Abstract
Objectives
We have previously reported the results of a dose-finding phase II trial showing that HGF angiogenic gene therapy can increase TcPO2 compared with placebo in patients with critical limb ischemia (CLI). The purpose of this randomized placebo controlled multi-center trial was to further assess the safety and clinical efficacy of a modified HGF gene delivery technique in patients with CLI and no revascularization options.
Methods
Patients with lower extremity ischemic tissue loss (Rutherford 5 and 6) received three sets of eight intramuscular injections every 2 weeks of HGF plasmid under duplex ultrasound guidance. Injection locations were individualized for each patient based on arteriographically defined vascular anatomy. Primary safety end point was incidence of adverse events (AE) or serious adverse events (SAE). Clinical end points included change from baseline in toe brachial index (TBI), rest pain assessment by a 10 cm visual analogue scale (VAS) as well as wound healing, amputation, and survival at 3 and 6 months.
Results
Randomization ratio was 3:1 HGF (n = 21) vs placebo (n = 6). Mean age was 76 ± 2 years, with 56% male and 59% diabetic. There was no difference in demographics between groups. There was no difference in AEs or SAEs, which consisted mostly of transient injection site discomfort, worsening of CLI, and intercurrent illnesses. Change in TBI significantly improved from baseline at 6 months in the HGF-treated group compared with placebo (0.05 ± 0.05 vs −0.17 ± 0.04; P = .047). Change in VAS from baseline at 6 months was also significantly improved in the HGF-treated group compared with placebo (−1.9 ± 1.3 vs +0.06 ± 0.2; P = .04). Complete ulcer healing at 12 months occurred in 31% of the HGF group and 0% of the placebo (P = .28) There was no difference in major amputation of the treated limb (HGF 29% vs placebo 33%) or mortality at 12 months (HGF 19% vs placebo 17%) between groups.
Conclusion
HGF gene therapy using a patient vascular anatomy specific delivery technique appears safe, maintained limb perfusion, and decreased rest pain in patients with CLI compared with placebo. A larger study to assess the efficacy of this therapy on more clinically relevant end points is warranted.
Critical limb ischemia (CLI) remains a major unmet public health care need. At present, effective treatment options for this patient population involve some form of revascularization, either through endovascular approaches or bypass surgery. Unfortunately, up to 50% of patients with CLI are not candidates for revascularization because of unsuitable anatomy or associated comorbidities such as coronary disease, pulmonary disease, and renal failure.1 Options for patients who are not candidates for revascularization remain poor, as there is no US Food and Drug Administration-approved medical therapy for CLI. Patients who do not have the option of revascularization have a 20% to 40% incidence of major amputation or death within 1 year.2,3 The incidence of CLI is expected to increase over the course of the next 10 to 15 years.
Therapeutic angiogenesis is a developing biologic therapy that attempts to use various angiogenic growth factors or autologous stem cells to improve perfusion in areas of ischemia through the development of new blood vessels from pre-existing blood vessels. Because of the short half-life of recombinant proteins, current clinical trials have approached the delivery of angiogenic factors through either a stem cell or gene therapy approach. Stem cell therapies in the United States are currently in early development, whereas several randomized placebo controlled gene therapy trials have been reported.
We have recently reported the results of the intramuscular infection of hepatocyte growth factor plasmid to improve limb perfusion on patients with critical limb ischemia (HGF-STAT) angiogenic gene therapy trial.4 This was a placebo-controlled randomized multi-center phase II trial that assessed the safety of varying doses of human hepatocyte growth factor (HGF) gene therapy in patients with CLI. HGF is an angiogenic protein that has been shown to improve neovascularization and limb perfusion in animal models of hind limb ischemia.5-7 In addition, this study evaluated efficacy as determined by change in transcutaneous oxygen pressure (TcPO2) in the foot following treatment, a surrogate measure for change in limb perfusion. This study showed that HGF gene therapy was well tolerated and that the high dose gene therapy group had a significant improvement in TcPO2 from baseline compared with placebo. Based on the results of the HGF-STAT trial, we conducted a second follow-up phase II study to further define the safety and potential efficacy of the HGF gene therapy dose identified in the previous trial to improve limb perfusion in patients with CLI. This was done prior to considering a phase III pivotal trial in order to gain further safety data. In addition, questions remained after the initial trial regarding the optimal location and delivery technique best suited to efficiently deliver the HGF plasmid. The purpose of the current study was to assess the effect of HGF gene therapy using a novel patient-specific gene delivery technique on safety, wound healing, and toe brachial index (TBI) in patients with CLI.
