Abstract
A fragment of the gyrA gene was sequenced from 34 isolates of Campylobacter coli, including 23 isolates resistant to ciprofloxacin. All ciprofloxacin-resistant isolates examined by DNA sequencing carried a point mutation at position Thr-86 on the gyrA gene product, involving the replacement of Thr-86 by Ile. A combined PCR-restriction fragment length polymorphism technique using RsaI was developed to detect this mutation.
The thermotolerant species of Campylobacter are frequent causes of acute bacterial gastroenteritis in humans worldwide. More than 90% of these infections are caused by C. jejuni, with C. coli causing most of the remainder (5, 8). Gastroenteritis caused by C. jejuni and C. coli is normally self limiting. However, early antibiotic therapy is recommended to reduce severity and duration, mainly in severe cases with prolonged disease, in immunocompromised patients or if the infection is extraintestinal (2). In such cases, macrolides and fluoroquinolones, particularly ciprofloxacin, are the drugs of choice. Fluoroquinolones are also frequently used as prophylaxes for traveler's diarrhea. However, coinciding with the introduction of fluoroquinolones in veterinary medicine was the increased emergence of quinolone-resistant enteric bacteria strains (3, 9, 13). Such strains can be transmitted to humans. This is particularly important in Campylobacter because it is thought to be a food-borne pathogen. In fact, fluoroquinolone resistance in Campylobacter from food animals is now recognized as an emerging public health problem (4, 14).
The resistance of C. jejuni and C. coli to quinolones mainly depends on mutations in the quinolone resistance-determining region (QRDR) of the gyrA gene and occasionally on mutations of the parC gene. Mutations in gyrA contribute to confer decreased sensitivity of DNA gyrase to quinolone antibiotics and a corresponding increase in their MICs (7, 15). Point mutations in the gyrA gene product at position Thr-86 have been associated with high-level resistance to ciprofloxacin (17).
Although direct sequencing is the more accurate technique for the detection of nucleotide mutations, DNA sequencing cannot be used as a routine assay for diagnosis because sequencing protocols usually are expensive and time-consuming. Therefore, alternative protocols for the detection of single-nucleotide changes of genes have been developed that can be applied to diagnosis in a routine clinical laboratory or epidemiological studies (11, 18, 19). In Campylobacter, most of these alternative protocols are focused on C. jejuni, a major cause of Campylobacter infection in humans. However, C. coli is often isolated together with C. jejuni, and it is the second cause of campylobacteriosis in humans. Furthermore, C. coli appears to display higher rates of resistance to ciprofloxacin than C. jejuni at present (6, 16).
For these reasons it is of interest to us to develop a PCR-based restriction fragment length polymorphism (PCR-RFLP) assay allowing rapid and reproducible identification of major mutations mediating fluoroquinolone resistance in C. coli. This process involves the introduction of an artificial restriction enzyme cleavage site into the PCR product using a primer-specified restriction site modification method and restriction enzyme digestion of the PCR product. In this communication, we report the application of this nonculture method to the detection of mutants within the Thr-86 codon of the C. coli gene associated with decreased susceptibility to ciprofloxacin, gyrA.
In this study, 34 C. coli strains, 10 of human origin and 24 of food or animal origin, were first examined for ciprofloxacin susceptibility. MICs were determined by the agar dilution method on Mueller-Hinton agar (Oxoid) supplemented with 5% lysed horse blood (Oxoid), following the NCCLS recommendations (10), as there are no internationally accepted criteria for susceptibility testing for Campylobacter. The plates were incubated at 42°C under a microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) for 24 h. The breakpoint for ciprofloxacin was considered to be a MIC of ≥4 μg/ml. The results of ciprofloxacin susceptibility testing for the 34 strains are shown in Table 1. Twenty-three of the 34 isolates were resistant to ciprofloxacin, all of them showing high-level resistance (MICs from 16 to 64 μg/ml). Among the 23 ciprofloxacin-resistant strains, one strain was resistant to amoxicillin, nine strains were resistant to tetracycline, six strains were coresistant to tetracycline and erythromycin, and four strains were coresistant to tetracycline and amoxicillin.
TABLE 1.
