Abstract
Background
First-line treatment for patients with superficial femoral arterial (SFA) occlusive disease has yet to be determined. This study compared long-term outcomes between primary SFA stent placement and primary femoral-popliteal bypass. Periprocedural patient factors were examined to determine their effect on these results.
Methods
All femoral-popliteal bypasses and SFA interventions performed in consecutive patients with symptoms Rutherford 3 to 6 between 2001 and 2008 were reviewed. Time-dependent outcomes were analyzed using the Kaplan-Meier method and log-rank test. Cox proportional hazards were performed to determine predictors of graft patency. Multivariate analysis was completed to identify patient covariates most often associated with the primary therapy.
Results
A total of 152 limbs in 141 patients (66% male; mean age, 66 ± 22 years) underwent femoral-popliteal bypass, and 233 limbs in 204 patients (49% male; mean age, 70 ± 11 years) underwent SFA interventions. Four-year primary, primary-assisted, and secondary patency rates were 69%, 78%, and 83%, respectively, for bypass patients and 66%, 91%, and 95%, respectively, for SFA interventions. Six-year limb salvage was 80% for bypass vs 92% for stenting (P = .04). Critical limb ischemia (CLI) and renal insufficiency were predictors of bypass failure. Claudication was a predictor of success for SFA stenting. Three-year limb salvage rates for CLI patients undergoing surgery and SFA stenting were 83%. Amputation-free survival at 3 years for CLI patients was 55% for bypass and 59% for SFA interventions. Multivariate predictors (odds ratios and 95% confidence intervals) of covariates most frequently associated with first-line SFA stenting were TransAtlantic Inter-Society Consensus II A and B lesions (5.9 [3.4-9.1], P < .001), age >70 years (2.1 [1.4-3.1], P< .001), and claudication (1.7 [1.1-2.7], P= .01). Regarding bypass as first-line therapy, claudicant patients were more likely to have nondiabetic status (5.6 [3.3-9.4], P < .001), creatinine <1.8 mg/dL (4.6 [1.5-14.9], P = .01), age <70 years (2.7 [CI, 1.6-8.3], P < .001), and presence of an above-knee popliteal artery target vessel (1.9 [CI, 1.1-3.4] P = .02).
Conclusion
Indication, patient-specific covariates, and anatomic lesion classification have significant association when determining surgeon selection of SFA stenting or femoral-popliteal bypass as first-line therapy. Patients with SFA disease can have comparable long-term results when treatment options are well matched to patient-specific and anatomic characteristics. (J Vasc Surg 2011;54:714-21.)
Recent reports of improved short-term and midterm patency with endoluminal stent placement within the superficial femoral artery (SFA) have challenged the historical dogma that bypass results are categorically superior to endovascular therapy.1 Many authors have demonstrated that a variety of patient-specific factors, such as indication and comorbidities, affect the durability of outcome of SFA stent placement.1-3 Disease extent and TransAtlantic Inter-Society Consensus (TASC) II classification are also critical not only in predicting successful intervention after endovascular or open surgical therapy in the SFA but also in influencing decisions for the choice of first-line therapy.4,5
A myriad of patient-specific and anatomic variables can also affect results of treatment within the SFA, making therapeutic decision making all the more complex. There are minimal data comparing bypass vs primary SFA stent placement, and as such, determination of optimal patient selection for first-line therapy has not been clearly elucidated. Several series have reported equivalent short-term and midterm outcomes for SFA stenting compared with femoral-popliteal bypass.5,6 Whether the long-term outcomes of these two therapies are comparable is unknown.
The purpose of this study was to compare outcomes of consecutive cases of femoral-popliteal bypass with primary SFA stent placement and to determine long-term results. We also sought to identify patient characteristics associated with selection for primary SFA stent vs femoral-popliteal bypass to better understand factors that influence surgeon decision making.
Methods
A retrospective review of a prospectively maintained vascular database at Dartmouth-Hitchcock Medical Center was conducted of consecutive patients with symptoms Rutherford 3 to 6 undergoing treatment of SFA occlusive disease between 2001 and 2008. Demographic features, comorbidities, indications for intervention, indications for revision, and noninvasive laboratory data were recorded. Angiographic anatomic data and TASC II classification were also recorded.
SFA stent intervention
The study included patients who received primary SFA stenting and had no evidence of concomitant popliteal or tibial disease. All consecutive patients underwent primary SFA stent placement with no adjunctive interventions such as atherectomy, cryoplasty, or angioplasty alone. Patients had never previously been treated with endovascular intervention or bypass in the analyzed limb. All endovascular interventions were performed in an endovascular suite with fixed imaging or in the operating room with a portable C-arm.