METHODS
This trial was approved by each study site’s Institutional Investigation Review Board prior to patient enrollment. This was a double-blind, parallel-group, placebo-controlled multi-institutional study in which eligible subjects were randomized to receive placebo or 4.0 mg AMG0001 by intramuscular injection. After a 30-day screening period, eligible subjects entered a 28-day treatment period during which subjects were dosed on three separate occasions (Days 0, 14, 28). Following the treatment period, subjects were followed for the next 11 months. The safety analysis was analyzed on intent-to-treat, which included all randomized subjects who received at least one dose of treatment. The efficacy evaluable (EE) population included all subjects who received all three doses and had at least one follow-up visit after receiving all three doses but before having either a peripheral vascular intervention or a major amputation. Eight centers enrolled patients into the trial.
Injections were performed into the ischemic extremity based on vascular disease anatomy as depicted on arteriography, magnetic resonance angiography, or computed tomography angiography and determined by a central committee of vascular specialists. This gene delivery technique utilized ultrasound-guided injection into the muscle surrounding occluded tibial vessels based on the preprocedure arteriography.
The goal of the committee was to ensure that injections were delivered into regions of the anatomically most severe vascular disease.8 This resulted in all injections being placed below the knee in patients with only tibial occlusive disease and injections placed above and below the knee in patients with combined SFA and tibial disease. Each series of injections consisted of eight injections of 3.0 ml volume delivered under duplex guidance to confirm intramuscular delivery of the therapeutic.
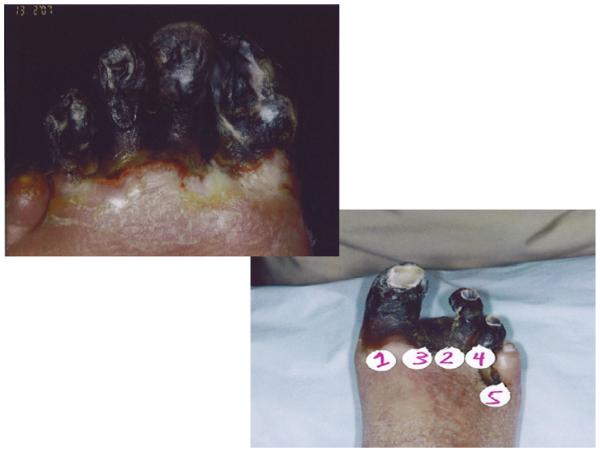
The efficacy end points were defined as wound healing as measured by change in size of ulcer at months 3 and 6, along with reduction in major amputation and improved pain at rest as measured by the visual analogue scale (VAS), and hemodynamic measurements as measured by anklebrachial index (ABI)/TBI at month 3 and month 6. Complete wound healing required complete epithelialization of the wound for at least 2 weeks. Complete wound healing in patients with gangrene involving the toes, as shown in Fig 1, was defined as healing of the surgical wound following amputation or excision. Additional study end points included survival and quality of life measures.
Fig 1.
Example of severity of tissue loss in two patients enrolled into HGF-0205 Trial.
Major inclusion criteria included:
Subjects needed to have appropriately sized ischemic peripheral ulcer(s) or tissue loss. Photographs of the wounds were reviewed by a vascular specialist prior to patient enrollment.
- Subjects needed to have one or both of the following hemodynamic indicators of severe peripheral arterial occlusive disease
- Ankle systolic pressure (in either the dorsalis pedis or posterior tibial arteries) of ≤70 mm Hg or
- Toe systolic pressure ≤50 mm Hg.
The subject was a poor candidate for standard revascularization treatment options for peripheral arterial disease, based on inadequate bypass conduit, unfavorable anatomy, or poor operative risk.
Major exclusion criteria included:
Subjects who, in the opinion of the investigator, had a vascular disease prognosis that indicated they may require a major amputation (at or above the ankle) within 4 weeks of start of treatment.
Subjects with a diagnosis of Buerger’s disease (thromboangitis obliterans).