C. coli strains tested in this study, PCR-RFLP results, and ciprofloxacin MICs for the corresponding strains
| Isolate no.a | Source/country of origin | gyrA mutation at codon 86b | Ciprofloxacin MIC (μg/ml) |
|---|---|---|---|
| CNET 051 | Human/France | Yes | 32 |
| CH 008 | Human/Spain | Yes | 32 |
| CH 024 | Human/Spain | No | 0.5 |
| CH 051 | Human/Spain | Yes | 32 |
| CH 056 | Human/Spain | No | 0.125 |
| CG001 | Human/Spain | Yes | 64 |
| CG002 | Human/Spain | Yes | 64 |
| CG003 | Human/Spain | No | 0.125 |
| CG004 | Human/Spain | Yes | 64 |
| CG005 | Human/Spain | Yes | 64 |
| CNET 019 | Flock outbreak/Netherlands | Yes | 16 |
| CNET 021 | Flock outbreak/Netherlands | Yes | 16 |
| CNET 066 | Pig/Netherlands | No | 0.25 |
| CNET 068 | Pig/North Ireland | No | 0.25 |
| CNET 069 | Pig/North Ireland | No | 0.25 |
| CNET 072 | Pig/Denmark | No | 0.25 |
| CNET 064 | Poultry/Denmark | No | 0.125 |
| CNET 082 | Poultry/North Ireland | No | 0.125 |
| CPM 011 | Poultry/Spain | Yes | 32 |
| CPM 021 | Poultry/Spain | Yes | 32 |
| CPM 023-1 | Poultry/Spain | Yes | 16 |
| CPM 042-7 | Poultry/Spain | Yes | 32 |
| CPM 043-3 | Poultry/Spain | Yes | 32 |
| CPM 043-4 | Poultry/Spain | Yes | 32 |
| CPM 045 | Poultry/Spain | Yes | 32 |
| CPM 053-3 | Poultry/Spain | Yes | 32 |
| CPM 057-1 | Poultry/Spain | Yes | 64 |
| CPM 058-2 | Poultry/Spain | Yes | 64 |
| CPM 063-1 | Poultry/Spain | Yes | 32 |
| CPM 063-4 | Poultry/Spain | Yes | 64 |
| CPM 064-2 | Poultry/Spain | No | 0.5 |
| CPM 069-2 | Poultry/Spain | Yes | 64 |
| CPM 072-1 | Poultry/Spain | Yes | 32 |
CNET, isolates from the CAMPYNET collection; CH and CG, isolates from humans with gastroenteritis; CPM, isolates from poultry samples at the retail level.
Mutants had a Thr-86-to-Ile (ACT-to-ATT) substitution.
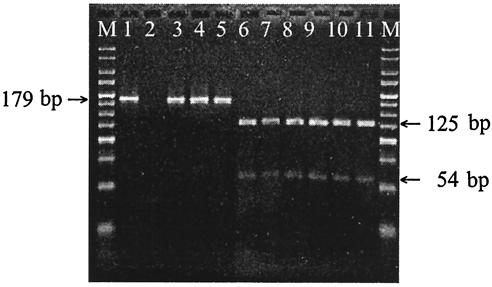
On the basis of the DNA sequence of the gyrA gene of C. coli (GenBank accession number AF092101), we have designed an oligonucleotide adjacent to the mutation sites within the Thr-86 codon nucleotide sequence that differ from the gene sequence by one base (underlined) to create an artificial RsaI leavage site. This nucleotide was used in forward primer colgyrA (5′-AAATCTGCTCGTATAGTAGGGGATGT TATCGGTAAGTATCATCCACATGGCGGT-3′). The reverse primer was cjgyrA2 as previously described (12). They were used to amplify a 179-bp fragment containing the QRDR of the gyrA gene of quinolone-resistant and -susceptible C. coli strains. Analysis of gyrA was started with the amplification of the 179-bp fragment by PCR using primers colgyrA and cjgyrA2, as mentioned above. The PCR was carried out in 25 μl of reaction mixture, containing 1× PCR buffer (10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl [pH 8.3]), 80 ng of genomic DNA, 10 pmol of each primer, 0.2 mM deoxynucleotide, and 1 U of Taq polymerase (Invitrogen). An initial denaturation step at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 45 s and a final extension at 72°C for 7 min were performed in a Robocycler 96 thermal cycler (Stratagene). Amplification products of the expected size (179 bp) were obtained for all strains, whether they had been resistant or susceptible to the ciprofloxacin. Finally, the PCR products were digested with RsaI (Roche) to screen for mutations at position Thr-86. Enzyme digestion was performed in a 10-μl mixture containing 7 μl of the PCR product and 10 U of enzyme. After digestion with RsaI and separation on a 3% MS-6 agarose gel (Pronadisa), two different profiles should be expected. As DNAs carrying mutations at the position corresponding to Thr-86 are amplified, the RsaI cleavage site is destroyed, and a 179-bp fragment identical in size to that of the nondigested PCR product after the digestion should be observed. As a wild-type gyrA gene is amplified, the PCR product should contain the artificial RsaI cleavage site. Consequently, RsaI digests the amplified 179-bp PCR product to produce two fragments of 54 and 125 bp, respectively. PCR-RFLP results from the 34 strains are recorded in Table 1 and shown in Fig. 1.
