Abstract
How genetic variations in the dopamine transporter (DAT) combined with exposure to environmental toxins modulate the risk of Parkinson’s disease (PD) remains unclear. Using unbiased stereology in DAT knock-down mice (DAT-KD) and wild-type (WT) littermates we found that decreased DAT caused a loss of tyrosine hydroxylase-positive (dopaminergic) neurons in subregions of the substantia nigra pars compacta (SNc) at 3–4 days, 5 weeks, and 18 months of age. Both genotypes lost dopaminergic neurons with age and remaining neurons at 11 months were resilient to paraquat/maneb. In 5 weeks old mice, the toxins decreased SNc dopaminergic neurons in both genotypes but less in DAT-KD. Regional analysis revealed striking differences in the subsets of neurons affected by low DAT, paraquat/maneb, and aging. In particular, we show that a potentially protective effect of low DAT against toxin exposure is not sufficient to reduce death of all nigrostriatal dopaminergic neurons. Thus, different regional vulnerability of nigrostriatal dopaminergic neurons may contribute to an increased risk of developing PD when multiple factors are combined.
Keywords: Parkinson’s disease, stereology, animal model, environmental toxins, neurodegeneration
1. Introduction
The cause and pathological mechanisms of Parkinson’s disease (PD) remain poorly understood. Even though the disease may originate outside the brain and affects multiple central and peripheral neurons (Del Tredici and Braak, 2012), a characteristic feature of PD is the progressive loss of nigrostriatal dopaminergic neurons, leading to the motor deficits that define PD. Dopamine is prone to oxidation into reactive molecules that can cause oxidative stress; accordingly, disruption of dopamine homeostasis may contribute to the vulnerability of dopaminergic neurons (Chesselet, 2003, Hastings, et al., 1996). The cytoplasmic dopamine transporter (DAT), which recycles extracellular dopamine into dopamine terminals, contributes to the control of intracellular dopamine levels. Mice with overexpression of DAT in dopaminergic cells lose neurons in the substantia nigra pars compacta (SNc) (Masoud, et al., 2015), supporting a role for DAT-mediated dopamine transport in modulating the survival of dopaminergic neurons.
Two epidemiological studies have concluded that DAT/SLC6A3 variations significantly contribute to PD risk in subjects with occupational exposure to pesticides (Kelada, et al., 2005, Ritz, et al., 2009). These include genetic variants in the 5′region of the gene together with 9 repeats of a 40-base pair variable number of tandem repeats (VNTR) polymorphism located in the 3′-untranslated region (3′UTR). Although the functional significance of these variants has been debated (Costa, et al., 2011, Drgon, et al., 2006, Kelada, et al., 2005), a large brain imaging study (van de Giessen, et al., 2009) revealed that variations in the DAT/SLC6A3 gene that are associated with increased PD susceptibility, especially the haplotype T-A-9R for the single-nucleotide polymorphisms (SNPs) rs2652511 and rs2937639 and the VNTR, increase the expression of DAT in striatum. A similar conclusion was reached by Faraone, et al. (2014) in a large meta-analysis of PET studies. Experimental studies suggest that the synergism between DAT/SLC6A3 variants and pesticide exposure (Kelada, et al., 2005, Ritz, et al., 2009) could be in part due to an increased uptake of the herbicide paraquat, as mice with greatly reduced levels of DAT (DAT hypomorphs) were resistant to paraquat-induced loss of dopaminergic neurons (Rappold, et al., 2011). However, little is known about the role of low DAT expression and environmental toxin exposure across the life span, and about their effects on subpopulations of nigrostriatal dopaminergic neurons within the SNc.
In the present study we examined the effects of low DAT levels on nigrostriatal dopaminergic neurons in young and old DAT knock-down mice (DAT-KD; Zhuang, et al., 2001) or wild-type (WT) littermates. In addition, we examined whether low DAT levels modulate the effects of a single exposure to the herbicide paraquat and the fungicide maneb, at different ages. This toxin combination was chosen because it increases paraquat toxicity in animals (Kachroo, et al., 2010, Thiruchelvam, et al., 2003) and increases PD risk in humans with DAT variants (Ritz, et al., 2009). We administered paraquat and maneb to either young adult or middle age male DAT-KD and WT mice and examined their effects on different subsets of nigrostriatal dopaminergic cells in the SNc, as well as on dopaminergic terminals in the striatum with histochemical and biochemical approaches.
2. Material and Methods
2.1 Animals
Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were approved by the Institutional Animal Care and Use Committee at the University of California Los Angeles (UCLA). DAT-KD mice, in which the insertion of an extra 4-kb DNA sequence in the promoter region resulted in a reduction in DAT expression levels, were obtained from Dr Zhuang, U. Chicago (Zhuang, et al., 2001). Heterozygous mice on a 129 Sv/J background were bred to generate WT, heterozygous, and homozygous mutant mice. Male DAT-KD mice at 4 different ages (pups at 3–4 days postnatal; young adult at 5 weeks; middle age adult at 11 months; and aged mice at 18 months) and corresponding littermates were used (n=5–10 in all groups). The genotype of all mice was verified with polymerase chain reaction (PCR) amplification analysis of tail DNA. Animals were maintained on a reverse 12/12-h light/dark cycle with lights off at 10 am. All behavioral testing was performed between 12–4 pm during the dark cycle under low light in an allocated room for behavioral experiments adjacent to the animal holding room. DAT-KD mice and WT littermates were tested in parallel in alternating sequence by an investigator unaware of the genotypes. Mice were housed socially (up to 5 mice per cage, conventional housing) with littermates on standard bedding (shredded wood) and material for nest-building (Nestlets, Ancare, NY, USA) was provided. Mice had ad libitum access to water and rodent diet (NIH-31 modified 7013, Harlan). Room temperature in the mouse holding room was 23°C ± 2°C and relative humidity was about 60%; values were recorded during the daily animal check.