Subjects with hemodynamically significant aorto-iliac occlusive disease.
Subjects who have had a revascularization procedure within 12 weeks prior to treatment initiation that remained patent. Revascularization procedures that were evidenced to have failed (completely occluded) for >2 weeks prior to treatment initiation were acceptable.
Subjects with deep ulcerations with bone or tendon exposure, or clinical evidence of invasive infection (eg, cellulitis, osteomyelitis, etc) uncontrollable by antibiotic therapy.
Evidence or history of malignant neoplasm (clinical, laboratory, or imaging), except for fully resolved basal cell carcinoma of the skin. Patients who underwent successful tumor resection or radio-chemotherapy of breast cancer more than 10 years prior to inclusion in the study, and with no recurrence, could be enrolled in the study. Patients who had successful tumor resection or radio-chemotherapy of all other tumor types more than 5 years prior to inclusion in the study, and with no recurrence, could be enrolled in the study.
Subjects who had proliferative diabetic retinopathy, severe non-proliferative retinopathy, recent (within 6 months) retinal vein occlusion, macular degeneration with choroidal neovascularization, macular edema on fundus evaluation by ophthalmologist, or intraocular surgery within 3 months.
Subjects with history of end stage renal disease (ESRD) defined as significant renal dysfunction evidenced by a creatinine of >2.5 mg/dL, or receiving chronic hemodialysis therapy.
Statistical plan
The sample size planned for this study was a maximum of 48 randomized subjects, with 32 subjects randomized to the AMG0001 arm and 16 subjects randomized to the placebo arm. Allowing for subjects who prematurely discontinued or were excluded from the efficacy evaluable population, it was expected that there would be at least 39 evaluable subjects (26 in the AMG0001 arm and 13 in the placebo arm) at the month 3 follow-up visit. This latter number is considered appropriate to determine a statistically significant difference in the reduction of total wound area between the AMG0001 treatment group and the placebo treatment group at month 3.
A Japanese study with the same AMG0001 dosage and administration methods showed a mean reduction in total wound area of 2.5 cm2 at month 3 compared with baseline in the AMG0001-treated arm.8 Standard deviation estimates for these means ranged from 1.05 (AMG0001) to 4.7 (placebo). Assuming the common standard deviation was 3.0, the proposed sample size would provide sufficient power (80%) at an alpha = 0.05 level to detect a minimum mean difference in total wound area of 2.9 cm2 from baseline to month 3 between the subjects in the AMG0001-treated arm and the subjects in the placebo-treated arm.
Analyses for continuous variables were performed with t tests, and analyses for categorical variables were performed with Fisher’s exact tests. P < .05 was considered significant. For missing data, last observation carried forward (LOCF) was used in the analysis.
RESULTS
Demographics
The trial was stopped early after enrolling 27 subjects that were randomized to either HGF treatment (n = 21) or placebo (n = 6). Patient demographics were not different between the groups and are shown in the Table. All patients in the HGF-treated group had undergone either a failed open surgical revascularization (85%) and or endovascular therapy (24%). In the placebotreated group, surgical revascularization had been performed in 67% of subjects and endovascular therapy in 17%. Prior to enrollment, major contralateral amputation had been performed in 14% of HGF-treated patients compared with 0% of placebo (P = 1.0). Minor amputation had been performed in 29% of HGF-treated subjects compared with 0% of placebo (P = .28).
Table.
Patient characteristics
|
Placebo
(n = 6) |
HGF-treated
(n = 21) |
P value | |
|---|---|---|---|
| Age | 78 ± 2 years | 76 ± 2 years | .64 |
| Race | |||
| White | 5 (83%) | 18 (86%) | 1.0 |
| Black | 1 (17%) | 3 (14%) | |
| Gender | |||
| Male | 2 (33%) | 13 (62%) | .36 |
| Tobacco | 3 (50%) | 14 (67%) | |
| Diabetes | 3 (50%) | 13 (62%) | .66 |
| Creatinine clearance <60 ml/min |
4 (67%) | 12 (57%) | 1.0 |
Safety
The injections were well-tolerated. There was no difference in adverse or serious adverse events between the two groups at 12-month follow-up. Adverse events occurred in 95% of HGF-treated subjects and 83% of placebo-treated subjects (P = .40). The majority of adverse events consisted mostly of transient injection site discomfort, worsening of CLI, and intercurrent illnesses. There was no progression of diabetic retinopathy, and no patients developed new malignancy during follow-up.
