frequency matched cohort (1 to 2 U vs 0 U: adjusted odds ratio [OR] = 1.4; 95% CI, 0.8–2.5; p 0.263; ≥3 U vs 0 U: OR = 3.5; 95% CI, 1.8–6.7; p = 0.0002; overall p = 0.002) and increased graft at thrombosis discharge (1 to 2 U vs 0 U: OR = 2.1; 95% CI, 1.2–3.6; p = 0.01; ≥3 U vs 0 U: OR = 4.8; 95% CI, 2.5–9.2; p < 0.0001, overall p < 0.0001).
CONCLUSIONS
Perioperative transfusion in patients undergoing LEB is associated with increased perioperative wound infection and graft thrombosis. From this observational study, it appears transfusion does not have major consequences during mid-term follow-up, but the presumed benefits of blood replacement should be weighed carefully because of the increased risk of perioperative complications with transfusion.
Patient outcomes after cardiac and noncardiac surgery have been correlated with perioperative transfusion of packed RBCs. Transfusion has been shown to be associated with increased mortality, postoperative ischemic morbidity, and cost after cardiac surgery.1 Infections, including severe postoperative bacterial infection,2–4 mediastinitis,2,5 sternal wound infection, and nosocomial pneumonia,2 are more common in cardiac surgery patients who received blood products, especially packed RBCs. Postoperative bacterial infection is also noted to be higher after transfusion in the setting of colorectal6,7 and orthopaedic surgery.8 In critically ill patients, randomized controlled trials have shown that transfusion does not improve outcomes in ICU settings, with the possible exception of patients who have acute cardiac events.9
Despite increasingly being recognized as a potential risk factor for adverse patient outcomes, transfusion remains relatively common in vascular patients. In a recent NSQIP review looking at intraoperative transfusion and perioperative outcomes during lower extremity revascularization procedures, transfusion rates varied from 14.5% to 27.1%.10 Perioperative transfusion can occur either intraoperatively, in the setting of acute blood loss to replace blood volume, or postoperatively in patients with anemia to improve tissue perfusion and oxygenation.10 The practice of transfusion has been under great scrutiny during the last 2 decades, but the significance of transfusion with respect to short and long-term outcomes after lower extremity bypass (LEB) remains poorly understood. Although most studies have concentrated on the influence of intraoperative transfusion on short-term outcomes,10 little is known about the effects of perioperative transfusion on mid-term outcomes and bypass graft patency.
The purpose of our study was to use the Vascular Study Group of New England (VSGNE) database to examine the LEB cohort, specifically to analyze the association of intraoperative and postoperative blood transfusion with 30-day and mid-term outcomes, including patient survival, wound infection, and bypass graft patency.
METHODS
Patients
Patients who underwent infrainguinal LEB in the VSGNE from 2003 to 2010 by 71 surgeons in academic and community hospitals were included in the study cohort. The VSGNE is a regional cooperative quality-improvement initiative developed in 2002 to prospectively study outcomes in patients undergoing vascular surgery. The details on this registry have been published previously11 and are available online at www.vsgne.org. The Institutional Review Board at Boston University School of Medicine and Public Health has approved the use of the deidentified data for this study.
We included all patients who underwent open infrainguinal revascularization procedures for critical limb ischemia (rest pain/tissue loss). In the cohort, inflow origin could be common femoral artery, profunda femoris artery, superficial femoral artery, and above- and below-knee popliteal artery. Outflow arteries included above- and below-knee popliteal artery, tibial, and pedal vessels. Conduits used were in situ vein, reversed vein, nonreversed vein, arm vein, and prosthetic graft. There were a total of 3,554 LEBs performed in VSGNE during the study interval. One thousand six hundred and seventy-four LEBs were excluded from this analysis for the following reasons: patient age younger than 40 years (n = 18), emergent (n = 91), acute ischemia (n = 292), claudication (n = 702), iliac graft origin (n = 63), superficial femoral artery/profunda femoris artery recipient (n = 31), concomitant procedures (n = 300), bilateral procedures (n = 115), functioning transplant (n = 23), and absence of transfusion data (n = 39). One thousand eight hundred and eighty LEBs were included in the final sample for analysis (Fig. 1).
Figure 1.
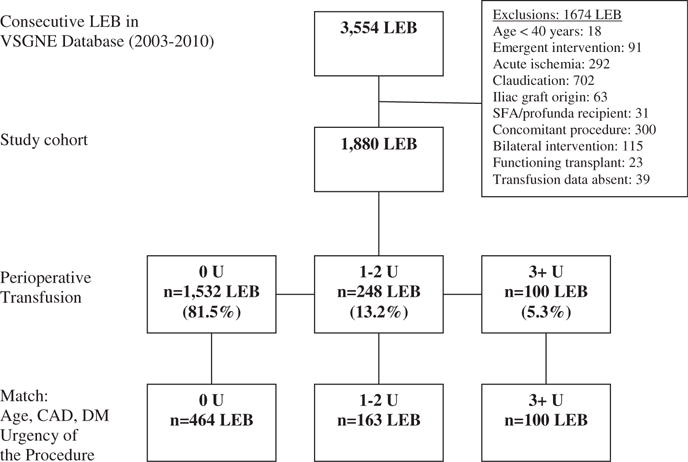
Flow diagram showing selection of the lower extremity bypass study cohort. CAD, coronary artery disease; DM, diabetes mellitus; LEB, lower extremity bypass; SFA, superficial femoral artery.