2.2 Toxin exposure
Mice of each litter were assigned to treatment groups to balance litters in order to avoid any possible litter effect (n=5–10 per group). Paraquat (10 mg/kg) and maneb (30 mg/kg) were dissolved separately in sterile preservative-free saline and injected intraperitoneally (i.p.) in two successive injections (first paraquat followed by maneb) in DAT-KD and WT mice at 5 weeks of age. A separate group of DAT-KD and WT at 11 months of age was injected with the same protocol and doses. Respective control mice received two saline injections. Body weight and general health was recorded daily. Motoric behavior was assessed 7 days after toxin injection in 5 weeks old mice. Mice were sacrificed 7 days (5 week-old mice) or 21 days (11 month-old mice) after toxin injection (Fig. 1A summary study plan).
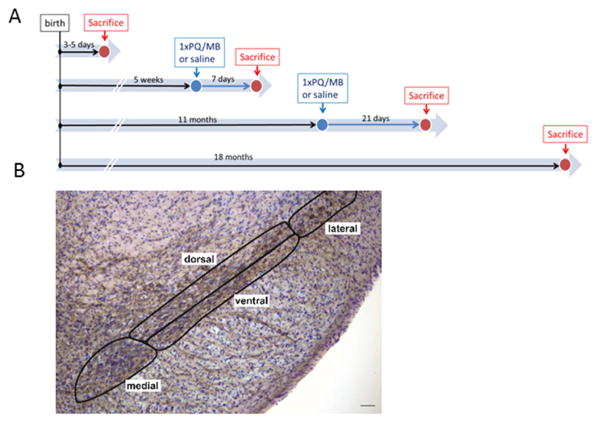
Fig. 1.
(A) Study plan for the four cohorts. Pups (3–5 days of age) and aged mice (18 months) were used naïve for quantification of SNc neuron numbers and striatal proteins. Two further cohorts of mice at 5 weeks or 11 months received a single i.p. injection of paraquat (PQ) and maneb (MB). Mice were sacrificed 1–3 weeks afterwards and brains processed for histology or biochemistry. (B) Image of a coronal brain section with the SNc labeled for TH (brown) and Nissl (blue-purple). The 4 subregions defined for stereological analysis of neuron counts are outlined. Scale bar = 50 μm.
2.3 Behavior
Spontaneous activity and motor coordination were measured with the cylinder and pole test, respectively, in 5 weeks old mice as described (Fleming et al. 2004 and supplemental information). Sensorimotor responses were measured in 18 month-old mice using the adhesive removal test according to (Fleming, et al., 2004, Lam, et al., 2011) with small modifications: the cut off time was set to 2 min as the mice of this background (129 Sv/J) required more time to remove the sticker than C57BL/6-DBA2 mice used in the previous study. The inter-trial interval was reduced from 2 to 1 minute as the longer latency to remove the sticker overall increased the interval between handling to place the sticker (see supplemental information for further details).
2.4 Histology
Immunohistochemisty for tyrosine hydroxylase (TH) was performed as described (Fernagut et al 2007 and supplemental information). We used unbiased stereological analysis with the optical fractionator probe to estimate the number of TH-positive neurons and the total number of Nissl stained neurons in the SNc as previously described (Fernagut, et al., 2007). Briefly, Stereo Investigator software (Microbrightfield, Williston, VT) coupled to a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12, Hendenheim, Traunreut, Germany) was used for stereological sampling. The SNc was delineated at 5X magnification and TH-positive neurons as well as total neuron counts (TH-positive plus TH-negative/Nissl positive neurons) were performed at 40X magnification. For regional analysis, the SNc of 5 weeks and 11–18 months old mice was subdivided into medial, lateral, dorsal and ventral regions as described in Fernagut et al. (2007) and illustrated in figure 1B.
2.4.1 Quantification of iron-containing cells in the substantia nigra
Sections at the level of the SNc were processed for Perls’ Prussian blue reaction and iron positive cells were counted (Morris, et al., 1992 and supplemental information).
2.4.2 Quantification of TH, DAT, IBA-1 and alpha-synuclein protein
Striatal sections (40 μm; rostral, medial and caudal striatum) and sections of the SNc (for alpha-synuclein staining) were processed as described (supplemental information) with the following primary antibodies: TH, Millipore; DAT, Millipore; anti-IBA-1, Wako, 019-19741; alpha-synuclein, BD Biosciences and images were analyzed with a microarray scanner as described (Richter, et al., 2014 and supplemental information).
2.4.3 Quantification of activated versus resting IBA-1-positive microglia in the SN
Immunostaining for IBA-1 was performed as described previously, and analyzed based on differences in diameters between resting and activated microglia (Watson, et al., 2012 and supplemental information).
2.4.4 Analysis of aggregated alpha-synuclein
Immunostaining for alpha-synuclein was performed as described previously (Fernagut, et al., 2007, Richter, et al., 2014) and sections examined for the presence of aggregates; no quantification was performed as no aggregates were detected in this study (see supplemental information for details).
2.5 HPLC analysis of monoamine content in the striatum
Tissue punches (2 mm diameter, between Bregma +1.70 to +0.86 mm) from the striata of all mice were analyzed for serotonin and catecholamines using high performance liquid chromatography coupled with an electrochemical detector (Antec Decade, Antec, Boston) fitted with a glassy carbon cell referenced against a silver/silver chloride electrode with an applied voltage of +0.75 V as described previously (Lam, et al., 2011) with modifications (supplemental information).