FIG. 1.
PCR-restriction fragment length polymorphism patterns obtained after digestion with RsaI in C. coli strains. Lanes: M, 25-bp DNA Ladder (Bioline); 1, undigested PCR product of gyrA gene; 2, negative control; 3 to 5, ciprofloxacin-resistant strains; 6 to 11, ciprofloxacin-sensitive strains.
A complete correlation was observed between the PCR-RFLP results and the MICs of ciprofloxacin for the corresponding strains. Analysis of restriction patterns after digestion with RsaI showed that all resistant strains had the same RFLP, the 179-bp fragment. These strains were assumed to have mutations at Thr-86. The susceptible strains had two fragments of 54 and 125 bp, respectively, produced by RsaI digestion. These strains were assumed to have no mutation at Thr-86.
In order to confirm the mutation associated with resistance, an extended 235-bp fragment of the QRDR of gyrA from all tested strains was amplified using primers clgyrA1 and cjgyrA2, as previously described (12). The PCR products were purified with a QIAquick PCR purification kit (QIAGEN) and sequenced by Sistemas Genómicos SA (Valencia, Spain). The nucleotide sequence of the 235-bp fragment was compared to that of the wild-type C. coli isolate in the GenBank (accession number AF092101). On the basis of this comparison, a point mutation at codon 86 (ACT→ATT), leading to the replacement of Thr by Ile, was identified in all ciprofloxacin-resistant strains but not in ciprofloxacin-sensitive strains. Despite all isolates having identical amino acid substitutions in the QRDR of the gyrA product, the ciprofloxacin MICs varied from 16 to 64 μg/ml, suggesting that other factors could contribute to the resistance phenotype. It is possible that mutations outside the QRDR or in other genes were responsible for the increased ciprofloxacin MICs (12). Additional silent mutations were found among isolates that were resistant or susceptible strains. Three isolates each had a silent mutation at the position corresponding to Gly-84 (GGC→GGT), and all but three isolates had one at the position corresponding to Phe-99 (TTT→TTC).
As in C. jejuni, fluoroquinolone resistance in C. coli is primarily associated with a single Thr-86-to-Ile mutation in GyrA in isolates from both humans and animals (1, 12, 17). Our results agree with this criterion, since the Thr-86-to-Ile mutation correlated with high-level resistance to ciprofloxacin in the C. coli isolates examined, whether isolated from humans, animals, or food. Thus, the validity of this assay for detection of point mutations in the C. coli gyrA gene associated with decreased susceptibilities to fluoroquinolones was confirmed. This molecular approach was therefore able to overcome technical problems of MIC determination and to detect high-level fluoroquinolone-resistant C. coli strains regardless of the MICs for them.
The PCR-RFLP methodology described here is a simple and rapid method (it can be performed within 6 h) for the detection of ciprofloxacin-resistant C. coli strains. It should serve as a portable alternative to methods such as sequence-specific oligonucleotide hybridization and nonradioisotopic single-strand conformation polymorphism, which have been previously described (11, 18, 19). The present study provides sufficient data suggesting that this rapid and simple assay proves useful for clinical diagnosis or epidemiological studies of C. coli isolates of different origins with decreased susceptibilities to ciprofloxacin.
