Abstract
Background
Although open surgical bypass remains the standard revascularization strategy for patients with critical limb ischemia (CLI), many centers now perform peripheral endovascular intervention (PVI) as the first-line treatment for these patients. We sought to determine the effect of a prior ipsilateral PVI (iPVI) on the outcome of subsequent lower extremity bypass (LEB) in patients with CLI.
Methods
A retrospective cohort analysis of all patients undergoing infrainguinal LEB between 2003 and 2009 within hospitals comprising the Vascular Study Group of New England (VSGNE) was performed. Primary study endpoints were major amputation and graft occlusion at 1 year postoperatively. Secondary outcomes included in-hospital major adverse events (MAE), 1-year mortality, and composite 1-year major adverse limb events (MALE). Event rates were determined using life table analyses and comparisons were performed using the log-rank test. Multivariate predictors were determined using a Cox proportional hazards model with multilevel hierarchical adjustment.
Results
Of 1880 LEBs performed, 32% (n = 603) had a prior infrainguinal revascularization procedure (iPVI, 7%; ipsilateral bypass, 15%; contralateral PVI, 3%; contralateral bypass, 17%). Patients with prior iPVI, compared with those without a prior iPVI, were more likely to be women (32 vs 41%; P = .04), less likely to have tissue loss (52% vs 63%; P = .02), more likely to require arm vein conduit (16% vs 5%; P = .001), and more likely to be on statin (71% vs 54%; P = .01) and beta blocker therapy (92% vs 81%; P = .01) at the time of their bypass procedure. Other demographic factors were similar between these groups. Prior PVI or bypass did not alter 30-day MAE and 1-year mortality after the index bypass. In contrast, 1-year major amputation and 1-year graft occlusion rates were significantly higher in patients who had prior iPVI than those without (31% vs 20%; P = .046 and 28% vs 18%; P = .009), similar to patients who had a prior ipsilateral bypass (1 year major amputation, 29% vs 20%; P = .022; 1 year graft occlusion, 33% vs 18%; P = .001). Independent multivariate predictors of higher 1-year amputation and graft occlusion rates were prior iPVI, prior ipsilateral bypass, dialysis dependence, prosthetic conduit and distal (tibial and pedal) bypass target.
Conclusions
Prior iPVI is highly predictive for poor outcome in patients undergoing LEB for CLI with higher 1-year amputation and graft occlusion rates than those without prior revascularization, similar to prior ipsilateral bypass These findings provide information, which may help with the complex decisions surrounding revascularization options in patients with CLI.
Although open surgical bypass provides the most durable option for limb salvage in patients with critical limb ischemia (CLI),1 it has substantial morbidity and mortality. Thus, many centers prefer to initially treat CLI patients with a less-invasive endovascular peripheral endovascular intervention (PVI) instead of open bypass. Results of the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial suggest that overall differences in amputation-free survival (AFS) after PVI vs bypass are insignificant.2,3
Although the potential benefit of less-invasive PVI is clear, the potential negative impact of PVI on ultimate limb salvage in CLI patients is unclear. In the BASIL Trial, patients undergoing bypass after a failed PVI had worse AFS than those undergoing primary bypass.3,4 Additionally, previous studies have suggested that patients who failed PVI and went on to require bypass needed longer bypasses to more distal targets.5,6 These findings have stimulated debate over PVI vs bypass as the optimal first-line strategy for the treatment of CLI.
The outcome of secondary lower extremity bypass after failed primary bypass is known to be poor, with significantly higher graft occlusion and amputation rates.7,8 However, the outcome of lower extremity bypass after failed ipsilateral PVI (iPVI) is not established. The purpose of this study is to assess the effect of a failed prior PVI on the outcome of infrainguinal bypass performed for CLI and to compare the magnitude of this effect with the impact of a failed previous bypass graft.