2.6 Statistics
An analysis of variance (ANOVA) followed by post-hoc comparisons with Holm-Sidak method was used to analyze all measures (normality confirmed prior with Shapiro-Wilks test), except the adhesive removal test and the pole test (not normally distributed due to cut off) and the ratios in the HPLC data (not normally distributed according to Shapiro-Wilk test) which were analyzed with the non-parametric Mann-Whitney U or Wilcoxon Signed Rank test. A genotype x age between-subject ANOVA was used to analyze the genotype effects across the different age groups (pups, aged mice). A genotype x toxin between-subject ANOVA was used to analyze the toxin (paraquat/maneb) effects in young adult and middle age DAT-KD and WT mice. A genotype x subregion mixed design ANOVA was used for analysis of SNc and striatal subregional data with subregion as repeated measure. A genotype x time mixed design ANOVA was used for analysis of body weights after toxin exposure with time as repeated measure. A value of p<0.05 was considered significant (SigmaPlot 12, Systat Software, Inc., San Jose, CA).
3. Results
DAT-KD mice weighed slightly more than WT mice at 5 weeks of age (non-significant) and 6.2 g more than WT mice at 18 months of age (p<0.01, Table S1), an effect previously related to higher food and water intake (Pecina, et al., 2003). No increased mortality or morbidity was observed in DAT-KD mice compared to WT up to 18 months of age.
3.1 Nigrostriatal dopaminergic neurons in DAT-KD mice and WT: effects of genotype and aging
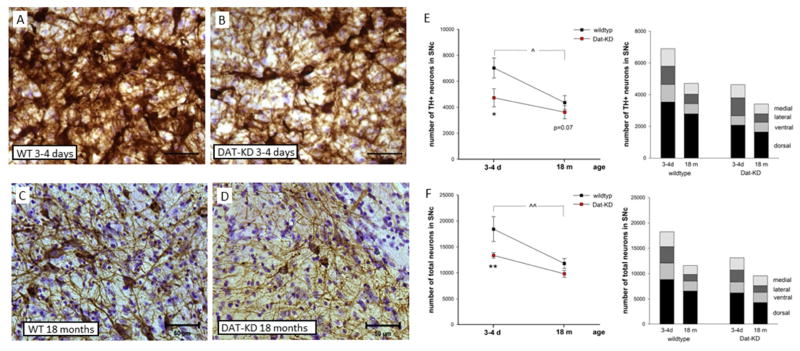
First we used naïve WT and DAT-KD pups (3–4 days of age) and aged mice (18 months of age) to determine whether low expression of DAT and aging affected nigrostriatal dopaminergic neurons in the absence of toxin exposure. Representative photomicrographs of TH-positive neurons are shown in figure 2A–D. We used unbiased stereology to estimate the number of TH-positive neurons in the SNc. DAT-KD mice showed a strong genotype effect (2×2 genotype x age ANOVA for TH-positive neurons, main effect of genotype: F(1,19)=11.1, p=0.004). Pups had 30% fewer TH-positive neurons in the SNc when compared with WT (Fig. 2E), but this decrease was reduced to a trend in old mice (Holm-Sidak: pups p=0.012, aged p=0.07). The total number of neurons (TH-positive plus TH-negative/Nissl-positive neurons) in the SNc of DAT-KD pups was significantly less than that of WT mice (Figure 2F; main effect of genotype: F(1,19)=11.7, p=0.003). Again, the effect was highly significant in pups, indicating neuronal death rather than simply a decrease in TH expression (Holm-Sidak: pups p=0.005, aged p=0.148).
Fig. 2.
Reduced number of nigral neurons in DAT-KD mice and with age. Representative microscopic images of the SNc stained for TH (brown) and Nissl (blue-purple) of (A) WT and (B) DAT-KD pups (3–4 days of age) and (C, D) aged mice (18 months of age) are shown. Scale bar = 50 μm. The number of (E) TH-positive and (F) total neurons (TH-positive and -negative) were estimated using unbiased stereology in pups (3–4 days (d) of age; WT n=4, Dat-KD n=5) and aged mice (18 months (m) of age; n=7 WT, n=7 DAT-KD) (2×2 genotype x age ANOVA, Holm-Sidak *p<0.05, **p<0.01 compared to respective WT, ^p<0.05, ^^p<0.01 compared to pup (WT); mean ± SEM. Stacked bars represent the SNc subregion counts for each respective group (for statistics see Table 1).
To further characterize the pattern of neuron loss induced by low DAT expression we analyzed the number of TH-positive and total neurons in subregions of the SNc that are known to be differentially vulnerable to PD and to toxin exposure (Fernagut, et al., 2007, Kordower, et al., 2013). As shown in stacked bars in figure 2E and F with detailed statistical analyses in table 1, there was a subregional specific loss of TH-positive neurons in pups with the ventral (−47%) and dorsal (−41%) region most affected by DAT-KD. This loss was also present when the number of total neurons was estimated, supporting a lower number of neurons (Table 1).
Table 1.
Effects of DAT-KD and aging on number of SNc neurons
| region | group | pups (3–4 days) | aged (18 months) | ||
|---|---|---|---|---|---|
| TH+ neurons | Total neurons | TH+ neurons | Total neurons | ||
| SNc complete | WT | 6898 ± 484 | 18258 ± 1396 | 4713 ± 449* | 11580 ± 662** |
| DAT | 4628 ± 577^ | 13120 ± 957^^ | 3411 ± 478° | 9576 ± 530* | |
| SNc medial | WT | 1100 ± 130 | 2943 ± 182 | 682 ± 125* | 1735 ± 215** |
| DAT | 823 ± 118 | 2403 ± 200 | 616 ± 106 | 1937 ± 272 | |
| SNc lateral | WT | 1152 ± 80 | 3240 ± 220 | 597 ± 104** | 1302 ± 221** |
| DAT | 1139 ± 137 | 2370 ± 153^^ | 531 ± 71** | 1335 ± 180** | |
| SNc ventral | WT | 1110 ± 93 | 3244 ± 350 | 644 ± 115** | 1965 ± 228** |
| DAT | 592 ± 132^^ | 2146 ± 205^ | 616 ± 137 | 2012 ± 202 | |
| SNc dorsal | WT | 3536 ± 236 | 8830 ± 756 | 2790 ± 293 | 6577 ± 354** |
| DAT | 2074 ± 323^^ | 6201 ± 687^^ | 1647 ± 230^^ | 4291 ± 407*^^ | |
Stereological estimates (n, means ± SEM) of TH-positive (TH+) neurons and TH-positive and –negative neurons (total neurons) in the SNc of pups (3–4 days old), WT (n=4) and DAT-KD (n=5) and aged mice (18 months old), WT (n=7) and DAT-KD (n=7) (2×2 genotype x age ANOVA for each region, Holm-Sidak, *p<0.05, **p<0.01 compared to respective pups group, °p=0.07, ^p<0.05, ^^p<0.01 compared to respective WT).