Wound healing
At 6-month follow-up, wounds had healed in 19% of patients in the HGF group compared with 0% in the placebo group. This was not a significant difference. By 12 months, 31% of patients had wounds healed in the HGF group compared with 0% in the placebo group (P = .28). There was no significant difference in rate of wound healing between the groups at any time point studied.
TBI
As shown in Fig 2, change in TBI from baseline in the HGF-treated patients was significantly improved at 6 months compared with placebo. The TBI in HGF-treated patients increased 0.05 ± 0.05 compared with a decrease in TBI of −0.17 ± 0.04 in placebo-treated patients (P = .047).
Fig 2.
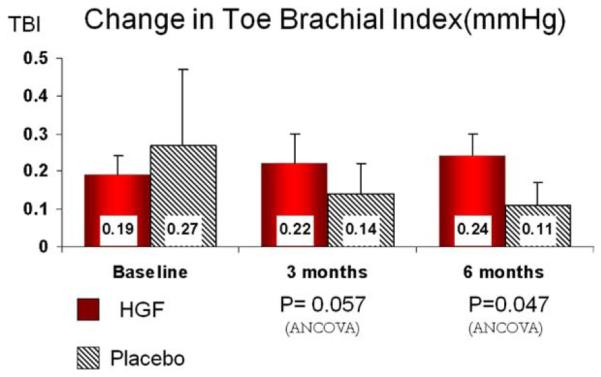
Toe brachial index at baseline and 3 and 6 months following first injections (solid columns) compared with placebo (hatched columns). Data presented as mean ± standard error.
Lower extremity pain
As shown in Fig 3, pain, as measured by VAS, was significantly improved from baseline at 6 months in HGF-treated patients compared with placebo. VAS improved (decreased) from baseline at 6 months in the HGF-treated group compared with a worsening (increase in VAS) in placebo (−1.9 ± 1.3 HGF vs +0.06 ± 0.2 placebo; P = .04).
Fig 3.
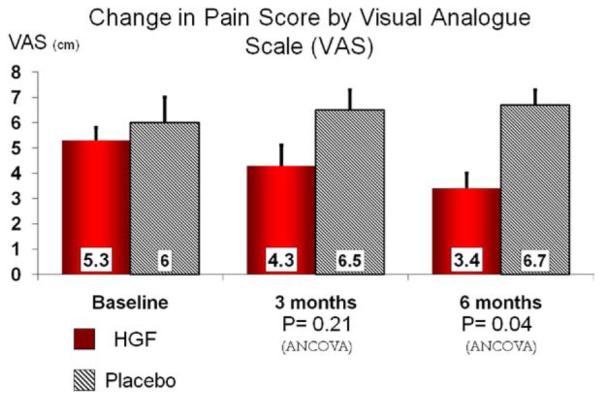
Pain measured as visual analogue scale (VAS) at baseline and 3 and 6 months following first injections (solid columns) compared with placebo (hatched columns). Data presented as mean ± standard error.
Amputation and survival
There was no difference in major amputation of the treated limb (HGF 29% vs placebo 33%) or mortality (HGF 19% vs placebo 17%) at 12 months between groups. There was no difference in minor amputation between the groups.
DISCUSSION
There is currently no US Food and Drug Administration-approved medical therapy for CLI. Therapies currently employed to treat CLI include endovascular therapy, open surgical revascularization, and primary amputation. Both open surgery and endovascular therapy are effective revascularization strategies.1,9,10 Unfortunately, endovascular options are frequently limited in this patient population due to the extent of occlusive disease, which frequently occurs in the distal femoral-popliteal and tibial segment and often presents as total occlusions. As shown in the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial, open surgical options were not available in up to 50% of patients with CLI due to associated medical comorbidities, lack of suitable autogenous conduit, or distal target vessel.1 In the absence of acceptable endovascular or surgical therapy, patients frequently require major amputation to control pain or infection. Therefore, there remains a dire need for a less invasive medical therapy to treat patients with CLI.