METHODS
Study design, sample, and database
This is a retrospective cohort analysis of 1880 infrainguinal bypass grafts performed between January 2003 and December 2009 for CLI (rest pain or tissue loss) in 10 centers participating in the Vascular Study Group of New England (VSGNE). The VSGNE is a regional cooperative quality improvement initiative developed by community and academic centers in 2002 to study regional outcomes in vascular surgery. Further details on this registry have been published previously9 and are available at http://www.vsgne.org. In the VSGNE registry, we collect over 70 preoperative, intraoperative, and postoperative variables, as well as key outcomes at 1-year follow-up, as described in previous publications.9,10
Historical information collected at the time of surgery indicated that 32% (n = 603) of patients had undergone an infrainguinal revascularization procedure at some time prior to the index bypass procedure being entered into the VSGNE registry. These consisted of 7% (n = 132) iPVI, 15% (n = 275) ipsilateral bypass, 3% (n = 62) contralateral PVI, and 17% (n = 326) contralateral bypass. One hundred ninety-four patients (10%) underwent multiple revascularization procedures. These are shown in the Appendix (online only). The number of patients undergoing bypass with a history of a prior ipsilateral PVI varied substantially among centers, ranging from 2% to 13% (data not shown) and the observed variation was used to adjust for outcomes in our statistical modeling. Details of these previous procedures were not further recorded, so timing and precise type of previous bypass or PVI is not known.
Study endpoints
To evaluate the outcomes of lower extremity bypass (LEB) following a failed PVI, we chose our study endpoints to target safety and efficacy of the procedure as previously described.11 The principal efficacy outcomes of this study were major amputation (above or below knee) and graft occlusion within the first year postoperatively to evaluate efficacy of LEB. The safety outcomes included in-hospital major adverse events (myocardial infarction, congestive heart failure, dysrhythmia requiring treatment, wound infection, renal insufficiency, respiratory failure, pneumonia, graft infection, return to the operating room, death, graft occlusion, or major amputation). Additional efficacy outcomes included 1-year mortality and 1-year major adverse limb events (major adverse limb events [MALE] – major above-knee amputation, new bypass, jump graft/interposition graft, graft occlusion or major reintervention requiring thrombectomy or thrombolysis).11
Definitions of patient medical comorbidities in the VSGNE have been defined elsewhere.12 These include chronic obstructive pulmonary disease (COPD; chart history), congestive heart failure (CHF; chart history or documented ejection fraction <50% on preoperative testing), coronary artery disease (CAD; any history of angina, myocardial infarction [MI], prior coronary intervention, or electrocardiogram [EKG] changes consistent with previous MI), chronic renal insufficiency (CRI; Cr ≥1.8 mg/dL), end-stage renal disease (ESRD; on dialysis), diabetes mellitus (DM; chart history, designated as diet-controlled, on oral hypoglycemic agents, or on insulin), and hypertension (HTN; chart history or blood pressure ≥140/90), hyperlipidemia (HLIP; chart history). For our analysis, distal bypass included tibial and pedal targets.
Analysis
Demographic data were compared using a t test for continuous variables and χ2 with Fischer exact correction for categorical or dichotomous variables. Rates were determined using Kaplan–Meier life table analyses. Comparisons of rates were made using the log-rank test. Multivariate predictors of major amputation and graft occlusion were determined using Cox proportional hazards models. Univariate predictors for outcomes identified in Table I were tested in our multivariate model and those that were significant were included in the multivariate model, including graft type and target vessel. Multilevel (hierarchical) modeling was performed to account for differences in outcomes across centers. All analyses were performed using Microsoft Excel (Redmond, Wash) and Stata (College Station, Tex). The Institutional Review Board at Dartmouth Medical School approved the analysis of deidentified data from our registry for this study.
Table I.
Patient characteristics
| No prior revasca (n=1277) | Ipsilateral PVI (n=134) | Bypass (n=275) | Contralateral PVI (n=62) | Bypass (n=326) | |
|---|---|---|---|---|---|
| Age | 70 | 68 | 68 | 67 | 70 |
| Male | 68% | 59%b | 64% | 65% | 70% |
| Tobacco use | 80% | 82% | 88%b | 84% | 82% |
| Hypertension | 86% | 90% | 89% | 95% | 94%b |
| Diabetes | 56% | 58% | 57% | 66%b | 68%b |
| CAD | 40% | 42% | 47%b | 47% | 44% |
| CHF | 23% | 24% | 16%b | 19% | 21% |
| COPD | 31% | 34% | 34% | 39% | 29% |
| Dialysis | 10% | 5%b | 5%b | 15% | 9% |
| Renal insufficiency | 20% | 13% | 12%b | 17% | 19% |
| Tissue loss | 63% | 52%b | 49%b | 53% | 63% |
| Pre-op beta blocker | 81% | 92%b | 84% | 94%b | 88% |
| ASA | 68% | 73% | 66% | 76% | 69% |
| Statin | 54% | 71%b | 59% | 69%b | 61%b |
| Prosthetic conduit | 24% | 29% | 46%b | 39%b | 32%b |
| Arm vein conduit | 5% | 16%b | 17%b | 11%b | 15%b |
| Crural distal target | 47% | 53% | 54% | 29%b | 49% |
ASA, Aspirin; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PVI, peripheral vascular intervention.