TH-positive neurons in the SNc decreased with age (Fig. 2E) in both genotypes, with a significant difference in WT mice. Between 4 days after birth and 18 months of age, WT mice lost 32% and DAT-KD mice 26% of their SNc TH-positive neurons (2×2 genotype x age ANOVA for TH-positive neurons, main effect of age: F(1,19)=10.1, p=0.005, Holm-Sidak, p=0.01 in WT, p=0.125 in DAT-KD). This was corroborated by a reduction of the total number of neurons in the SNc with age in both genotypes (Figure 2F; main effect of age: F(1,19)=23.7, p<0.001; Holm-Sidak, p<0.001 in WT, p=0.025 in DAT-KD). Analysis of age related decline in SNc subregions revealed a TH-positive neuron loss in the medial, lateral and ventral subregion in WT mice, and in the lateral subregion in DAT-KD mice thereby adding a further loss to the effect of genotype in these mice (Table 1 with details on statistics). In affected subregions, this loss of TH-positive neurons corresponded to a significant loss of total neurons (Table 1).
3.2 Alterations in striatal proteins in DAT-KD mice
Striatal tissue sections were processed for immunohistochemistry to confirm the loss of DAT in all DAT-KD mice and to determine whether the loss of nigrostriatal neurons resulted in decreased TH-positive axons in their target region. Tissue sections for pups and aged mice were processed separately; therefore, staining intensities were only compared for a genotype effect within each age group. As expected, DAT-KD mice showed a marked decrease in DAT immunoreactivity in the striatum in pups and 18 months old mice across all striatal levels (2×3 genotype x subregion ANOVA, main effect of genotype: pups F(1,19)=13.9, aged F(1,23)=43.4, p<0.01; Holm-Sidak WT vs DAT-KD p<0.01 for each age group). Table 2 shows the normalized values to WT levels for each age group for easier comparison. No significant differences in striatal TH immunoreactivity were found in DAT-KD pups or aged mice when compared with respective WT mice (Table 2).
Table 2.
Effects of DAT knock-down on striatal DAT and TH-immunoreactivity
| striatal DAT-immunoreactivity | ||||||
|---|---|---|---|---|---|---|
| rostral | medial | caudal | ||||
| Age | WT | DAT-KD | WT | DAT-KD | WT | DAT-KD |
| Pups (3–4 days) | 1.00 ± 0.10 | 0.54 ± 0.05** | 1.00 ± 0.10 | 0.57 ± 0.05** | 1.00 ± 0.09 | 0.66 ± 0.07* |
| aged (18 months) | 1.00 ± 0.09 | 0.42 ± 0.02** | 1.00 ± 0.06 | 0.42 ± 0.04** | 1.00 ± 0.11 | 0.41 ± 0.04** |
| striatal TH-immunoreactivity | ||||||
| rostral | medial | caudal | ||||
| Age | WT | DAT-KD | WT | DAT-KD | WT | DAT-KD |
| Pups (3–4 days) | 1.00 ± 0.05 | 1.03 ± 0.05 | 1.00 ± 0.05 | 1.10 ± 0.07 | 1.00 ± 0.04 | 1.16 ± 0.07 |
| aged (18 months) | 1.00 ± 0.11 | 0.94 ± 0.09 | 1.00 ± 0.11 | 0.98 ± 0.13 | 1.00 ± 0.13 | 0.88 ± 0.12 |
Striatal DAT and TH immunoreactivity. A coronal section of the rostral, medial and caudal striatum was used for each mouse (pups, WT n=7, DAT-KD =6; 18 months, WT=7, DAT-KD n=7). Data are represented as ratios to respective mean of WT (mean ± SEM) for comparison purposes only; all statistical analyses were performed on raw numbers (2×3 genotype x striatal subregion ANOVA for each age group, Holm-Sidak *p<0.05, **p<0.01 compared to respective WT). Tissue processing and immunohistochemistry for pups and aged mice were performed separately; therefore fluorescence intensities were not compared between ages. Intensity values were not saturated providing similar signal to noise ratio for all age groups.
3.3 Sensorimotor behavior in DAT-KD mice
DAT-KD mice were previously shown to be hyperactive in an open field (Zhuang, et al., 2001). In order to further explore behavioral deficits in 18 months old DAT-KD mice we used a sensorimotor test that is sensitive to dopaminergic system dysfunction, feasible in aged mice and not affected by differences in body weight: the ability to remove an adhesive sticker placed on the snout (Fleming, et al., 2004, Lam, et al., 2011, Schallert, 1988). WT mice took longer to remove the sticker with each subsequent trial (median trial 1: 35 s, trial 3: 120 s, W=21, p=0.031, Wilcoxon Signed Rank test), likely due to habituation to the sticker (Fig. S1). In contrast, the performance of the DAT-KD mice did not change between the first and last trials (median trial 1: 9.4 s, trial 3: 42 s, W=10, p=0.47, Wilcoxon Signed Rank test). This paradoxically led to a shorter latency to remove the sticker in the third trial in DAT-KD mice when compared with WT (Fig. S1; U=3.5, p=0.008, Mann Whitney U test) but reveals a failure to habituate to the sensory stimulus, in addition to the impaired response habituation in novel environments described by Zhuang et al. (2001).