Prior studies that attempted to utilize angiogenic gene therapy to treat claudication have met with disappointing results. Numerous randomized placebo-controlled claudication trials using various VEGF isoforms, FGF, and hypoxia inducible factor-1 alpha have failed to show a clinically meaningful improvement in peak walking time compared with placebo-treated subjects.11,12
However, unlike claudication, clinical trials using angiogenic gene therapy to treat CLI have been met with somewhat more promising results. A recent randomized placebo-controlled clinical trial using plasmid-delivered FGF-1, the Talisman 201 Trial, has been completed and has shown an improved major amputation-free survival in FGF-1-treated subjects compared with controls.13 In this trial, 12-month amputation-free survival was 73% in the FGF-treated subjects compared with 48% in the subjects receiving placebo. In a subsequent study, these investigators have gone on to show proof of plasmid uptake and protein expression by muscle cells in the region of FGF plasmid injection by studying tissues from patients treated with FGF plasmid prior to lower extremity amputation.14 The results of the current study support the concept that HGF gene therapy can improve limb perfusion in patients with CLI with tissue loss. HGF gene therapy resulted in improved TBI and decreased rest pain when compared with subjects treated with placebo. These findings are in line with those reported from the HGF-STAT trial, which demonstrated an increase in TcPO2 from baseline in patients treated with high-dose HGF gene therapy compared with placebo. In the current study, there was no difference in major amputation between subjects treated with HGF gene therapy or placebo. This was not surprising for two reasons. First, given that our goal was to demonstrate safety and efficacy of the HGF plasmid therapy, the study was not powered to identify differences in an infrequent end-stage outcome measure such as major amputation. Second, the extent of tissue necrosis present in the CLI patient population was highly variable, as many patients had extensive tissue loss, an example of which is shown in Fig 1. Because of the variation in tissue loss at presentation, it was not surprising that there was no difference in major amputation between the groups.
A recent study completed in Japan using HGF gene therapy to treat patients with relatively small ischemic ulcers was stopped early by the Data Safety Monitoring Board. In this trial, patients treated with HGF gene therapy had a 70% improvement in ulcer healing or rest pain compared with 30% in subjects treated with placebo.8 In summary, these three HGF trials have together shown an increase in limb perfusion as measured by TcPO2 and TBI as well as improvements in wound healing compared with placebo. Furthermore, there have been no safety concerns. It is important to point out that these studies have not shown an improvement in the more clinically meaningful outcome of amputation-free survival; however, the studies were small-sized and not powered to show such a difference.
The current phase II trial was stopped early following an interim analysis at 6 months. The reasons for stopping the trial early were several. Sufficient numbers of patients had been enrolled into the study to adequately assess the safety of the utilized dose of HGF gene therapy to support a pivotal trial. The purpose of the second phase II trial was to determine if there was sufficient signal of efficacy to warrant advancing to a pivotal trial. This does not necessarily require statistical significance in light of previous phase II trials. In the current trial, there was a signal of efficacy based on hemodynamic data and wound healing that, when taken into account with the previous US phase II trial that showed improved TcPO2 in treated patients and the previously described Japanese trial, there was sufficient evidence of efficacy to proceed with a pivotal trial. This decision was also influenced by the difficulty and typically slow recruitment inherent in conducting CLI gene therapy trials in no-option CLI patients.
Due to many trial design issues, performing CLI clinical trials using gene therapy remains difficult. The CLI patient represents a widely disparate population in terms of both extent of disease, which can range from rest pain to extensive tissue loss, but also rate of progression of disease. CLI patients can present with a vast spectrum of wounds that vary from extensive gangrene and deeply penetrating heel wounds to mild superficial skin erosions. Because of this heterogenous patient population, outcomes are more variable, and, as a result, larger numbers of patients may be needed to discover proof of efficacy. In addition, despite our encouraging results in this trial, it remains unlikely that attempts at limb revascularization using biologic approaches are going to result in the same level of improved limb perfusion as a successful lower extremity bypass. In the future, CLI trials must standardize acceptable initial wound size and wound care treatments to ensure that study populations are homogenous and comparable.
The CLI patient population is associated with a high prevalence of severe comorbidities and limited life expectancy. In the current study, 93% of patients suffered at least one adverse event during the course of the study, further underscoring the extensive nature of vascular disease present in the CLI cohort.