Renal insufficiency defines as creatinine >1.8. Coronary artery disease (CAD), congestive heart failure (CHF), and chronic obstructive pulmonary disease (COPD) defined by chart history.
Revasc, revascularization procedure (PVI or bypass).
P <.05 (compared with no prior revascularization group).
RESULTS
Most demographic factors were similar when compared between those who underwent prior iPVI and those who did not (Table I). However, patients who had been treated with prior iPVI were less likely to have tissue loss, were more likely to be women, were more likely to be on statin therapy and beta blocker therapy at the time of their index bypass, and were more likely to require arm vein for conduit (P < .05). Although there was a tendency for these patients to require more distal, tibial bypass targets, this finding was not statistically significant and their subsequent outcome was not changes.
Overall, in-hospital major adverse events occurred in 27% of patients after bypass for CLI in VSGNE with no prior revascularization procedures. This event rate was not affected by whether or not a patient had undergone previous iPVI or bypass (Table II). Similarly, 1-year mortality (12%) was not different among these groups (Table II).
Table II.
Major adverse events (MAE) and 1-year mortality based on prior revascularization
| Type of prior revascularization | In hospital MAE | Pa | One-year mortality (P valueb) | Pb |
|---|---|---|---|---|
| No prior revascularizationa | 27% | 12% | ||
| Ipsilateral | ||||
| Prior PVI | 30% | .54 | 13% | .95 |
| Prior bypass | 28% | .83 | 13% | .51 |
| Contralateral | ||||
| Prior PVI | 34% | .22 | 13% | .85 |
| Prior bypass | 35% | .54 | 12% | .36 |
PVI, Peripheral vascular intervention.
Major adverse events include myocardial infection, congestive heart failure, dysrhythmia requiring treatment, wound infection, renal insufficiency, respiratory failure, pneumonia, graft infection, return to the operating room, death, graft occlusion, or major amputation.
Comparison by χ2 statistic.
Comparison by log-rank statistic.
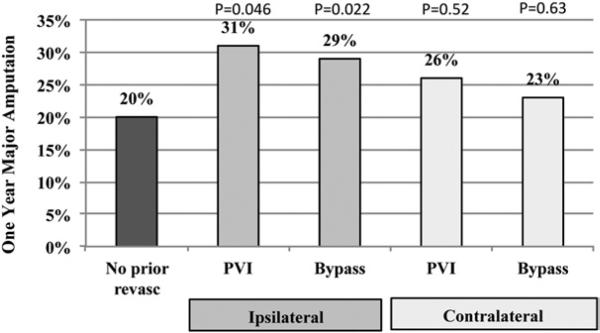
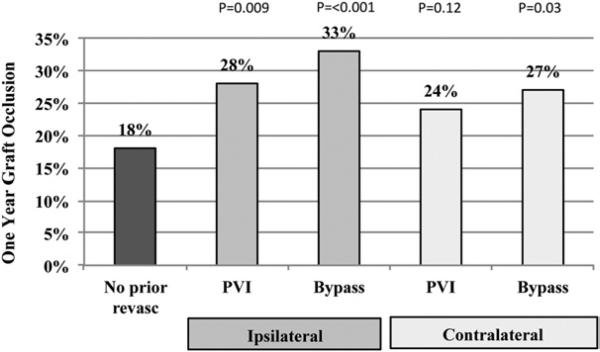
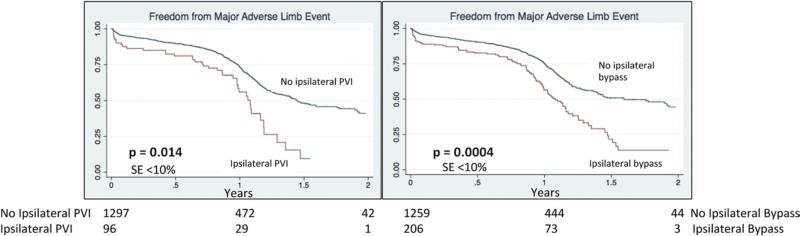
Major amputation by 1 year postoperatively, however, was significantly more likely after infrainguinal bypass if patients had a prior iPVI, at 31% (P = .046), or prior bypass, at 29% (P = .022), compared with 20% in patients without prior revascularization procedures (Fig 1). Prior contralateral procedures (PVI or bypass) were not associated with a higher rate of amputation (Fig 1). Similarly, the 1-year graft occlusion rate was significantly higher in patients who had prior iPVI, at 28% (P = .009), or prior bypass at 33% (P < .001), compared with only 18% in patients without prior ipsilateral revascularization (Fig 2). As a combined endpoint, MALE were significantly more likely in patients with prior iPVI, or bypass (Fig 3).