Contralateral femoral access was used in most cases unless a steep aortic bifurcation or prior abdominal graft (endovascular or Dacron) precluded this approach. Heparin (80-100 U/kg) was routinely administered intraprocedurally with selective protamine usage. Flush SFA occlusions were not considered candidates for SFA stenting. Lesions were typically crossed with 0.035-inch systems, and the type of self-expanding nitinol stent used was determined by operator preference and available inventory.
Most patients were maintained on aspirin and clopidogrel for a minimum of 30 days after the procedure and on aspirin monotherapy thereafter. Postprocedural surveillance consisted of ankle-brachial index (ABI) measurements at 1 month, 6 months, and annually thereafter. Among patients with significant tibial calcification and falsely elevated ABIs, toe pressures were obtained along with ABIs. Any decrease in ABIs of 0.15 or recurrent symptoms prompted duplex imaging or arteriography, or both, to determine stenosis and patency. Primary patency within the SFA stent patients was defined as freedom from reintervention or thrombosis.
Femoral-popliteal bypass
All patients who underwent primary SFA bypass procedures had never previously undergone an endovascular or surgical intervention. All bypass patients whose conduit was the ipsilateral leg vein underwent bypass to the above-knee (AK) or below-knee (BK) popliteal position. Claudication patients whose bypass required a prosthetic conduit received a distal anastomosis only to the AK position. A prosthetic graft was used selectively to the AK or BK position in patients with a preoperative indication of critical limb ischemia (CLI).
Surveillance consisted of graft duplex imaging and ABI measurements at intervals of 1, 3, 6, and 12 months. Toe pressures were obtained in patients who demonstrated significant tibial artery incompressibility. Bypass patency was determined by postoperative duplex imaging and ABI. Primary patency was defined as freedom from reintervention or thrombosis. Graft failure was defined as bypass thrombosis, restenosis of >50% of the treated arterial segment immediately above or below the bypass, intragraft restenosis >50%, or a decrease in the ABI of ≥0.15.
Statistical analysis
Main outcome measure and overall analysis
Univariate comparisons were made between patient characteristics and loss of primary patency, which was our main outcome measure. Data were maintained in an Excel spreadsheet (Microsoft Inc, Redmond, Wash) and analyzed using STATA 9.2 software (StataCorp, College Station, Tex). Kaplan-Meier survival analysis was performed to assess time-dependent outcomes, and comparisons were made with the log-rank test.
Multivariable model to predict graft failure
Cox proportional hazards regression analysis was used to determine predictors of failure for the bypass and stenting groups. The eligibility criterion for evaluation for inclusion in the model was a univariate analysis value of P < .20. Backward stepwise regression was used to construct Cox proportional hazards models, and these models were used to determine the patient-specific and anatomic variables associated with graft failure. Individual models were constructed for the overall cohort, as well as specific models for bypass patients and endovascular patients.
Multivariable model to predict choice of intervention
Because the decision for open surgery or endovascular intervention was not random, we adjusted for confounding factors by the type of revascularization chosen by the surgeon. To accomplish this, we created a multivariable logistic regression model to predict, from patient characteristics, whether the patient would receive open or endovascular revascularization. Patient scores for this model were included as a covariate in the Cox proportional hazards models described subsequently to adjust for the possible confounding factors between the choice of revascularization and graft failure, which was our main outcome measure.
Power analysis
Power analysis was performed with 345 total patients, comprising 141 in bypass group and 204 in the endovascular group. This gave us 69% power to detect a hazard ratio of ≥1.5 between the intervention groups on graft patency at the .05 significance level.
Results
Patient cohort
During the study interval, 152 limbs in 141 bypass patients and 233 limbs in 204 endovascular patients were analyzed. Bypass patients were typically younger (66 ± 22 vs 70 ± 11 years, P = .001) and male sex (66% vs 55%, P = .03) compared with the primary SFA stent group. Claudication patients were less likely to undergo bypass than CLI patients (56% vs 46%, P = .07). TASC D patients underwent more bypass procedures, whereas TASC B patients had more primary SFA stent interventions (Table I). The overall 6-year survival was 64% for the bypass cohort vs 67% in the stent cohort.
Table I. Demographics of patients undergoing revascularization for superficial femoral artery (SFA) occlusive disease.
| Variablea | Bypass | SFA stent | P |
|---|---|---|---|
| Total patients | 141 | 204 | |
| Total limbs | 152 | 233 | |
| Age, years | 66 ± 22 (38-87) | 70 ± 11 (37-99) | .0013 |
| Male sex | 101 (66) | 129 (55) | .0302 |
| Indication | |||
| Claudication | 70 (46) | 130 (56) | .0773 |
| CLI | 82 (54) | 103 (44) | |
| Diabetes | 64 (42) | 105 (45) | .5686 |
| Creatinine >1.8 mg/dL | 17 (11) | 21 (9) | .5594 |
| Hypertension | 115 (76) | 192 (82) | .0897 |
| Hyperlipidemia | 105 (69) | 160 (69) | .9813 |
| Statin use | 101 (66) | 106 (55) | .0197 |
| Smoking | 75 (49) | 70 (30) | <.0001 |
| CAD | 53 (35) | 110 (47) | .0165 |
| TASC lesion | <.0001 | ||
| A | 3 (2) | 41 (18) | |
| B | 50 (33) | 145 (62) | |
| C | 33 (22) | 32 (14) | |
| D | 66 (43) | 15 (6) |
CAD, Coronary artery disease; CLI, critical limb ischemia; TASC, TransAtlantic Inter-Society Consensus.