3.4 Toxins exposure in young adult DAT-KD mice
To determine if lower DAT function protects nigrostriatal dopaminergic neurons from paraquat/maneb toxicity, as found previously for paraquat alone (Rappold, et al., 2011), we exposed young DAT-KD mice to a combined administration of the herbicide paraquat (10 mg/kg) and fungicide maneb (30 mg/kg). This regimen was chosen because epidemiological data have shown an effect of DAT haplotypes in individuals exposed to this combination of toxins (often used together), and adding maneb to paraquat increases the maximum loss of dopaminergic neurons from 25 to about 40% in WT mice (Kachroo, et al., 2010, Thiruchelvam, et al., 2003). All mice showed weight gain seven days after either saline or toxin injection, and there was no significant effect of paraquat/maneb injections on bodyweight in WT (2×4 toxin x time ANOVA, main effect of time F(3,27)=39.5, p<0.001; no main effect of toxin) or DAT-KD mice (main effect of time F(3,39)=38.4, p<0.001; no main effect of toxin).
3.5 Toxin effects on dopamine neurons in the SNc
Table 3 shows the values obtained by unbiased stereological analysis in the SNc of young adult (5 weeks old) WT and DAT-KD mice. First we confirmed a significant effect of genotype on the number of TH-positive neurons in the complete SNc (−28%) and in the medial (−30%), lateral (−35%) and ventral (−27%) subregions of the SNc, with a similar trend that did not reach significance in the dorsal region (−24%) (Table 3 for means ± SEM and statistics). The reduction of the total number of neurons (TH-positive and TH-negative/Nissl positive) was similar to the loss of TH expression:−25% (complete), −28% (medial), −32% (lateral), −11% (ventral, not significant) and −26% (dorsal, p=0.069) (Table 3).
Table 3.
Effects of DAT-KD and paraquat/maneb (PQ/MB) exposure on number of SNc neurons in 5 weeks old mice
| region | group | TH+ neurons (n, Means ± SEM) | % loss (to saline) | neuron loss (compared to saline) | Total neurons (n, Means ± SEM) | % loss (to saline) | neuron loss (compared to saline) |
|---|---|---|---|---|---|---|---|
| SNc complete | WT_SAL | 6212 ± 709 | 11291 ± 849 | ||||
| WT_PQ/MB | 3908 ± 309** | 37 | 2310 | 7863 ± 808** | 30 | 3428 | |
| DAT_SAL | 4483 ± 310^ | 8526 ± 528^^ | |||||
| DAT_PQ/MB | 3261 ± 508* | 27 | 1222 | 7335 ± 333 | 14 | 1191 | |
| SNc medial | WT_SAL | 1652 ± 152 | 2567 ± 255 | ||||
| WT_PQ/MB | 1073 ± 49** | 35 | 579 | 1955 ± 141* | 24 | 612 | |
| DAT_SAL | 1162 ± 97^^ | 1837 ± 147^^ | |||||
| DAT_PQ/MB | 592 ± 76**^^ | 49 | 570 | 1311 ± 149*^ | 29 | 526 | |
| SNc lateral | WT_SAL | 1007 ± 181 | 2133 ± 273 | ||||
| WT_PQ/MB | 737 ± 100 | 27 | 270 | 1494 ± 281* | 30 | 639 | |
| DAT_SAL | 658 ± 47^ | 1455 ± 101^^ | |||||
| DAT_PQ/MB | 531 ± 78 | 19 | 127 | 1420 ± 95 | 2 | 35 | |
| SNc ventral | WT_SAL | 1303 ± 177 | 2383 ± 404 | ||||
| WT_PQ/MB | 718 ± 105** | 45 | 585 | 1889 ± 182 | 21 | 494 | |
| DAT_SAL | 948 ± 83^ | 2113 ± 153 | |||||
| DAT_PQ/MB | 592 ± 110* | 38 | 356 | 1821 ± 167 | 14 | 292 | |
| SNc dorsal | WT_SAL | 2249 ± 356 | 4208 ± 466 | ||||
| WT_PQ/MB | 1381 ± 356 | 39 | 868 | 2524 ± 466* | 40 | 1684 | |
| DAT_SAL | 1715 ± 251 | 3121 ± 330 | |||||
| DAT_PQ/MB | 1714 ± 301 | 0 | 1 | 3230 ± 394 | 0 | 0 |
Stereological estimates (means ± SEM) of TH-positive (TH+) neurons and TH-positive and –negative neurons (total neurons) in the SNc of 5 weeks old WT and DAT-KD mice injected with saline (WT_SAL n=5, DAT_SAL n=10) or PQ/MB (WT_PQ/MB n=5, DAT_PQ/MB n=7) as well as the percentage and the number of neurons lost compared to respective saline injected group (2×2 genotype x toxin between-subject ANOVA, Holm-Sidak *p<0.05, **p<0.01 compared to respective saline group; ^p<0.05, ^^p<0.01 compared to WT; significant interaction (p=0.04) of genotype x toxin in dorsal subregion for total neurons).