Outcomes and variable definitions
In our analysis, we reviewed demographics, pre-existing medical comorbidities, and index operation details. More than 100 clinical and demographic variables were collected prospectively for each procedure and entered into the VSGNE database.11 Definitions of medical comorbidities in VSGNE have been described previously.11 Perioperative transfusion requirements are defined as transfusion during the surgery or postoperatively within the index hospitalization. These were categorized into the following groups: 0 U, 1 to 2 U, and ≥3 U. Our main outcomes measures were perioperative mortality, wound infection, and loss of primary graft patency at discharge. In addition, we analyzed mortality and loss of primary graft patency at the time of midterm follow-up, which was at a mean of 381 days (range 0 to 3,050 days) in these patients. These were evaluated using life-table analysis. Perioperative wound infection was defined as culture-positive requiring antibiotic treatment or reoperation. Graft patency was defined as primary bypass graft patency only.
Baseline hemoglobin level was collected by VSGNE as one of the preoperative variables in the last 2 years of the study cohort only (2008 to 2010). Additional analysis incorporating baseline hemoglobin as an adjuster was performed to evaluate the association of transfusion with perioperative mortality, wound infection, and loss of primary patency using the data from those 2 years.
Statistical analysis
Outcomes were compared based on perioperative transfusion among patients who received 0 U, 1 to 2 U, and ≥3 U. Additional analyses were performed using cohort frequency group matching to select patients for comparison among these 3 groups. Each patient who received ≥3 U transfusion was cohort frequency group matched for age, coronary artery disease, diabetes, urgency, and indication of revascularization, with 2 patients receiving 1 to 2 U and 5 patients receiving 0 U transfusion. These matches were randomly selected from the cohort of patients with the same values of the matching variables. The goal of cohort frequency group matching was to reduce potential bias due to covariates when estimating effects from observational data.12,13 The final cohort frequency group matched study cohort included 727 LEBs: 464 LEBs received no transfusion, 163 LEBs received 1 to 2 U, and 100 LEBs received ≥3 U (this represented the 5:2:1 ratio of patients in each of the transfusion groups described here) (Fig. 1).
Patient baseline demographics were compared using chi-square test for categorical variables and Student’s t-test for continuous variables. Those variables with a p value <0.2 were entered into logistic regression models to analyze the outcomes of wound infection, mortality, and bypass graft patency, with p < 0.05 considered to be statistically significant. Multivariate analysis was performed in both the entire study cohort and in the matched cohort, adjusting in both cases for variables that were unbalanced across the treatment groups. Analysis was performed using SAS 9.1 software (SAS Institute).
RESULTS
There were 1,880 LEBs in this study cohort. Sixty-seven percent of patients were male and 98% of LEBs were performed in white patients. The indication for LEB was tissue loss in 66.5% and 76.9% of LEBs were performed electively. Forty percent of patients had a history of revascularization. Overall perioperative mortality was 2.1% in this study cohort, and wound infection rate was 5.5% (Table 1). Perioperative transfusion was administered in 18.5%; 13.2% received 1 to 2 U transfusion and 5.3% received ≥3 U (Table 1).
Table 1.
Patient Demographic and Operative Characteristics in the Study Cohort with p Values from the Study Cohort and Group Frequency Matched Cohort
| Patient and operative characteristics | 0 U (n = 1,532) | 1 to 2 U (n = 248) | 3+ U (n = 100) | p Value study cohort | p Value matched cohort |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Male sex, n (%) | 1,048 (68.4) | 152 (61.3) | 67 (67) | 0.085 | 0.023 |
| White race, n (%) | 1,508 (99.3) | 245 (100) | 98 (98) | 0.123 | 0.223 |
| Age, y, mean ± SD | 69.5 ± 11.4 | 73.9 ± 10.5 | 75 ± 10.8 | <0.0001 | 0.64 |
| Smoking (past/current), n (%) | 1,257 (82.2) | 186 (75) | 77 (77.8) | <0.0001 | 0.027 |
| Hypertension, n (%) | 1,350 (88.1) | 237 (96) | 94 (94) | 0.0003 | 0.112 |
| COPD, n (%) | 448 (29.2) | 59 (23.8) | 34 (34) | 0.105 | 0.209 |
| Diabetes, n (%) | 892 (58.2) | 167 (67.3) | 76 (76) | 0.0001 | 0.818 |
| Coronary disease, n (%) | 605 (39.5) | 120 (48.4) | 49 (49) | 0.008 | 0.879 |
| Congestive heart failure, n (%) | 289 (18.9) | 71 (28.6) | 38 (38) | <0.0001 | 0.012 |
| End-stage renal failure, n (%) | 140 (9.1) | 29 (11.7) | 13 (13) | 0.232 | 0.596 |
| Creatinine, mg/dL, mean ± SD | 1.6 ± 1.6 | 1.9 ± 1.7 | 2 ± 2 | 0.0007 | 0.181 |
| Hemoglobin, g/dL, mean ± SD | 12.7 ± 2.1 | 10.7 ± 2 | 10.8 ± 2 | <0.0001 | <0.0001 |
| Indication, n (%) | |||||
| Rest pain | 546 (35.6) | 61 (24.6) | 23 (23) | 0.0002 | 0.251 |