Data are shown as number (%) or mean ± standard deviation (range).
A comparison of other common vascular comorbidities, such as diabetes, chronic renal insufficiency (CRI, creatinine >1.8 mg/dL), hypertension, or dyslipidemia, detected no significant difference between groups. Tobacco use (P < .0001) and statin use (P = .02) was more common in the bypass patients. Coronary artery disease, as defined by prior myocardial infarction, was more common in the SFA stenting group (P = .02). The demographic and clinical data are summarized in Table I.
Bypass
The 152 limbs in 141 patients that underwent bypass were treated for claudication (46%), rest pain (30%), and tissue loss (24%). Popliteal bypasses were performed AK in 55% of limbs and BK in 45%. In the CLI cohort, 45 patients (55%) underwent prosthetic bypass reconstruction; however, only 12 (15%) of these patients received an anastomosis to the BK position. An AK anastomosis was created in 28 patients (39%) with a preoperative indication of claudication who underwent femoral-popliteal bypass reconstruction with a prosthetic graft. The conduit was great saphenous vein in 54% of bypasses and expanded polytetrafluoroethylene in 46%. Concomitant iliofemoral endarterectomy was performed in 31% of limbs.
Patency and reintervention
Four-year primary, primary-assisted, and secondary patency rates (95% confidence interval [CI]) were 69% (79%-99%), 78% (68%-88%) and 83% (74%-92%), respectively, for bypass patients (Fig 1). The 6-year primary patency rate was 49% (35%-63%) for AK vs 75% (59%-81%) for BK popliteal bypass. The 6-year primary patency rates were 67% (56%-78%) for great saphenous vein vs 32% (17%-48%) for prosthetic bypass. The overall 6-year limb salvage rate for all bypass patients was 80% (81%-95%). Only 4% of limbs in CLI patients with rest pain or tissue loss required major amputation, and minor amputations were performed in 8% of limbs.
Fig 1.
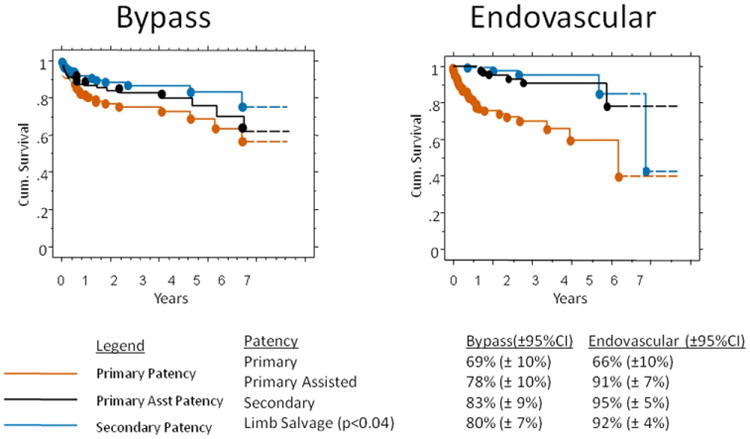
Overall, cumulative 4-year patency is shown for the (left) femoral-popliteal bypass and (right) primary superficial femoral artery (SFA) stent placement cohorts. Left, Standard error for all survival curves for the femoral-popliteal bypass was 0.05 at 4 years. The femoral-popliteal bypass number at risk was 152 at the start and 36 at 4 years. Right, The standard error for survival curves in the primary SFA stent group was 0.06 at 4 years. The primary SFA stent number at risk was 233 at the start and 58 at 4 years. CI, Confidence interval.
Reintervention was required in 24% of bypass patients. Within that group, 72% were open reintervention (thrombectomy, vein patch, graft removal), 22% were endovascular (angioplasty, stent, thrombolysis), and 6% comprised repair of lymph leak and wound debridement. The overall complication rate was 21%, consisting of 20% bleeding/hematoma, 3% emergency reoperation, 3% wound infection, 14% pneumonia, 1% lymph leak, 2% urinary tract infection, 1% respiratory failure, and 9.8% transient ischemic attack (Table II).