A single injection of paraquat/maneb reduced the number of TH-positive neurons by 37% in WT and 27% in DAT-KD mice when compared with the respective vehicle group (2×2 genotype x toxin ANOVA, effect of toxin on TH-positive neurons: F(1,23)=14.1, p=0.001; Holm-Sidak WT saline vs toxin p=0.005, DAT-KD saline vs toxin p=0.045; Table 3). Notably, because DAT-KD mice have significantly less neurons than WT, the average number of neurons lost was much smaller in DAT-KD than in WT mice (1222 versus 2310) and the number of remaining neurons in DAT-KD mice was no longer different from WT after exposure to paraquat/maneb. This was also reflected in the total number of SNc neurons, which was reduced significantly by 30% in WT but only by 14% in DAT-KD mice, which was not significant (2×2 genotype x toxin ANOVA, effect of toxin on total SNc neurons: F(1,23)=13.4, p<0.001; Holm-Sidak WT saline vs toxin p=0.002, DAT-KD saline vs toxin p=0.139), resulting in similar number of remaining neurons in both genotypes. Thus paraquat/maneb caused loss of TH phenotype in both WT and DAT-KD mice, but only a significant neuronal death in WT mice. Importantly, the toxin exposure abolished the difference in TH-positive and in neuron numbers observed in mice that only received saline. Thus, both low DAT and paraquat/maneb alter dopamine neurons but their effects are not additive. Rather, a smaller number of neurons were lost in the DAT-KD mice, supporting a partial protection against the toxin effects as proposed by Rappold, et al. (2011).
Subpopulations of nigrostriatal dopaminergic neurons are differentially vulnerable in PD (Kordower, et al., 2013) and to various experimental insults, including paraquat (Fernagut, et al., 2007) and aging (this study). The effects of paraquat/maneb on TH-positive neurons also differed markedly between subregions of the SNc in 5 week old WT and DAT-KD mice (Table 3) with strong effects in the medial and ventral regions in both genotypes, a mild effect in the lateral region, only significant on total number of neurons in WT mice, and a striking lack of toxin effects in the dorsal region of the SNc in DAT-KD mice. Figure S2 illustrates the loss of TH-positive neurons in the medial region of the SNc in DAT-KD mice, in which the toxins induced not only a loss of TH phenotype but a loss of neurons in DAT-KD mice. Figure S3 illustrates the losses due to genotype and toxin in the dorsal and ventral subregions. Thus, even though dopaminergic neurons in some regions of the SNc, notably its dorsal tier, may be less sensitive to toxin in mice with low DAT than in WT mice, the combination of toxin exposure and genotype effect results in a lower remaining number of dopaminergic neurons in the medial region of the SNc in DAT–KD, suggesting that the proposed protective effect of low DAT against toxin exposure is not sufficient to reduce death of all nigrostriatal dopaminergic neurons, in particular those of the most vulnerable regions of the SNc.
3.6 Toxin effects on striatal proteins and monoamines in young adult DAT-KD mice
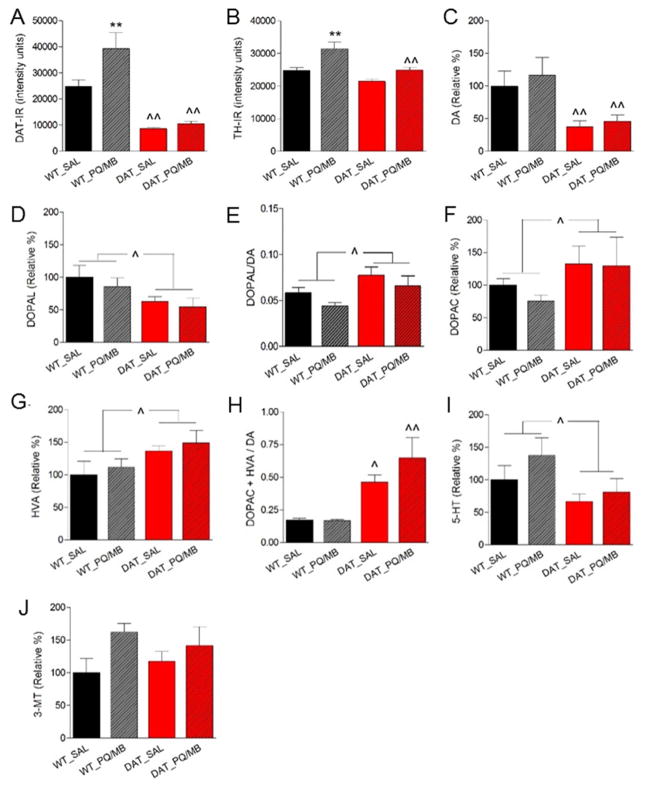
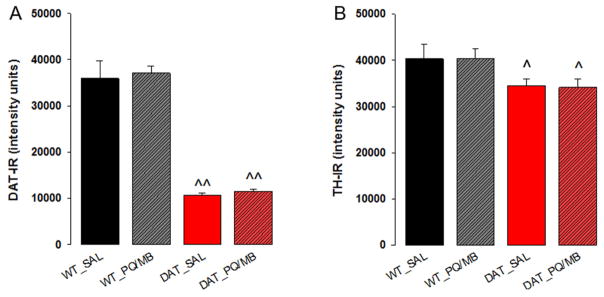
As expected, saline exposed young adult DAT-KD mice showed a marked decrease in DAT immunoreactivity in the striatum across all striatal levels (2×3 genotype x subregion ANOVA, main effect of genotype: F(1,16)=44.7, p<0.01; Holm-Sidak WT vs DAT-KD p<0.01). While pups and aged DAT-KD mice did not show differences in TH immunoreactivity as described above, young adult DAT-KD mice had 17% less TH immunoreactivity in the caudal striatum compared to WT with a strong trend to reduction in the medial striatum (2×3 genotype x subregion ANOVA, main effect of genotype: F(1,16)=6.231, p=0.037, Holm-Sidak WT vs DAT-KD caudal p=0.032, rostral p=0.99, medial p=0.057). Figure 3A and B show the level of immunoreactivity (arbitrary units) for DAT and TH in the medial striatum. A single injection of paraquat/maneb increased DAT and TH immunoreactivity in the striatum of WT mice (Fig. 3A, B). In contrast, DAT-KD mice showed a non-significant trend toward an increase in TH immunoreactivity and no change in DAT immunoreactivity (2×2 genotype x toxin ANOVA, for TH, effect of toxin: F(1,16)=17.80, p<0.001, Holm-Sidak WT saline vs toxin p=0.001, DAT-KD saline vs toxin p=0.057; for DAT, effect of toxin: F(1,16)=5.96, p=0.027, Holm-Sidak WT saline vs toxin p=0.008, DAT-KD saline vs toxin p=0.689).