Fig 1.
One-year major amputation rates in patients undergoing lower extremity bypass who have undergone prior revascularization procedure. P values compared with patients with no prior revascularization procedure. PVI, Peripheral vascular intervention.
Fig 2.
One-year graft occlusion rates in patients undergoing lower extremity bypass who have undergone prior revascularization procedure. P values compared with patients with no prior revascularization procedure. PVI, Peripheral vascular intervention.
Fig 3.
Freedom from major adverse limb events after lower extremity bypass. Major adverse limb event includes amputation, graft occlusion, or reintervention. PVI, Peripheral vascular intervention.
By multivariate analysis, predictors of 1-year major amputation rate were prior ipsilateral PVI or bypass, dialysis dependence, prosthetic conduit, and tibial level bypass target. Aspirin was found to be protective. Prior ipsilateral PVI or bypass similarly increased the risk of major amputation by approximately 50% in addition to dialysis dependence, prosthetic conduit, and tibial level target. Aspirin use was protective of amputation at 1 year (Table III). Finally, prior ipsilateral PVI and bypass also increased the risk of 1-year graft occlusion by 50% (Table IV). Dialysis dependence, prosthetic conduit, arm vein conduit, a tibial level bypass target, and female gender also increased the risk of graft occlusion by multivariate analysis (Table IV).
Table III.
Independent predictors of major amputation at 1 year
| HR | 95% CI | ||
|---|---|---|---|
| Dialysis | 2.7 | 2.2 | 3.3 |
| Prosthetic conduit | 2.1 | 1.3 | 3.4 |
| Prior ipsilateral PVI | 1.5 | 1.1 | 2 |
| Prior ipsilateral bypass | 1.4 | 1.1 | 1.7 |
| Tibial target | 1.4 | 1.2 | 1.7 |
| Aspirin | 0.8 | 0.7 | 0.9 |
CI, Confidence interval; HR, hazard ratio; PVI, peripheral vascular intervention.
Table IV.
Independent predictors of graft occlusion at 1 year
| HR | 95% CI | ||
|---|---|---|---|
| Tibial target | 1.9 | 1.6 | 2.4 |
| Dialysis | 1.8 | 1.3 | 2.5 |
| Arm vein conduit | 1.7 | 1.3 | 2.1 |
| Prosthetic conduit | 1.6 | 1.3 | 1.8 |
| Prior ipsilateral PVI | 1.5 | 1.1 | 2.1 |
| Prior ipsilateral bypass | 1.4 | 1.2 | 1.6 |
| Female gender | 1.3 | 1.1 | 1.5 |
CI, Confidence interval; HR, hazard ratio; PVI, peripheral vascular intervention.
DISCUSSION
Our study shows that a prior ipsilateral infrainguinal PVI is an important predictor of poor outcomes after lower extremity bypass for CLI. According to multivariate analysis, a prior iPVI increased both the risk of limb loss and the risk of occlusion of a subsequent infrainguinal bypass by approximately 50% at 1 year. This magnitude of effect was very similar to the negative predictive effect of a prior ipsilateral bypass that has reported patency rates of only 50% to 60% and limb salvage rates of 80% at 1 year.13 Other factors identified in our risk-prediction model have been previously reported to confer a negative effect on lower extremity bypass outcomes. These factors include dialysis dependence, prosthetic conduit, tibial (as opposed to popliteal) target and female gender,14-16 lending face validity to the risk prediction model. Aspirin was found to be protective as previously noted by others.17 These findings lend support to the general applicability of the VSGNE findings. In contrast to the impact of prior ipsilateral PVI or bypass, contralateral PVI or bypass did not affect the risk of either amputation or graft occlusion. Thus, systemic factors such as a hypercoagulable state are less likely to have been responsible for the negative impact of a prior ipsilateral procedure. Further, there were no differences in postoperative major adverse events or 1-year mortality between patients having primary bypass vs those who had prior ipsilateral revascularization, suggesting that prior ipsilateral revascularization primarily impacts graft-related events, rather than global patient-related outcomes such as medical, graft, or wound complications.