Acknowledgments
We thank Susan Barrow for her assistance in the English language.
This work was supported by the Dirección General de Investigación (MCyT) of the Spanish Government (AGL2002-04480-C03-02). R. Alonso is the recipient of a postdoctoral fellowship from the Basque Government.
REFERENCES
- 1.Bachoual, R., S. Ouabdesselam, F. Mory, C. Lascols, C. J. Soussy, and J. Tankovic. 2001. Single or double mutational alterations of gyrA associated with fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Microb. Drug Resist. 7:257-261. [DOI] [PubMed] [Google Scholar]
- 2.Blaser, M. J. 2000. Campylobacter jejuni and related species, p. 2276-2285. In G. L. Mandell, J. E. Bennett, and R. D. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 3.Endtz, H. P., G. J. Ruijs, B. van Klingeren, W. H. Jansen, T. van der Reyden, and R. P. Mouton. 1991. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J. Antimicrob. Chemother. 27:199-208. [DOI] [PubMed] [Google Scholar]
- 4.Engberg, J. F., M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman, J., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 6.Ge, B., D. G. White, P. F. McDermott, W. Girard, S. Zhao, S. Hubert, and J. Meng. 2003. Antimicrobial-resistant Campylobacter species from retail raw meats. Appl. Environ. Microbiol. 69:3005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gootz, T. D., and B. A. Martin. 1991. Characterization of high-level quinolone resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 35:840-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovo, J., E. Mateo, A. Muñoz, M. Urquijo, S. L. W. On, and A. Fernandez-Astorga. 2003. Molecular typing of Campylobacter jejuni isolates involved in a neonatal outbreak indicates nosocomial transmission. J. Clin. Microbiol. 41:3926-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luber, P., J. Wagner, H. Hahn, and E. Bartelt. 2003. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli strains isolated in 1991 and 2001-2002 from poultry and humans in Berlin, Germany. Antimicrob. Agents Chemother. 47:3825-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. Approved standard M7-A5. NCCLS document M100-S12, vol. 22, no. 1. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Niwa, H., T. Chuma, K. Okamoto, and K. Itoh. 2003. Simultaneous detection of mutations associated with resistance to macrolides and quinolones in Campylobacter jejuni and C. coli using a PCR-line probe assay. Int. J. Antimicrob. Agents 22:374-379. [DOI] [PubMed] [Google Scholar]
- 12.Piddock, L. J. V., V. Ricci, L. Pumbwe, M. J. Everett, and D. J. Griggs. 2003. Fluoroquinolone resistance in Campylobacter species from man and animals: detection of mutations in topoisomerase genes. J. Antimicrob. Chemother. 51:19-26. [DOI] [PubMed] [Google Scholar]
- 13.Saenz, Y., M. Zaraga, M. Lantero, M. J. Gastanares, F. Baquero, and C. Torres. 2000. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997-1998. Antimicrob. Agents Chemother. 44:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith, K. E., J. M. Besser, C. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, and M. T. Osterholm. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 15.Taylor, D. E., and P. Courvalin. 1988. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob. Agents Chemother. 32:1107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thwaites, R. T., and J. A. Frost. 1999. Drug resistance in Campylobacter jejuni, C. coli and C. lari isolated from humans in North West England and Wales, 1997. J. Clin. Pathol. 52:812-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, Y., W. M. Huang, and D. E. Taylor. 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob. Agents Chemother. 37:457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson, D. L., S. R. Abner, T. C. Newman, L. S. Mansfield, and J. E. Linz. 2000. Identification of ciprofloxacin-resistant Campylobacter jejuni by use of a fluorogenic PCR assay. J. Clin. Microbiol. 38:3971-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zirnstein, G., L. Helsel, Y. Li, B. Swaminathan, and J. Besser. 2000. Characterization of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli by DNA sequence analysis and MAMA PCR. FEMS Microbiol. Lett. 190:1-7. [DOI] [PubMed] [Google Scholar]