Fig. 3.
Striatal protein (A, DAT; B, TH) quantification via immunohistochemistry and striatal monoamine (C, D) or dopamine metabolite (E–J) quantification via HPLC (see methods section for definition of abbreviations) in 5-week-old WT or DAT-KD mice (n=5 per group) injected with saline (WT_SAL or DAT_SAL) or paraquat/maneb (WT_PQ/MB or DAT_PQ/MB). Data are shown as the mean + SEM, 2×2 genotype x toxin ANOVA, Holm-Sidak, *p<0.05, **p<0.01 compared to respective saline group, ^p<0.05, ^^p<0.01 compared to respective WT).
To further describe PQ/MB effects on the striatum we next determined monoamines and their metabolites. DOPAL has recently emerged as an important dopamine metabolite because of its toxicity to dopamine neurons (Burke, et al., 2003, Fitzmaurice, et al., 2013). Therefore, we measured DOPAL levels in right striatal punches of the striatum together with dopamine and DOPAC using an optimized protocol for DOPAL detection (see supplemental methods). Congruent with previously published results (Zhuang, et al., 2001) we observed a significant genotype effect on the levels of dopamine (−62% in DAT-KD), but no alteration by toxin exposure in WT or DAT-KD mice (Fig. 3C). The toxic dopamine metabolite DOPAL was also decreased in DAT-KD (Fig. 3D) but the ratio DOPAL/dopamine was increase in DAT-KD compared to WT animals (Fig. 3E), and DOPAC was increased (Fig. 3F). There was no effect of toxin administration on these measures neither in WT nor in DAT-KD mice. Data on additional monoamines and metabolites determined in the left striatum further support increased dopamine metabolism in DAT-KD mice (figure 3G–J): HVA (increased in DAT-KD), DOPAC+HVA/dopamine (2.9 fold increased in saline treated DAT-KD), 5-HT (decrease in DAT-KD), 3-MT (unchanged). These measures were not affected by toxin exposure compared to the same genotype exposed to saline.
3.7 Toxin effects on motor behavior
The moderate loss of dopamine neurons of the SNc after toxin exposure is likely insufficient to cause detectable parkinsonism. In agreement with the presence of high extracellular dopamine levels in the striatum, DAT-KD mice are hyperactive in an open field (Zhuang, et al., 2001). We used the cylinder test which enabled us to distinguish between fore- and hindlimb activity and the pole test, both sensitive tests for detection of motor deficits in PD models (Fleming, et al., 2004). The results confirmed hyperactivity of DAT-KD mice and showed that this includes increased use of fore- and hindlimbs but no change in grooming activity at 5 weeks of age in the cylinder test (Fig. S4A; 2×2 genotype x toxin ANOVA, genotype effect forelimb steps F(1,24)=21.5, p<0.001, hindlimb steps F(1,24)=7.76, p=0.01, grooming F(1,24)=2.1, p=0.162; Fig S4A–C). There were no effects of PQ/MB injection on these parameters (Fig S4A–C). On the vertical pole time to turn face down (Fig. S4D) was not altered by genotype (WT vs DAT-KD, Mann Whitney U p>0.05), time of test (pre vs post toxin exposure, Wilcoxon signed rank test p>0.05) or toxin exposure (saline vs PQ/MB, Mann Whitney U p>0.05). During the second trial (one week post toxin exposure) the time to descend after the turn (Fig. S4E) decreased in all groups, likely as an effect of learning, but in WT injected with PQ/MB this improvement was not significant (*p<0.05, **p<0.01 signed rank test). These PQ/MB injected WT mice were significantly slower compared to corresponding saline injected mice (^p<0.05 rank sum test, Fig. S4E).
3.8 Toxin exposure increased iron accumulation in young adult DAT-KD mice
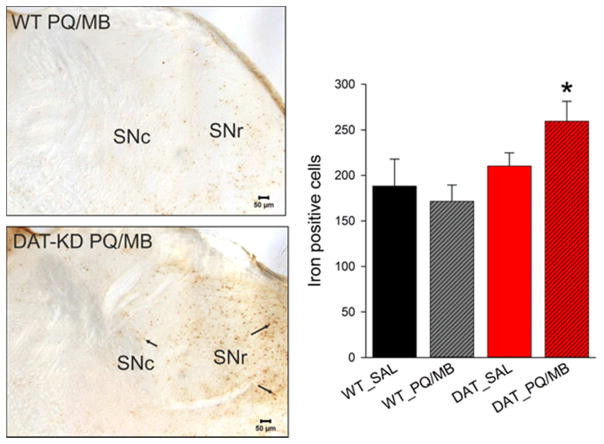
To begin identifying mechanisms by which low DAT expression and/or toxin exposure may affect dopaminergic neurons, we measured IBA-1-positive microglial activation, alpha-synuclein accumulation and aggregation, and iron accumulation in the striatum and/or SN. The number of activated or resting IBA-1-positive microglia in the SN or IBA-1 immunoreactivity in the striatum did not show either genotype or toxin effects (Table S2). Similarly, we did not find alterations in alpha-synuclein immunoreactivity in the SNc or substantia nigra pars reticulata (SNr) in response to low DAT expression or toxin exposure (Table S2). Furthermore, no alpha-synuclein-positive proteinase K-resistant aggregates were detected in the brains of WT or DAT-KD mice (data not shown). In contrast, a single exposure of paraquat/maneb in 5 week-old mice resulted in the accumulation of iron in DAT-KD but not WT mice (Fig. 4, 2×2 genotype x toxin ANOVA, effect of genotype F(1,13)=6.87, p=0.02, Holm-Sidak toxin WT vs DAT-KD p=0.013).