| Tissue loss | 986 (64.4) | 187 (75.4) | 77 (77) | ||
| Previous bypass | 477 (31.1) | 82 (33.1) | 42 (42) | 0.072 | 0.112 |
| Operative characteristics | |||||
| Urgency, n (%) | |||||
| Elective | 1,201 (78.4) | 175 (70.6) | 69 (69) | <0.004 | 0.519 |
| Urgent | 331 (21.6) | 73 (29.4) | 31 (31) | ||
| Estimated blood loss, mL, mean ± SD | 220.7 ± 238.7 | 372.9 ± 309.1 | 724 ± 807.9 | <0.0001 | <0.0001 |
| Graft origin, n (%) | |||||
| Common femoral | 1,000 (65.4) | 172 (69.4) | 63 (63) | 0.636 | 0.951 |
| Profunda/superficial femoral | 359 (23.5) | 53 (21.4) | 23 (23) | ||
| AK pop/BK pop/tibial | 170 (11.1) | 23 (9.3) | 14 (14) | ||
| Graft recipient, n (%) | |||||
| AK pop/BK pop | 777 (50.7) | 107 (43.7) | 38 (38) | 0.037 | 0.621 |
| TP trunk/AT/PT/peroneal | 552 (36) | 105 (42.3) | 46 (46) | ||
| DP, PT ankle/tarsal/plantar | 203 (13.3) | 36 (14.5) | 16 (16) | ||
| Conduit type, n (%) | |||||
| Greater saphenous | 1,045 (68.2) | 170 (68.5) | 63 (63) | 0.513 | 0.858 |
| Arm vein | 133 (8.7) | 27 (10.9) | 12 (12) | ||
| Prosthetic | 383 (25) | 61 (24.6) | 28 (28) | ||
| Anesthesia, n (%) | |||||
| Spinal | 236 (15.4) | 45 (18.1) | 8 (8) | 0.044 | 0.023 |
| Epidural | 127 (8.3) | 15 (6) | 4 (4) | ||
| General | 1,169 (76.3) | 188 (75.8) | 88 (88) | ||
| Preoperative medications, n (%) | |||||
| Aspirin | 1,069 (69.8) | 178 (71.8) | 76 (76) | 0.366 | 0.754 |
| Clopidogrel | 152 (9.9) | 37 (14.9) | 18 (18) | 0.005 | 0.03 |
| Statin | 926 (60.5) | 146 (58.9) | 74 (74) | 0.021 | 0.036 |
AK pop, above-knee popliteal; AT, anterior tibial; BK pop, below-knee popliteal; DP, dorsalis pedis; PT, posterior tibial; TP, tibioperoneal.
Patients who received perioperative transfusion were considerably older, more likely to be smokers, and had more underlying medical comorbidities (eg, hypertension, diabetes, coronary artery disease, and congestive heart failure) (Table 1). They were also more likely to have LEB performed for tissue loss (64.4% vs 75.4% vs 77%; p = 0.0002). These patients had lower baseline hemoglobin levels (0 U: 12.7 ± 2.1 g/dL vs 1 to 2 U: 10.7 ± 2 g/dL vs ≥3 U: 10.8 ± 2 g/dL; p < 0.0001) and higher baseline creatinine levels (1.6 ± 1.6 mg/dL vs 1.9 ± 1.7 mg/dL vs 2 ± 2 mg/dL; p = 0.0007). The group of patients who required perioperative transfusion had higher in-hospital mortality (1.7% vs 2.4% vs 7%; p = 0.002) and a higher incidence of perioperative wound infection (4.8% vs 6.5% vs 14%; p = 0.0004) after LEB. Cardiac events, including perioperative MI (3.9% vs 8.5% vs 20%; p < 0.0001) and dysrhythmia (3.9% vs 8.5% vs 16%; p < 0.0001), were significantly higher with transfusion (Table 2). Transfusion is associated with a higher incidence of reoperation during the same admission (12.6% vs 18.5% vs 38%; p < 0.0001). Of the 277 reoperations, 36 were for bleeding, 16 for infection, 51 for thrombosis, 44 for bypass revision, and 130 for unknown causes. The discharge bypass graft thrombosis rates (including patients who underwent reoperation) were also significantly higher (0 U: 4.5% vs 1 to 2 U: 7.7% vs ≥3 U: 15.3%; p < 0.0001).
Table 2.
Postoperative Morbidity, Mortality, and Bypass Graft Patency in Study Cohort with p Values of the Study Cohort and Group Frequency Matched Cohort
| 0 U (n = 1,532) | 1 to 2 U (n = 248) | 3+ U (n = 100) | p Value study cohort | p Value matched cohort | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Mortality | 26 | 1.7 | 6 | 2.4 | 7 | 7 | 0.002 | 0.125 |
| Wound infection | 73 | 4.8 | 16 | 6.5 | 14 | 14 | 0.0004 | 0.0008 |
| Graft infection | 2 | 0.1 | 0 | 0 | 3 | 3 | <0.0001 | <0.0001 |
| MI | 60 | 3.9 | 21 | 8.5 | 20 | 20 | <0.0001 | <0.0001 |
| Dysrhythmia | 59 | 3.9 | 21 | 8.5 | 16 | 16 | <0.0001 | 0.001 |
| Reoperation | 193 | 12.6 | 46 | 18.5 | 38 | 38 | <0.0001 | <0.0001 |
| Loss of primary patency | 69 | 4.5 | 19 | 7.7 | 15 | 15.3 | <0.0001 | 0.0003 |
There were 727 LEBs in the cohort frequency group matched study group, which was matched using age, coronary artery disease, diabetes, urgency, and indication of revascularization. Age, race, history of hypertension, coronary artery disease, diabetes, indication, and urgency of revascularization were similar across 3 group matched groups (Table 1, Appendix 1 [online only]). Patients who received transfusion were more likely to be male (0 U: 70% vs 1 to 2 U: 58.3% vs ≥3 U: 67%; p = 0.023), have congestive heart failure (0 U: 24.4% vs 1 to 2 U: 31.3% vs ≥3 U: 38%; p = 0.012), have lower baseline hemoglobin levels (12.2 ± 2.1 g/dL vs 10.4 ± 2.1 g/dL vs 10.8 ± 2 g/dL; p < 0.0001), and have higher estimated blood loss during surgery (209.4 ± 183.5 mL vs 346.4 ± 283.6 mL vs 724 ± 807.9 mL; p < 0.0001). They were also more likely to have been taking clopidogrel preoperatively (9.3% vs 13.5% vs 18%; p = 0.029), but there was no difference in use of aspirin (72.4% vs 72.4% vs 76%; p = 0.755). Revascularization was more likely to be performed under general anesthesia in the transfusion groups (74.6% vs 71.2% vs 88%; p = 0.023).