The BASIL trial is the only large-scale randomized control trial to evaluate the outcomes of PVI vs bypass in patients eligible for both. The results of our study regarding the impact of prior PVI on AFS are similar to those published in the BASIL Trial,3 which reported AFS at 1 year of only 40% for bypass after failed angioplasty vs 70% after initial bypass. Though not an endpoint of our study, corresponding 1-year results for AFS in VSGNE were 53% for bypass after failed angioplasty vs 76% after initial bypass (P = .03), very similar to the BASIL results.
The outcomes of prior failed PVI have been described in other studies,4-6,18 but few evaluate the impact of the PVI on subsequent bypass, as most are treated with a repeat PVI.6,18 In a review of failed PVI, five of 46 patients with failed PVI required subsequent bypass with a 20% amputation rate. Similarly, of 39 early (<30 day) PVI failures, Galaria et al reported that 19 required subsequent bypass with a similar 17% rate of amputation.18 In a study of 25 bypasses after failed PVI, Böckler et al demonstrated similar outcomes to the present study with a bypass failure rate of 50% and an amputation rate of 44% over a median of 6 months.4
The effect of a failed PVI on a distal anastomotic site is less well understood. Joels et al reported that a failed PVI resulted in a more distal target for bypass in 28% of cases and half of these patients had compromised runoff as a result of PVI failure.5 Conversely, others have reported the opposite. One study of 46 failed PVIs reported no change in distal anastomosis site over a mean follow-up of 8.7 months.6 Another reported that of 39 PVI failures, there was no change in distal anastomotic site. This discrepancy in the literature may be due to the small number of cases in each study where a failed PVI required a subsequent bypass. While the present study is the largest to date to report on bypass outcome after a prior PVI, we are unable to determine if the PVI failure affected the distal target since information of the index procedure is not tracked in our database.
Why is prior ipsilateral PVI or bypass associated with worse results for infrainguinal bypass? Several possible explanations exist. First, failure of prior PVI or bypass could be a surrogate for patient-specific risk factors such as hypercoagulability15 that are not measured in our registry. However, this seems unlikely given that a previous failed contralateral revascularization procedure was not associated with any negative effects. Second, a prior failed PVI or bypass could compromise runoff for a future bypass by embolization or thrombosis. While this has been demonstrated in previous reports,5 we were only able to identify a trend (P = .15) in this cohort for secondary bypasses to require a more distal target artery than primary bypasses. Finally, marginal vein quality may have dictated the initial choice of PVI or the type of previous bypass (for example, initial use of prosthetic graft if vein quality was marginal). If marginal vein was then resorted to for the subsequent bypass, this could negatively influence graft outcome.19 Vein quality used for bypass is not recorded in our registry beyond the use of arm vein or spliced veins, which were included in our analyses. In our multivariate models, arm vein was significantly associated with graft occlusion at 1 year but not with major amputation.
Our study has several limitations. Most notably, we know only that patients in our registry had undergone a prior bypass or PVI, but we do not have data on the type of procedure or when it occurred. Others reporting on similar complications after PVI note that patients with failure present within 4 to 6 months after their procedure.4,5 Additionally, we do not have data on the total number of PVI procedures previously performed, which might provide a better understanding of the association between a failed PVI and later bypass. The VSGNE is now tracking all PVIs prospectively to assist in answering these questions. Finally, without a randomized trial, the effect of unknown confounders exists. Yet, we have attempted to minimize the effect of this with sound statistical methods in our multivariate model and adjust for potential confounders such as differences across centers or distal target vessels. When controlling for all of these known factors, a prior PVI still appears to be associated with a risk of major graft events at 1 year.
In conclusion, while it is well known that a prior failed ipsilateral infrainguinal bypass is a negative predictor for future LEB success, this study demonstrates that a prior failed infrainguinal ipsilateral PVI has a similar negative prognostic effect on subsequent LEB. While we are unable to definitively prove causation, prior failed ipsilateral PVI is associated with 1-year major amputation or graft occlusion in the same manner that other established factors such as dialysis dependence, synthetic conduit and distal target arteries are. While some patients may benefit from an initial PVI, those who are eligible for either procedure may benefit from bypass given our findings and those of the BASIL trial. Further work is necessary to clarify the durability, utility, and cost-effectiveness of a PVI-first approach for patients presenting with CLI.