Table II. Preoperative complications.
| Variable | Bypass (%) | SFA stent (%) | P |
|---|---|---|---|
| Emergency re-op | 0.0 | 0.4 | .45 |
| Bleeding | 3.3 | 0.8 | .09 |
| Urinary tract infection | 0.7 | 0.4 | .70 |
| Wound infection | 13.8 | 0.0 | <.0001 |
| Pneumonia | 1.3 | 0.0 | .10 |
| Transient ischemic attack | 0.7 | 0.0 | .23 |
| Lymph leak | 2.0 | 0.0 | .04 |
| Respiratory failure | 0.7 | 0.0 | .23 |
| Death | 0.0 | 0.8 | .27 |
| Total | 21.0 | 2.1 | <.0001 |
SFA, Superficial femoral artery.
With regard to the SFA stent cohort, 4-year primary, primary-assisted, and secondary patency rates (95% CI) were 66% (56%-76%), 91% (84%-98%), and 95% (90%-99%) for SFA stent interventions. The 6-year limb salvage rate was 92% in the endovascular cohort vs 80% (log-rank, P = .04); however, a higher proportion of these patients had claudication (Fig 1). The reintervention rate was 14%. Within that group, 52% had open interventions (bypass, thrombectomy, endarterectomy) and 48% had endovascular reintervention (angioplasty, stenting, thrombolysis).
No major amputations were performed on limbs undergoing SFA intervention, and minor amputation was required in 4% of extremities. The complication rate in this cohort was 2.1%, comprising 0.8% bleeding/hematoma, 0.4% emergency operation, and 0.4% urinary tract infection (Table II).
Predictors of patency
Cox regression analysis was used to determine predictors of failure for the entire cohort of patients. Poor runoff, renal insufficiency, and CLI were predictors of failure for any intervention performed in this patient cohort. The type of intervention, SFA stenting or femoral-popliteal bypass, was not a predictor of failure (Table III).
Table III. Predictors of patency by Cox proportional hazard models.
| Variable | HR (95% CI) | P |
|---|---|---|
| Overall cohort | ||
| One-vessel outflow | 53.311 (4.938-575.497) | .0011 |
| Creatinine >1.8 mg/dL | 1.890 (1.059-3.378) | .0316 |
| Critical limb ischemia | 2.976 (1.375-6.410) | .0133 |
| Bypass vs stent | 1.079 (0.596-1.947) | .7997 |
| Procedure-specific model | ||
| Bypass | ||
| One-vessel outflow | 47.609 (4.077-555.952) | .0021 |
| Creatinine >1.8 mg/dL | 3.012 (1.199-7.576) | .0189 |
| Not taking statin | 2.573 (1.243-5.326) | .0109 |
| Endovascular | ||
| Critical limb ischemia | 10.753 (3.831-30.030) | <.0001 |
CI, Confidence interval; HR, hazard ratio.
To further look at predictors of patency, we used a Cox regression analysis for the bypass and stent groups independently. Predictors of failure of bypass grafts were decreased renal function, poor outflow, and lack of statin therapy. Predictors of failure for the stent group were indication, such that CLI patients had worse patency compared with claudicant patients. Previously reported predictors of SFA or bypass failure, such as TASC classification or diabetes, did not predict patency (Table III). Because indication was determined to be an important predictor of patency, and the patients treated for claudication vs CLI are different, we stratified our results by the diagnosis of claudication or CLI.
Outcomes for claudication cohort
The primary, primary-assisted, and secondary patencies were similar in the bypass and SFA stent groups. Specifically, the primary patency rate (95% CI) at 3 years was 78% (61%-95%) for claudicant patients receiving femoral-popliteal bypass vs 89% (70%-99%) for primary SFA stenting (Fig 2). Limb salvage was 100% for bypass as well as SFA stents. The 3-year amputation-free survival (AFS) was 92% in the claudicant patients undergoing bypass vs 97% for the SFA stent group. No outcomes of primary patency, limb salvage, or amputation-free survival were significantly different between intervention groups for claudicant patients by log-rank analysis. No predictors of long-term patency were identified.
Fig 2.
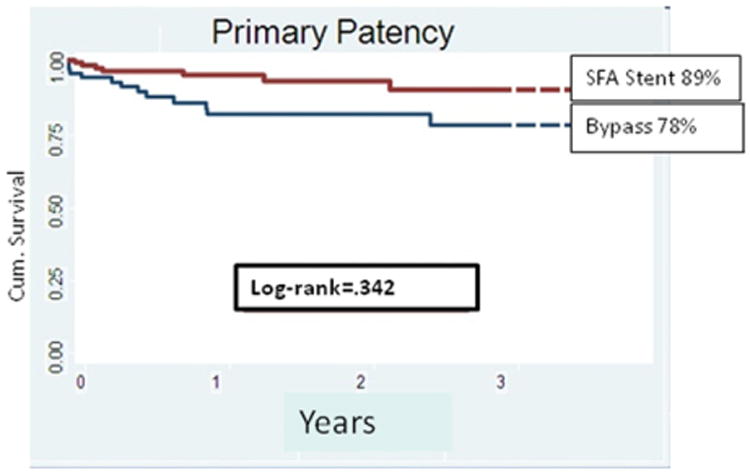
Cumulative 3-year patency is shown for the claudicant patients undergoing femoral-popliteal bypass and primary superficial femoral artery (SFA) stenting. Number of claudicant patients with femoral-popliteal bypass at risk was 70 at the start and 22 at 3 years. The standard error was 0.09 at 3 years. Number of claudicant patients with primary SFA stent at risk was 130 at the start and was 41 at 3 years. The standard error was 0.089 at 3 years.