In bivariate analysis of the cohort frequency group matched patients, although there were no differences with hospital mortality, perioperative transfusion is noted to be associated with higher rates of in-hospital wound infection (0 U: 4.3% vs 1 to 2 U: 9.2% vs ≥3 U: 14%; p = 0.0008) (Table 2, Appendix 2 [online only]). Perioperative MI (5.2% vs 6.7% vs 20%; p < 0.0001) and cardiac dysrhythmia (5.4% vs 8% vs 16%; p = 0.001) were more common in the transfusion groups. Transfusion was associated with a higher incidence of reoperation (12.9% vs 17.8% vs 38%; p < 0.0001). Of the 127 reoperations, 17 were for bleeding, 9 for infection, 25 for thrombosis, 20 for bypass revision, and 56 for unknown causes. Transfusion was associated with significantly higher bypass graft thrombosis at discharge (4.4% vs 8.6% vs 13%; p = 0.0003).
Follow-up data are collected as a routine practice to conform to VSGNE standards. In this study, patients from 2010 were excluded from 1-year follow-up evaluations because of high levels of missing data (most have not had follow-up visits or had their follow-up data entered into the VSGNE database). For patients undergoing LEB from 2003 to 2009, only 5.2% were missing follow-up data for mortality, and 17.9% were missing data for graft patency. Despite these missing data, certain trends were still apparent. In the unadjusted patient cohort (on life-table analysis), 1-year mortality rates remained higher in patients who required transfusion (Fig. 2) (16.4% vs 20.6% vs 31%; p = 0.0004), but loss of primary graft patency (Fig. 3) (33.1% vs 29.6% vs 40.7%; p = 0.285) was similar across the 3 groups, with mean follow-up of 381 days (range 0 to 3,050 days). Mid-term outcomes were not calculated for the group frequency matched cohort because, given the required exclusion of patients from 2009 to 2010 due to missing data, the matching algorithms used previously became less meaningful and accurate.
Figure 2.
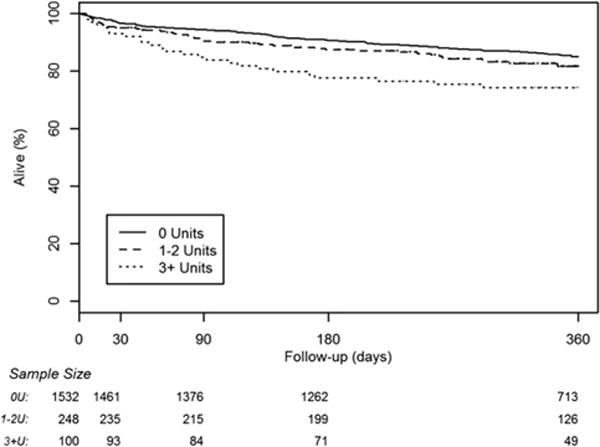
Life-table analysis of mid-term patient survival for the entire study cohort.
Figure 3.
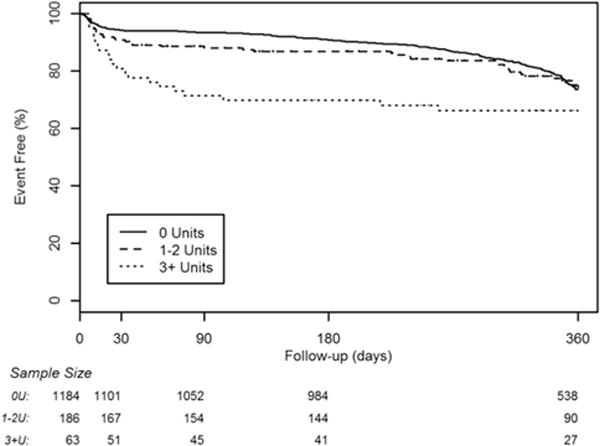
Life-table analysis of mid-term primary bypass graft patency for the entire study cohort.
In multivariate analysis of the study cohort, transfusion was associated with increased perioperative wound infection (1 to 2 U vs 0 U: adjusted odds ratio [OR] = 1.4; 95% CI, 0.8–2.5; p = 0.263; ≥3 U vs 0 U: OR = 3.5; 95% CI, 1.8–6.7; p = 0.0002, overall p= 0.002) and bypass graft thrombosis at discharge (1 to 2 U vs 0 U: OR = 2.1; 95% CI, 1.2–3.6; p = 0.01; ≥3 U vs 0 U: OR = 4.8; 95% CI, 2.5–9.2; p < 0.0001, overall transfusion p < 0.0001) (Table 3, Fig. 4). There was no association between transfusion and perioperative mortality, mid-term patient survival, or mid-term loss of primary patency. In multivariate analysis of the cohort frequency group matched patients, transfusion was associated with increased perioperative wound infection (1 to 2 U vs 0 U: OR = 2.1; 95% CI, 1.0–4.3; p = 0.04; ≥3 U vs 0 U: OR = 3.8; 95% CI, 1.8–8.1; p = 0.0005, overall transfusion p = 0.002), as well as higher perioperative bypass graft thrombosis (1 to 2 U vs 0 U: OR = 1.7; 95% CI, 0.8–3.6; p = 0.15; ≥3 U vs 0 U: OR = 4.0; 95% CI, 1.9–8.5; p = 0.0002, overall transfusion p = 0.0016) (Table 4).
Table 3.