Supplementary Material
Footnotes
Competition of interest: none.
Additional material for this article may be found online at www.jvascsurg.org
Presented at the 2010 Vascular Annual Meeting of the Society for Vascular Surgery, June 10-13, 2010, Boston, Mass.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: BN, DS, AS, PG, JC
Analysis and interpretation: BN, DS, PG, DW, LC
Data collection: BN, AS, PG, JC
Writing the article: BN, RD
Critical revision of the article: BN, RD, JC
Final approval of the article: BN, LC
Statistical analysis: BN, PG
Obtained funding: JC
Overall responsibility: BN
REFERENCES
- 1.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–41. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 2.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplastyfirst revascularization strategy. J Vasc Surg. 2010;51:5S–17S. doi: 10.1016/j.jvs.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51:18S–31S. doi: 10.1016/j.jvs.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 4.Böckler D, Blaurock P, Mansmann U, Schwarzbach M, Seelos R, Schumacher H, et al. Early surgical outcome after failed primary stenting for lower limb occlusive disease. J Endovasc Ther. 2005;12:13–21. doi: 10.1583/04-1252.1. [DOI] [PubMed] [Google Scholar]
- 5.Joels CS, York JW, Kalbaugh CA, Cull DL, Langan EM III, Taylor SM, et al. Surgical implications of early failed endovascular intervention of the superficial femoral artery. J Vasc Surg. 2008;47:562–5. doi: 10.1016/j.jvs.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Ryer EJ, Trocciola SM, DeRubertis B, Lam R, Hynecek RL, Karwowski J, et al. Analysis of outcomes following failed endovascular treatment of chronic limb ischemia. Ann Vasc Surg. 2006;20:440–6. doi: 10.1007/s10016-006-9101-4. [DOI] [PubMed] [Google Scholar]
- 7.Rossi PJ, Skelly CL, Meyerson SL, Bassiouny HS, Katz D, Schwartz LB, et al. Redo infrainguinal bypass: factors predicting patency and limb salvage. Ann Vasc Surg. 2003;17:492–502. doi: 10.1007/s10016-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 8.Biancari F, Railo M, Lundin J, Albäck A, Kantonen I, Lehtola A, et al. Redo bypass surgery to the infrapopliteal arteries for critical leg ischaemia. Eur J Vasc Endovasc Surg. 2001;21:137–42. doi: 10.1053/ejvs.2000.1290. [DOI] [PubMed] [Google Scholar]
- 9.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE). J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. Discussion:101-2. [DOI] [PubMed] [Google Scholar]
- 10.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52:674–83. 683, e1–83, e3. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–73. e1–3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE). J Vasc. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 101-2. [DOI] [PubMed] [Google Scholar]
- 13.Belkin M, Conte MS, Donaldson MC, Mannick JA, Whittemore AD. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21:282–93. doi: 10.1016/s0741-5214(95)70269-5. discussion:93-5. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Sidawy AN, DeZee KJ, Neville RF, Akbari C, Henderson W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008;47:556–61. doi: 10.1016/j.jvs.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 15.Giswold ME, Landry GJ, Sexton GJ, Yeager RA, Edwards JM, Taylor LM, Jr, et al. Modifiable patient factors are associated with reverse vein graft occlusion in the era of duplex scan surveillance. J Vasc Surg. 2003;37:47–53. doi: 10.1067/mva.2003.4. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119:123–30. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins TC, Souchek J, Beyth RJ. Benefits of antithrombotic therapy after infrainguinal bypass grafting: a meta-analysis. Am J Med. 2004;117:93–9. doi: 10.1016/j.amjmed.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 18.Galaria SSM, II, Rhodes JM, Shortell CK, Illig KA, Davies MG. Implications of early failure of superficial femoral artery endoluminal interventions. Ann Vasc Surg. 2005;19:787–92. doi: 10.1007/s10016-005-7972-4. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson MC, Mannick JA, Whittemore AD. Causes of primary graft failure after in situ saphenous vein bypass grafting. J Vasc Surg. 1992;15:113–8. doi: 10.1067/mva.1992.32984. discussion:8-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.