Outcomes for the CLI cohort
The CLI patients who underwent primary bypass or SFA stent placement had comparable primary, primary-assisted, and secondary patencies. Primary patency (95% CI) at 3 years for CLI patients was 67% (59%-85%) for bypass surgery and 43% (23%-63%) for SFA stenting. No significant differences were found when limb salvage or amputation-free survival was compared by log-rank analysis. Limb salvage for bypass surgery and SFA stenting for CLI patients was 83% for both at 3 years (Fig 3, A). More importantly, bypass and primary stent patients with CLI had similar outstanding results for AFS. AFS at 3 years was 55% for bypass patients compared with 59% for the primary SFA stent patients (Fig 3, B).
Fig 3.
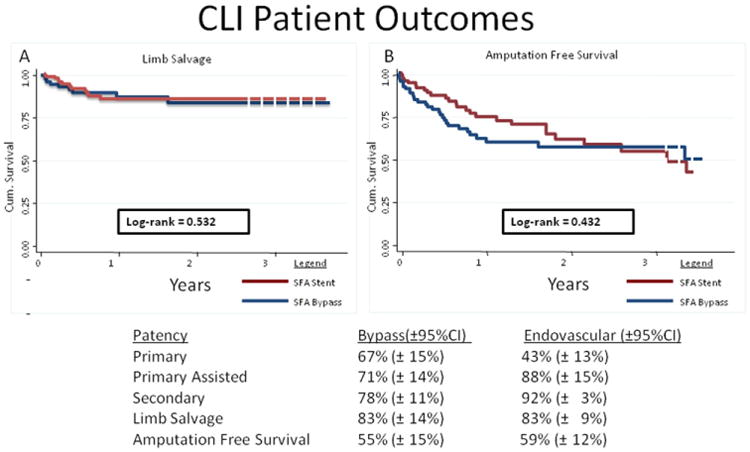
A, Limb salvage and (B) amputation-free 3-year survival are shown for the femoral-popliteal bypass and primary superficial femoral artery (SFA) stent groups in patients with critical limb ischemia (CLI). For limb salvage after femoral-popliteal bypass, number at risk was 82 at the start and 22 at 3 years, and the standard error was .088. For amputation-free survival, the number was 82 at risk to start and 24 at 3 years, and the the standard error was 0.097. For limb salvage after primary SFA intervention, the number at risk was 103 at the start and 35 at 3 years was 35. The standard error was 0.088 at 3 years. For amputation-free survival, the number at risk was 103 to start and 37 at 3 years. The standard error was 0.089 at 3 years. CI, Confidence interval.
Selection of revascularization strategy among patients with claudication
Because the overall results of the primary SFA stent and bypass experience in this series are among some of the best reported outcomes in the literature and indication was a predictor of patency, multiple logistic regression models were used to better understand patient selection bias that may have led to these data. Logistic regression was performed to determine characteristics of claudication patients that were associated with bypass being chosen compared with endovascular therapy. Nondiabetic status, age <70 years, normal renal function (glomerular filtration rate >60 mL/min/1.73 m2), and presence of an AK target were covariates associated with surgeon choice of bypass as first-line therapy in the claudication cohort. Hence, claudicant patients who underwent bypass were more likely to be younger and healthier with a good target for bypass (Table IV).
Table IV. Characteristics of claudication patients receiving femoral-popliteal bypass.
| Covariate | OR (95% CI) | P |
|---|---|---|
| Nondiabetic | 5.6 (3.3-9.4) | <.001 |
| Age <70 years | 2.7 (1.6-8.3) | <.001 |
| Creatinine <1.8 mg/dL | 4.6 (1.5-14.9) | .01 |
| Above-knee target | 1.9 (1.1-3.4) | .02 |
CI, Confidence interval; OR, odds ratio.