One-Year Results for Mortality and Loss of Primary Patency in the Entire Study Cohort
| Entire cohort | 0 U, % | 1 to 2 U, % | 3+ U, % | p Value |
|---|---|---|---|---|
| 1-Year mortality | 16.4 | 20.6 | 31 | 0.0005 |
| Loss of 1-year primary patency | 33.1 | 29.6 | 40.7 | 0.285 |
Data exclude lower extremity bypass patients from 2009–2010.
Figure 4.
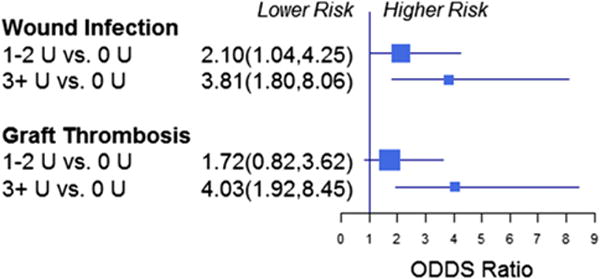
Multivariable analysis of perioperative wound infection and discharge graft patency of the entire study cohort.
Table 4.
Multivariate Analysis of Perioperative Wound Infection and Loss of Primary Patency in the Group Frequency Matched Cohort
| Adjusted odds ratio | Lower confidence limit | Upper confidence limit | p Value | |
|---|---|---|---|---|
| Wound infection* | ||||
| 1–2 U vs 0 U | 2.10 | 1.04 | 4.26 | 0.04 |
| ≥3 U vs 0 U | 3.81 | 1.80 | 8.06 | 0.0005 |
| General anesthesia vs epidural/spinal | 0.56 | 0.29 | 1.08 | 0.083 |
| Graft thrombosis at discharge† | ||||
| 1–2 U vs 0 U | 1.72 | 0.82 | 3.62 | 0.151 |
| ≥3 U vs 0 U | 4.04 | 1.93 | 8.46 | 0.0002 |
| General anesthesia vs epidural/spinal | 1.08 | 0.52 | 2.25 | 0.845 |
Adjusted for smoking, congestive heart failure, previous bypass, and clopidogrel.
Overall transfusion p = 0.002.
Overall transfusion p = 0.0016.
Subgroup analysis was performed by incorporating baseline hemoglobin data into the multivariate regression model for the last 2 years of the study cohort (2008 to 2010) and adjusting based on these values. With these data from the last few years, the estimates of the effects of transfusion were still the same. In multivariate analysis, transfusion was associated with higher trends of discharge bypass graft thrombosis (1 to 2 U vs 0 U: OR = 2.4; ≥3 U vs 0 U: OR = 3.1; overall p = 0.08) and perioperative wound infection (1 to 2 U vs 0 U: OR = 0.9; ≥3 U vs 0 U: OR = 1.6; overall p = 0.63). There were higher trends of hospital mortality with transfusion, despite adjusting for preoperative hemoglobin level (1 to 2 U vs 0 U, OR = 2.7; ≥3 U vs 0 U, OR = 2.2; overall p = 0.45).
DISCUSSION
Our study has shown that the practice of transfusion in the VSGNE cohort of patients is similar to practice patterns in other groups of vascular patients after LEB.10 Up to 18.5% of patients required transfusion either intra- or postoperatively after lower extremity revascularization, with the majority of patients receiving 1 to 2 U of transfusion. In the study cohort and group frequency matched cohort, transfusion for LEB was associated with increased perioperative wound infection and bypass graft thrombosis at discharge.
It is essential to recognize the potential cofounders in this study when evaluating transfusion and patient outcomes. Patients who required perioperative transfusion during LEB are different from those who did not receive transfusions. Baseline characteristics (ie, sex, smoking, hypertension, coronary artery disease, diabetes, baseline hemoglobin, indication and urgency of LEB), as well as operative factors (ie, type of anesthesia and bypass recipient), were different among the 3 groups (Table 1). To try to account for these cofounders, regression analysis incorporating these factors were performed for the entire study cohort (1,880 LEBs) and after cohort frequency group matching (727 LEBs). The aim of cohort frequency group matching was to reduce potential bias due to covariates when estimating causal effects using this observational data. Age, coronary artery disease, diabetes, indication for revascularization, and urgency for revascularization were selected for matching. These variables were significantly different among the 3 transfusion groups on bivariate analysis and are important factors that can independently affect patient outcomes after revascularization. Patients who required transfusion were more likely to be taking clopidogrel preoperatively. Clopidogrel has previously been shown not to be associated with major bleeding complications during peripheral arterial surgery14 and therefore was not selected for matching. The findings were similar after multivariate analysis in both the unadjusted and cohort frequency matched cohort. Perioperative transfusion appears to be independently associated with increased perioperative wound infection and bypass graft thrombosis.
In their study of 8,799 patients from the NSQIP database, O’Keeffe and colleagues found that there was a higher risk of postoperative mortality and pulmonary and infectious complications after intraoperative transfusion in patients undergoing lower extremity revascularization.10 They examined the effect of intraoperative transfusion on 30-day morbidity and mortality after risk adjustment using regression models. Wound occurrences, including superficial, deep, and organ-space surgical site infection, as well as wound dehiscence, were higher with transfusion. In their model, they accounted for potential cofounders by incorporating factors such as preoperative hematocrit, procedure type, and duration of operation using transfusion propensity scores. Due to the limitation of the database, they were not able to evaluate the effect of postoperative transfusion after revascularization and concentrated solely on intraoperative transfusion. Their outcomes were perioperative outcomes only and not mid-term or long-term outcomes. In our study, we were able to evaluate perioperative blood transfusion, including intraoperative and immediate postoperative transfusion, to try to study the influences of transfusion during and after LEB. After cohort frequency group matching, our study showed that there were no differences with hospital mortality; perioperative transfusion was associated with increased short-term wound infection, but had no effect on mid-term infectious complications.