At present, all CLI studies have enrolled “no-option” subjects with ischemic ulcers or rest pain. These are patients in whom all endovascular or open surgical options have been exhausted or who are too high-risk medically to undergo revascularization procedures. Gene therapy within this population may be biased toward failure, as these patients may be biologically less likely to respond to angiogenic gene therapy when compared with the general CLI patient population. In the current trial, 96% of the study population had undergone at least one failed attempt at either open surgical revascularization or endovascular therapy prior to enrollment into the trial. In future CLI trials, consideration should be given to the enrollment of “poor-option” CLI patients who have a higher risk for revascularization with expected decreased bypass success such as a synthetic tibial bypass or spliced arm vein graft.
At the present time, amputation-free survival remains the gold standard end point for a CLI trial (TASC II).15 This particular end point has several shortcomings that include the lack of any measurable effect on quality of life as well as the inclusion of death, which is a non-limb-specific end point. The development of more limb-specific end points, such as major adverse limb events in end points proposed by an SVS CLI working group, would help to better define the role of future therapies for CLI.16
CONCLUSIONS
The current trial has shown that HGF gene therapy is safe and well tolerated. HGF gene therapy has been shown to maintain limb perfusion and decrease ischemic rest pain in patients with CLI. Persistent obstacles exist with current study designs that complicate the ability to successfully perform clinical CLI trials. Preliminary trials using HGF gene therapy are promising, and a larger study addressing clinically relevant end points is warranted and being planned.
We acknowledge the efforts of Julie Vanas and Carol Pound in the monitoring and supervision of study activities.
Footnotes
Supported by AnGes Inc.
Competition of interest: Drs Powell and Annex are paid consultants by AnGes.
Presented at the 2009 Vascular Annual Meeting of the Society for Vascular Surgery, June 11-14, 2009, Denver, Colo.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RP, BA
Analysis and interpretation: RP, BA
Data collection: RP, PG, FM, EM
Writing the article: RP, PG
Critical revision of the article: RP, PG, BA, FM, EM
Final approval of the article: RP, PG, BA, FM, EM
Statistical analysis: RP, PG, BA, sponsor AnGes
Obtained funding: RP
Overall responsibility: RP
REFERENCES
- 1.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicentre, randomized controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 2.Dormandy J, Heeck L, Vig S. The fate of subjects with critical leg ischemia. Sem Vasc Surg. 1999;12:142–7. [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 5.Taniyama Y, Morishita R, Aoki M, Nakagami H, Yamamoto K, Yamazaki K, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hind limb ischemia models: preclinical study for treatment of peripheral arterial disease. Gen Ther. 2001;8:181–9. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 6.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–84. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- 7.Morishita R, Aoki M, Yo Y, Ogihara T. Hepatocyte growth factor as cardiovascular hormone: role of HGF in the pathogenesis of cardiovascular disease. Endocr J. 2002;49:273–84. doi: 10.1507/endocrj.49.273. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17:1152–61. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 9.Giles KA, Pomposelli FB, Spence TL, Hamdan AD, Blattman SB, Panossian H, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of transatlantic intersociety concensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128–36. doi: 10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Conte M, Bandyk D, Clowes A, Moneta G, Seely L, Lorenz T, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Olin J, Deitcher S, Pieczek A, Laird J, Grossman PM, et al. Use of a constitutively active hypoxia-inducible factor-1a transgene as a therapeutic strategy in no-option critical limb ischemia patients. Circulation. 2007;115:1234–43. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 12.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. TRAFFIC Investigators. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication: the TRAFFIC study. Lancet. 2002;359:2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 13.Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi MC, et al. TALISMAN 201 investigators. Therapeutic angiogenesis with intramuscular NV1FGF improved amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–8. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner I, Chronos N, Camerota A, Henry T, Pasquet JP, Finiels F, et al. Local gene transfer and expression following intramuscular administration of FGF-1 plasmid DNA in patients with critical limb ischemia. Mol Ther. 2009;17:914–21. doi: 10.1038/mt.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. TASC II Working Group. Trans-Atlantic Inter-society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–73. doi: 10.1016/j.jvs.2009.09.044. e1-3. [DOI] [PubMed] [Google Scholar]