Logistic regression was then done to determine characteristics of patients given the option of primary stenting by the surgeon. Factors that influenced surgical decisions about the likelihood of receiving SFA stenting as a first-line therapy were the presence of TASC A and B lesions (odds ratio [OR] 5.9; 95% CI, 3.9-9.1; P < .001), age >70 years (OR, 2.1; 95% CI, 1.4-3.1; P < .001), and claudication as a preoperative indication (OR, 1.7; 95% CI, 1.1-2.7; P = .01; Table IV). Therefore, stented patients were more likely older claudicant patients with limited SFA disease.
Discussion
These results represent one of the largest and longest follow-ups to date for primary SFA stent vs bypass outcomes. Because the results for primary SFA stenting and bypass for claudicant patients are comparable, when patients are chosen in a similar fashion, a less-invasive intervention, such as primary SFA stent placement, should be offered for first-line therapy in this patient cohort. In a similar manor, CLI patients can have equivalent outcomes for primary SFA stent placement vs femoral-popliteal bypass as long as age, renal function, diabetic status, and presence of a BK popliteal target are considered in the treatment algorithm. This conclusion is further supported by the Cox regression analysis to determine predictors of failure for the entire cohort. Choice of type of intervention did not predict failure; therefore, if patient selection is appropriate, either procedure to treat patients with femoral-popliteal disease should lead to equivocal results.
Owing to the durable and comparable outcomes between the two treatment modalities, regardless of indication, our group sought to understand the clear selection bias that must be present when determining which patients receive the given treatment. After analyzing the anatomic and comorbid attributes of the various CLI and claudicant cohorts, we were not surprised to find that patients with TASC A or B lesions, older patients (age >70 years), and claudicant patients were more likely to undergo primary SFA stent placement (Table IV).
Contemporary practice management of the claudicant patient should still center on modification of risk factors. Invariably, however, a small cohort of patients will be disabled despite these interventions.7 Once committed to treatment, one must weigh the attendant risks and benefits of invasive intervention. Prudence would dictate choosing a modality that has the greatest durability with the least amount of morbidity.8 Thus, it was not surprising to find that in our practice, open operations for claudicant patients typically occurred in those who were younger, had an AK popliteal artery target, and were less likely to have accelerated disease progression over time due to a nondiabetic status.9 Therefore, claudicant patients who were young and had limited comorbidities were more likely to have better long-term survival. Subsequently, given the historically more durable procedure of SFA bypass, this was more frequently offered as the primary intervention.
The converse analysis is that CLI patients offered femoral-popliteal bypass as a first-line option were more often older, had renal insufficiency, were diabetic, and had typically only a BK popliteal target. Absence of an AK popliteal target invariably heralds the presence of more complicated TASC C or D lesions, which have been shown in multiple series to have superior outcomes with bypass.6,9,10 An “endo-first” option is desirable for older patients with multiple comorbidities; however, they frequently have associated comorbidities, such as diabetes or renal insufficiency, that lead to more advanced arterial occlusive disease. This often predicts poorer outcomes with a percutaneous intervention, particularly in patients with tissue loss.11
Although this series compares well with previous large-scale series for CLI patients, it is important to note that equivalent limb salvage rates of 83% at 3 years were achieved in CLI patients undergoing primary bypass or SFA stent placement.3 In addition, 3-year AFS was no different in the CLI cohort of patients when the two therapies were compared (55% bypass vs 59% stent). None of the study patients had a popliteal intervention, so when clinicians are faced with the decision of which first-line therapy to offer, consideration of the anatomic and physiologic cost of failure, need for salvage or bail-out revascularization, and overall medical risk stratification of the patient affect the clinical selection bias.
A limitation of contemporary endovascular practice is that the conduit status is often unknown before clinical decision analysis that is made to determine which first-line therapy to offer. We contend that in patients with usable autologous conduit and CLI, particularly those with tissue loss, primary SFA bypass should be offered. Bypass should be the historical gold standard against which this decision should be weighed, and the threshold for this therapy over SFA stenting should be lowered in patients with CRI and diabetes given the effect of these comorbidities on durability of endovascular intervention.12,13
Another important finding in this series, in contrast to previously published data, is that TASC II classification was not a predictor of primary patency after primary SFA stent placement. Of the patients in this study, 55% of the bypass cohort and 76% of the endovascular cohort were TASC B or C patients. Previous reports of decision analysis modeling regarding the treatment of these lesions have advocated a first-line angioplasty with stenting approach for disabling claudication patients. However, low-risk TASC C patients likely gain greater benefit from a great saphenous vein bypass.14 Lesion morphology (stenosis vs occlusion) can affect outcome, and several reports identify lesion length with outcome in the historical and contemporary vascular surgical literature.15,16 Consensus guidelines have been published on the “best practice” treatment of SFA occlusive disease; however, many clinicians are influenced by additional patient-specific comorbidities when deriving solutions for intervention.4
This contemporary series has demonstrated that a number of clinical factors, both comorbid and anatomic, are more frequently associated with a given treatment strategy. We contend that these factors likely led to surgeon bias that ultimately resulted in the selected revascularization strategy. One important limitation, as mentioned previously, is the patient's preintervention conduit status. A patient who lacks an adequate conduit for lower extremity arterial reconstruction is more likely to undergo an endovascular intervention.12 This analysis did not capture this information; however, it likely reflects a real-world experience where patients present with complaints of lifestyle-limiting claudication, rest pain, or tissue loss and invariably undergo diagnostic arteriography. Once in the interventional suite, the vascular surgeon is left with the knowledge of the patient's comorbid status and TASC II classification to ultimately decide on the best initial therapy.