Blood transfusions have been shown to be associated with postoperative infections in patients undergoing cardiac surgery. Transfusion was associated with a higher incidence of bacterial infections after coronary artery bypass graft.1,3 Sternal wound infection,2,15 mediastinitis,5,16 and nosocomial pneumonia2,17 were associated with administration of RBCs after cardiac surgery. As in our study, transfusion has been demonstrated to have a dose-dependent association with postoperative infections in multiple studies.2,3 A possible cause for this association is the suppressive effect of transfusion on the human immune system. Allogenic blood transfusion generally causes down-regulation of macrophage and T-cell immunity.18 This immunomodulation is hypothesized to be the mechanism by which transfusion can cause increased rates of cancer recurrence and increased prevalence of postoperative bacterial and viral infections.18,19
The association of transfusion with bypass graft patency after cardiac or vascular surgery is poorly studied. In their study, Murphy and colleagues have shown that RBC transfusion is strongly associated with perioperative ischemic morbidities, including MI, stroke, and renal complications after cardiac surgery.1 To our knowledge, there have been no studies examining the significance of transfusion on graft patency. Our data showed that transfusion is associated with considerably lower primary bypass graft patency at discharge, but there were no differences with mid-term primary patency rates. One possible explanation for this observation is the association of transfusion with reoperation, because reoperation could potentially indicate a high-risk bypass graft that would be more prone to immediate failure. However, we are not able to evaluate this association because of the limitations of the VSGNE database. Another possible explanation is that systemic inflammation can occur in response to the transfusion of RBCs and therefore lead to early bypass graft failure.20 Stored red cells have also been shown to possess increased aggregability, rigidity, and overall viscosity, which can influence short-term bypass graft patency.21
Most available evidence describing postoperative patient outcomes and their associations with transfusion have been in patients undergoing cardiac surgery. A number of groups have observed decreased survival in patients exposed to transfusion after cardiac surgery.22–24 In their regional cohort study, Surgenor and colleagues reported a 16% increase in risk-adjusted 5-year mortality with exposure to limited transfusion (1 or 2 U) after cardiac surgery.24 Koch and colleagues and Engoren and colleagues made similar observations with decreased survival after packed RBC exposure in patients after coronary artery bypass graft.22,23 O’Keeffe and colleagues has noted that there was higher risk of postoperative mortality in lower extremity revascularization after receiving intraoperative transfusion but did not evaluate the association with longer-term survival.10 The perioperative mortality was relatively low in our LEB cohort and there were no differences in patient survival during mid-term follow-up after surgery in our study (in the frequency group matched cohort or on multivariate analysis for either cohort).
It is difficult to set a defined hemoglobin level as a trigger for transfusion in postoperative patients, and recent studies have suggested that a lower hemoglobin threshold should be used for transfusion guidelines.1,9 However, in their article, Wu and colleagues reported that blood transfusion is associated with a lower short-term mortality rate among elderly patients with acute MI if the hematocrit is ≤30.0%.25 In a follow-up study, Wu and colleagues showed that in elderly patients undergoing major noncardiac surgery, intraoperative transfusion is associated with lower 30-day postoperative mortality when there is substantial operative blood loss or low preoperative hematocrit (<24%).26 We performed additional subgroup analysis on the matched cohort and incorporated preoperative hemoglobin level into the multivariate regression model as an adjuster. Unfortunately, preoperative hemoglobin values were only available in the last 2 years of data collection and, therefore, not for the entire study cohort. With the incorporation of preoperative hemoglobin values, the effects of transfusion were still found to be the same in short and midterm results. We found that transfusion did not appear to affect short or mid-term patient survival after LEB, irrespective of preoperative hemoglobin level. This observation, however, might be subject to type II error because of the smaller number of patients available for study.
In this study, perioperative transfusion is associated with increased risk of perioperative wound infection. The incidence of perioperative wound infection after LEB in VSGNE appeared to be lower than other comparable literature,27,28 although this might relate to more accurate data recording in the other trials. Overall perioperative wound infection rate was 5.1% in the non-matched cohort and 6.3% in the matched cohort, with other reported rates of wound infection up to 20%.27,28 Perioperative transfusion is associated with a 2- to 4-fold increased risk of wound infection after LEB.
There are important limitations to this study. First, ours is an observational study of a prospectively collected database rather than a randomized controlled trial. Although we used matching to control for possible confounding factors, baseline hemoglobin, creatinine, and estimated blood loss still remained significantly different across the 3 groups. We adjusted for these observations by incorporating factors that were significant in bivariate analysis into our multivariate regression model for both the entire study cohort and the matched cohort. Second, the database was missing information for 5% to 18% of patients in various mid-term outcomes variables. Although there were no differences in mid-term outcomes across the entire cohort on bivariate and multivariate analysis, these analyses were likely subject to significant type II errors due to missing data. Third, because of the nature of the database, the majority of patients were white and the generalizability of the results should be made with caution in non-white patients. Fourth, we were not able to determine the exact cause and timing of blood transfusion in relation to the index LEB operation, and these would likely be important factors in determining the significance of transfusion. In addition, analysis was performed incorporating baseline hemoglobin into the analysis using only the last 2 years of the data rather than those for the entire LEB cohort. This analysis is also subject to type II error due to the smaller numbers of patients. Last, we were not able to determine the transfusion threshold in this cohort to evaluate outcomes in relation to hemoglobin level. The transfusion practice likely varied across different centers. Although this is not a randomized trial with defined transfusion triggers, our study is a true representation of actual practice in both community and academic settings.