A variety of authors have reported SFA stent outcomes in medically high-risk patient cohorts and patients who are functionally impaired or at extremes of age.10,17 These patients are likely to be unfit for bypass. However, determining which factors are most important when deciding an intervention is the key issue because there are historical reports of good outcomes for infrainguinal revascularization in medically high-risk patients.18 Patients often have multiple atherosclerotic risk factors and extensive coronary disease that stratify them into a high-risk cohort for conventional open infrainguinal arterial reconstruction.19 Multiple series have identified diabetes, age >80 years, and renal failure as independent physiologic predictors of bad outcomes for both endovascular and open surgical revascularization.13,20,21 In our series for the SFA stent cohort, we noted overall similar outcomes compared with bypass, and the only predictor of poor patency was indication. High-risk patients or patients with significant comorbidities who had claudication were given SFA stenting as a primary option. One would consider all CLI patients to be medically high risk because CLI could be considered a surrogate for worse coronary artery disease and cerebrovascular disease. That being said, CLI patients given the primary option of SFA stenting were those whose options for bypass were still available if SFA stenting failed. These reported experiences, plus the TASC II recommendations, are likely to be weighed differentially in the clinician's mind when attempting to navigate a clinical treatment algorithm for femoral-popliteal occlusive disease.
Although these data rival or exceed reported outcomes for primary SFA stenting vs bypass, the study has several limitations. This was not a randomized trial directly comparing primary SFA stent placement with femoral-popliteal bypass. A future goal would be to conduct such a trial in an attempt to better determine and more directly compare the two treatment strategies. A propensity score would have been a better method to compare the subgroups; however, the numbers were too small to perform this analysis.
Another limitation of our work is that the unit of analysis in our study was the procedure, not the patient, and some patients had procedures on both limbs, including 11 patients (7% of the total) who had a bypass procedure on their contralateral limb and 29 patients (12% of the total) who underwent stent placement in their contralateral limb. Although these patients represent a small proportion of all patients and were not systematically different than the population undergoing unilateral procedures, we cannot be sure that these “events” were truly independent of each other. Our observed standard errors do not take into account the possibility of within-patient correlation, although given the similarity of these populations, it seems unlikely these would change the main effects of our findings.
Better quantification of the claudication outcomes, such as walking distance rather than patency and limb salvage, would have been a more optimal comparative end point. In addition, outcome measures, such as major adverse limb events, will likely be more useful when determining the efficacy of the treatment options given to patients. Nonetheless, this report represents some of the longest-term follow-up and outcome reporting in a contemporary series of consecutive patients treated for superficial femoral arterial occlusive disease.
Conclusions
Understanding the different effect of the various physiologic and anatomic factors that can affect outcomes of femoral-popliteal revascularization is paramount to achieving optimal outcomes. When patient-specific and anatomic characteristics are matched appropriately, equivalent outcomes can be achieved between endovascular and open surgical revascularization of the SFA regardless of indication or TASC classification.
Footnotes
Competition of interest: none.
Presented at the Thirty-sixth Annual Meeting of the New England Society of Vascular Surgery, Boston, Massachusetts, October 4, 2009.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
Author Contributions: Conception and design: ER, SS, PG, AB
Analysis and interpretation: ER, SS, DS, DW, PG, RP
Data collection: ER, AB, PG
Writing the article: SS, ER, DS,DW
Critical revision of the article: SS, ER, AB, DS, DW, PG, RP
Final approval of the article: ER, SS, AB, DS, DW, PG
Statistical analysis: ER, PG
Obtained funding: ER
Overall responsibility: ER
References
- 1.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–88. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa Y, Yajima J, Oikawa Y, Ogasawara K, Kirigaya H, Nagashima K, et al. Clinical outcomes after percutaneous peripheral intervention for chronic total occlusion of superficial femoral arteries: comparison between self-expandable nitinol stent and stainless steel stent. J Cardiol. 2009;53:417–21. doi: 10.1016/j.jjcc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Surowiec SM, Shirley EW, Rhodes JM, Illig KA, Shortell CK, Lee DE, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41:269–78. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA. Fowkes FGR and on behalf of the TASC II Working Group Inter-Society Consensus for the Management of peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Dosluoglu HH, Cherr GS, Lall P, Harris LM, Dryjski ML. Stenting vs above knee polytetrafluoroethylene bypass for TransAtlantic Inter-Society Consensus-II C and D superficial femoral artery disease. J Vasc Surg. 2008;48:1166–74. doi: 10.1016/j.jvs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Dearing DD, Patel KR, Compoginis JM, Kamel MA, Weaver FA, Katz SG, et al. Primary stenting of the superficial femoral and popliteal artery. J Vasc Surg. 2009;50:542–7. doi: 10.1016/j.jvs.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Sabeti S, Mlekusch W, Amighi J, Minar E, Schillinger M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J Endovasc Ther. 2005;12:6–12. doi: 10.1583/04-1359.1. [DOI] [PubMed] [Google Scholar]
- 8.Almahameed A, Bhatt DL. Contemporary management of peripheral arterial disease: III Endovascular and surgical management. Cleve Clin J Med. 2006;73(suppl 4):S45–51. doi: 10.3949/ccjm.73.suppl_4.s45. [DOI] [PubMed] [Google Scholar]
- 9.Bakken AM, Palchik E, Rhodes JM, Saad WE, Davies MG. Impact of diabetes mellitus on outcomes of superficial femoral artery endoluminal interventions. J Vasc Surg. 2007;46:946–58. doi: 10.1016/j.jvs.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Brilakis ES, Das TS, Lichtenwalter CS. Treatment of Complex Superficial Femoral Artery Lesions with PolarCath cryoplasty. Am J Cardiol. 2009;104:447–9. doi: 10.1016/j.amjcard.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Setacci C, Chisci E, de sDonato G, Setacci F, Iacoponi F, Galzerano G, et al. Subintimal angioplasty with the aid of a re-entry device for TASC C and D lesions of the SFA. Eur J Vasc Endovasc Surg. 2009;38:76–87. doi: 10.1016/j.ejvs.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Ihnat DM, Duong ST, Taylor ZC, Leon LR, Mills JL, Sr, et al. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J Vasc Surg. 2008;47:967–74. doi: 10.1016/j.jvs.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Florian D, Galimanis A, Husmann M, Schmidli J, Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: Influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007;45:751–61. doi: 10.1016/j.jvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Nolan B, Finlayson S, Tosteson A, Powell R, Cronenwett J. The treatment of disabling intermittent claudication in patients with superficial femoral artery occlusive disease-Decision analysis. J Vasc Surg. 2007;45:1179–84. doi: 10.1016/j.jvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen B, Tønnesen KH, Holstein P. Late hemodynamic failure following percutaneous transluminal angioplasty for long and multifocal femoropopliteal stenoses. Cardiovasc Intervent Radiol. 1991;14:290–2. doi: 10.1007/BF02578452. [DOI] [PubMed] [Google Scholar]
- 16.Sabeti S, Schillinger M, Amighi J, Sherif C, Mlekusch W, Ahmadi R, et al. Primary patency of femoropopliteal arteries treated with nitinol versus stainless steel self-expanding stents: propensity score-adjusted analysis. Radiology. 2004;232:516–21. doi: 10.1148/radiol.2322031345. [DOI] [PubMed] [Google Scholar]
- 17.Nishibe T, Kondo K, Nishibe M, Muto A, Dardik A. Stent placement for superficial femoral arterial occlusive disease in high-risk patients: preliminary results. Surg Today. 2009;39:21–6. doi: 10.1007/s00595-008-3812-9. [DOI] [PubMed] [Google Scholar]
- 18.Hnath J, Roddy SP, Darling RC, 3rd, Paty PS, Taggert JB, Mehta M. Comparative results of open lower extremity revascularization in nonagenarians. J Vasc Surg. 2009;49:1463–4. doi: 10.1016/j.jvs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Ohta T, Hosaka M, Ishibashi H, Sugimoto I, Mihara E, Hida K, et al. Limb salvage and survival rates among elderly patients with advanced limb ischemia. Surg Today. 1998;28:151–61. doi: 10.1007/s005950050098. [DOI] [PubMed] [Google Scholar]
- 20.Vainio E, Salenius JP, Lepäntalo M, Luther M, Ylonen K. Endovascular surgery for chronic limb ischemia Factors predicting immediate outcome on the basis of a nationwide vascular registry. Ann Chir Gynaecol. 2001;90:86–91. [PubMed] [Google Scholar]
- 21.Ramdev P, Rayan SS, Sheahan M, Hamdan AD, Logerfo FW, Akbari CM, et al. A decade of experience with infrainguinal revascularization in a dialysis-dependent patient population. J Vasc Surg. 2002;36:969–74. doi: 10.1067/mva.2002.128297. [DOI] [PubMed] [Google Scholar]