Even with its limitations, the utility of the VSGNE database has been validated through numerous other studies,29–31 and its strength lies in its comprehensive repository of specific variables collected and its regular adjudication process. Using this prospectively maintained database, we were able to evaluate a large cohort of LEBs to study the association of perioperative transfusion with short and mid-term patient outcomes. Most other published studies have concentrated on intraoperative transfusion and study 30-day outcomes only. Ours is one of the few available studies to examine the association of perioperative transfusion with short and mid-term outcomes in vascular surgical patients, and one of the first to look at the significance of transfusion on LEB graft patency.
CONCLUSIONS
Perioperative packed RBC transfusion is associated with increased perioperative complications, including wound infection and bypass graft thrombosis during the initial hospital stay. Although transfusion might not have substantial mid-term consequences, additional studies are needed to evaluate the risks and benefits of perioperative transfusion in patients undergoing lower extremity revascularization.
Appendix 1. Patient Demographic and Operative Characteristics in Frequency Group Matched Study Cohort
| Patient and operative characteristics | 0 U (n = 464) | 1–2 U (n = 163) | 3+ U (n = 100) | p Value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male sex, n (%) | 325 (70) | 95 (58.3) | 67 (67) | 0.023 |
| White race, n (%) | 454 (99.1) | 161 (100) | 98 (98) | 0.223 |
| Age, y, mean ± SD | 74 ± 10.5 | 74.6 ± 10.3 | 75 ± 10.8 | 0.64 |
| Smoking (current/past), n (%) | 362 (78) | 123 (75.5) | 77 (77.8) | 0.027 |
| Hypertension, n (%) | 428 (92.2) | 157 (96.9) | 94 (94) | 0.112 |
| COPD, n (%) | 130 (28) | 39 (23.9) | 34 (34) | 0.209 |
| Diabetes, n (%) | 345 (74.4) | 125 (76.7) | 76 (76) | 0.818 |
| CAD, n (%) | 229 (49.4) | 84 (51.5) | 49 (49) | 0.879 |
| CHF, n (%) | 113 (24.4) | 51 (31.3) | 38 (38) | 0.012 |
| End-stage renal failure, n (%) | 47 (10.1) | 20 (12.3) | 13 (13) | 0.596 |
| Creatinine, mg/dL, mean ± SD | 1.7 ± 1.6 | 1.9 ± 1.7 | 2 ± 2 | 0.181 |
| Hemoglobin, g/dL, mean ± SD | 12.2 ± 2.1 | 10.4 ± 2.1 | 10.8 ± 2 | <0.0001 |
| Indication, n (%) | ||||
| Rest pain | 100 (21.6) | 26 (16) | 23 (23) | 0.251 |
| Tissue loss | 364 (78.4) | 137 (84) | 77 (77) | |
| Previous bypass, n (%) | 146 (31.5) | 51 (31.3) | 42 (42) | 0.112 |
| Operative characteristics | ||||
| Urgency, n (%) | ||||
| Elective | 338 (72.8) | 123 (75.5) | 69 (69) | 0.519 |
| Urgent | 126 (27.2) | 40 (24.5) | 31 (31) | |
| Estimated blood loss, mL, mean ± SD | 209.4 ± 183.5 | 346.4 ± 283.6 | 724 ± 807.9 | <0.0001 |
| Graft origin, n (%) | ||||
| Common femoral | 298 (64.2) | 109 (66.9) | 63 (63) | 0.951 |
| Profunda/superficial femoral artery | 108 (23.3) | 36 (22.1) | 23 (23) | |
| AK pop/BK pop/tibial | 58 (12.5) | 18 (11) | 14 (14) | |
| Graft recipient, n (%) | ||||
| AK pop/BK pop | 213 (45.9) | 69 (42.3) | 38 (38) | 0.621 |
| TP trunk/AT/PT/peroneal | 178 (38.4) | 68 (41.7) | 46 (46) | |
| DP, PT ankle/tarsal/plantar | 73 (15.7) | 26 (16) | 16 (16) | |
| Conduit type, n (%) | ||||
| GSV | 308 (66.4) | 111 (68.1) | 63 (63) | 0.858 |
| Arm vein | 51 (11) | 20 (12.3) | 12 (12) | |
| Prosthetic | 116 (25) | 36 (22.1) | 28 (28) | |
| Anesthesia, n (%) | ||||
| Spinal | 74 (15.9) | 33 (20.2) | 8 (8) | 0.023 |
| Epidural | 44 (9.5) | 14 (8.6) | 4 (4) | |
| General | 346 (74.6) | 116 (71.2) | 88 (88) | |
| Concomitant endarterectomy, n (%) | 95 (65.5) | 30 (69.8) | 27 (67.5) | 0.867 |
| Preoperative medications, n (%) | ||||
| ASA | 336 (72.4) | 118 (72.4) | 76 (76) | 0.755 |
| Clopidogrel | 43 (9.3) | 22 (13.5) | 18 (18) | 0.029 |
| Statin | 283 (61.1) | 97 (58.5) | 74 (74) | 0.036 |
AK pop, above-knee popliteal; ASA, aspirin; AT, anterior tibial; BK pop, below-knee popliteal; CAD, coronary artery disease; CHF, congestive heart failure; DP, dorsalis pedis; GSV, greater saphenous vein; PT, posterior tibial; TP, tibioperoneal.
Appendix 2. Postoperative Complications and Graft Patency of the Frequency Group Matched Cohort
| Postoperative complications | 0 U (n = 464) | 1 to 2 U (n = 163) | 3+ U (n = 100) | p Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Mortality | 15 | 3.2 | 4 | 2.5 | 7 | 7 | 0.125 |
| Wound infection | 20 | 4.3 | 15 | 9.2 | 14 | 14 | 0.0008 |
| Graft infection | 0 | 0 | 0 | 0 | 3 | 3 | <0.0001 |
| MI | 24 | 5.2 | 11 | 6.7 | 20 | 20 | <0.0001 |
| Dysrhythmia | 25 | 5.4 | 13 | 8 | 16 | 16 | 0.001 |
| Reoperation | 60 | 12.9 | 29 | 17.8 | 38 | 38 | <0.0001 |
| Loss of primary patency | 20 | 4.4 | 16 | 8.6 | 13 | 13 | 0.0003 |
Footnotes
CME questions for this article available at http://jacscme.facs.org
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
Presented at Annual Meeting of the New England Society for Vascular Surgery, Providence, RI, September 2011.
Author Contributions
Study conception and design: Tan, Farber, Kalish, Eldrup-Jorgensen, Goodney, Cronenwett
Acquisition of data: Tan, Rybin, Doros, Kalish
Analysis and interpretation of data: Tan, Farber, Hamburg, Eberhardt, Goodney, Kalish
Drafting of manuscript: Tan, Farber, Hamburg, Eberhardt, Eldrup-Jorgensen, Goodney, Cronenwett, Kalish
Critical revision: Tan, Farber, Hamburg, Eberhardt, Eldrup-Jorgensen, Goodney, Cronenwett, Kalish
References
- 1.Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 2.Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–1468. doi: 10.1378/chest.119.5.1461. [DOI] [PubMed] [Google Scholar]
- 3.Chelemer SB, Prato S, Cox PM, Jr, et al. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann Thorac Surg. 2002;73:138–142. doi: 10.1016/s0003-4975(01)03308-2. [DOI] [PubMed] [Google Scholar]
- 4.Banbury MK, Brizzio ME, Rajeswaran J, et al. Transfusion increased the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006;202:131–138. doi: 10.1016/j.jamcollsurg.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Fariñas MC, Gald Peralta F, Bernal JM, et al. Suppurative mediastinitis after open-heart surgery: a case-control study covering a seven-year period in Santander, Spain. Clin Infect Dis. 1995;23:272–279. doi: 10.1093/clinids/20.2.272. [DOI] [PubMed] [Google Scholar]
- 6.Edna TH, Bjerkeset T. Association between transfusion of stored blood and infection bacterial complications after resection for colorectal cancer. Eur J Surg. 1998;164:449–456. doi: 10.1080/110241598750004265. [DOI] [PubMed] [Google Scholar]
- 7.Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: a retrospective analysis of 1446 patients. Arch Surg. 2006;141:1014–1018. doi: 10.1001/archsurg.141.10.1014. [DOI] [PubMed] [Google Scholar]
- 8.Innerhofer P, Walleczek C, Luz G, et al. Transfusion of buffy coat-depleted blood components and risk of postoperative infection in orthopedic patients. Transfusion. 1999;39:625–632. doi: 10.1046/j.1537-2995.1999.39060625.x. [DOI] [PubMed] [Google Scholar]
- 9.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 10.O’Keeffe SD, Davenport DL, Minion DJ, et al. Blood transfusion is associated with increased morbidity and mortality after lower extremity revascularization. J Vasc Surg. 2010;51:616–621. doi: 10.1016/j.jvs.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Cronenwett JL, Liksoky DS, Russell MT, et al. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VS GNNE) J Vasc Surg. 2007;46:1093–1102. doi: 10.1016/j.jvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, Rosenbaum PR. Optimal pair matching with two control groups. J Comput Graph Stat. 2004;13:422–434. [Google Scholar]
- 13.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone DH, Goodney PP, Schanzer A, et al. for the Vascular Study Group of New England Clopidogrel is not associated with major bleeding complications during peripheral arterial surgery. J Vasc Surg. 2011;54:779–784. doi: 10.1016/j.jvs.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottino G, De Paulis R, Pansini S, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg. 1987;44:173–179. doi: 10.1016/s0003-4975(10)62035-8. [DOI] [PubMed] [Google Scholar]
- 16.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs superficial infection. Chest. 1996;110:1173–1178. doi: 10.1378/chest.110.5.1173. [DOI] [PubMed] [Google Scholar]
- 17.Leal-Noval SR, Marquez-Vácaro JA, García-Curiel A, et al. Nosocomial pneumonia in patients undergoing heart surgery. Crit Care Med. 2000;28:935–940. doi: 10.1097/00003246-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 19.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;12791:295–307. doi: 10.1378/chest.127.1.295. [DOI] [PubMed] [Google Scholar]
- 20.Fransen E, Massen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–1239. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 21.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–281. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 22.Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–1186. doi: 10.1016/s0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- 23.Koch CG, Li L, Duncan AL, et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81:1650–1657. doi: 10.1016/j.athoracsur.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Surgenor SD, Kramer RS, Olmstead EM, et al. Northern New England Cardiovascular Disease Study Group The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108:1741–1746. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- 25.Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 26.Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252:11–17. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
- 27.Adam DJ, Beard JD, Cleveland T, et al. BASIL trial participants Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicenter, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen LL, Brahmanandam S, Bandyk DF, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46:1191–1197. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nolan BW, De Martino RR, Stone DH, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54:730–735. doi: 10.1016/j.jvs.2011.03.236. discussion 735–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodney PP, Nolan BW, Schanzer A, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51:71–79. doi: 10.1016/j.jvs.2009.07.123. [DOI] [PubMed] [Google Scholar]
- 31.Simons JP, Goodney PP, Nolan BW, et al. Vascular Study Group of Northern New England Failure to achieve clinical improvement despite graft patency in patients undergoing infrainguinal lower extremity bypass for critical limb ischemia. J Vasc Surg. 2010;51:1419–1424. doi: 10.1016/j.jvs.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]