Fig. 4.
Number of iron deposits (Perls’ Prussian blue reaction) in the SN of 5-week-old WT and DAT-KD mice (n=4–5/group) injected with saline (WT_SAL or DAT_SAL) or paraquat/maneb (WT_PQ/MB or DAT_PQ/MB). Microscopic images (iron stain in brown) of one SN hemisphere (SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata) for WT and DAT-KD mice exposed to PQ/MB are shown. Scale bar = 50 μm. Data are shown as the mean + SEM (2×2 genotype x toxin ANOVA, Holm-Sidak, *p<0.05 compared to respective WT).
3.9 Effects of age on susceptibility to PQ/MB toxicity
Having determined that in young adult WT mice a single PQ/MB injection can induce a loss of nigral dopamine neurons and that this effect was partially modified by low DAT expression we thought to determine if age at exposure was critical for these effects. Therefore we injected 11 months old WT and DAT-KD mice with the single dose of PQ/MB. We chose a longer post injection interval of 3 weeks (compared to 1 week in young adult mice), to ensure that we capture the full extent of toxin-induced cell loss. As expected from our observations in 18 months old mice, the genotype effect (loss of TH-positive neurons in DAT-KD mice) at this age was less overt and only significant in the medial subregion when analyzed with multi-comparison correction (Table 4). Importantly, the effect of aging observed at 18 months is already overt in 11 months old mice (e.g. TH positive neurons decline from n=6212 in 5 weeks old WT mice to n=3663 in 11 months old WT mice, Table 4). Toxin exposure did not result in additional dopamine neuron loss in the SNc of 11 months old WT or DAT-KD mice neither in the total SNc nor in any of the subregions at 3 weeks post injection (Table 4). Based on this lack of neuron loss there was no reason to repeat the experiment with the 1 week post injection period used in young adult mice.
Table 4.
Effects of DAT-KD and paraquat/maneb (PQ/MB) exposure on number of SNc neurons in 11 months old mice
| region | group | TH+ neurons (n, Means ± SEM) | % loss (to saline) | neuron loss (compared to saline) | Total neurons (n, Means ± SEM) | % loss (to saline) | neuron loss (compared to saline) |
|---|---|---|---|---|---|---|---|
| SNc complete | WT_SAL | 3663 ± 393 | 10127 ± 755 | ||||
| WT_PQ/MB | 3632 ± 338 | 1 | 31 | 9260 ± 524 | 9 | 867 | |
| DAT_SAL | 3261 ± 243 | 8625 ± 586 | |||||
| DAT_PQ/MB | 3149 ± 370 | 3 | 112 | 8304 ± 1108 | 4 | 321 | |
| SNc medial | WT_SAL | 856 ± 77 | 2238 ± 109 | ||||
| WT_PQ/MB | 801 ± 51 | 6 | 55 | 2085 ± 182 | 7 | 153 | |
| DAT_SAL | 691 ± 79 | 2031 ± 185 | |||||
| DAT_PQ/MB | 552 ± 92 ^ | 20 | 139 | 1770 ± 182 | 13 | 261 | |
| SNc lateral | WT_SAL | 461 ± 82 | 1007 ± 131 | ||||
| WT_PQ/MB | 351 ± 88 | 24 | 110 | 944 ± 163 | 6 | 63 | |
| DAT_SAL | 428 ± 84 | 964 ± 146 | |||||
| DAT_PQ/MB | 523 ± 56 | 0 | 0 | 1258 ± 97 | 0 | 0 | |
| SNc ventral | WT_SAL | 560 ± 73 | 1738 ± 166 | ||||
| WT_PQ/MB | 554 ± 67 | 1 | 6 | 1772 ± 72 | 0 | 0 | |
| DAT_SAL | 545 ± 68 | 1580 ± 116 | |||||
| DAT_PQ/MB | 475 ± 89 | 13 | 70 | 1657 ± 131 | 0 | 0 | |
| SNc dorsal | WT_SAL | 1787 ± 283 | 5144 ± 498 | ||||
| WT_PQ/MB | 1925 ± 217 | 0 | 0 | 4460 ± 266 | 13 | 684 | |
| DAT_SAL | 1596 ± 156 | 4050 ± 377 | |||||
| DAT_PQ/MB | 1599 ± 170 | 0 | 0 | 3620 ± 908 | 11 | 430 |
Stereological estimates (means ± SEM) of TH-positive (TH+) neurons and TH-positive and –negative neurons (total neurons) in the SNc of 11 months old WT and DAT-KD mice injected with saline (WT_SAL n=5, DAT_SAL n=7) or PQ/MB (WT_PQ/MB n=6, DAT_PQ/MB n=9) as well as the percentage and the number of neurons lost compared to respective saline injected group (2×2 genotype x toxin between-subject ANOVA, Holm-Sidak, no effect of toxin; ^p<0.05 compared to respective WT).
In young adult WT mice PQ/MB had induced an increase in TH and DAT immunoreactivity one week after a single injection. Although PQ/MB had no effects on dopamine neurons in 11 months old mice, we asked if this increase in striatal proteins was still present. As expected, saline exposed 11 months old DAT-KD mice showed a marked decrease in DAT immunoreactivity in the striatum across all striatal levels (2×3 genotype x subregion ANOVA, main effect of genotype: F(1,26)=73.2, p<0.001; Holm-Sidak WT vs DAT-KD p<0.001; Fig. 5A for medial subregion). The single injection of paraquat/maneb did not alter DAT immunoreactivity in any of the subregions or genotypes (2×2 genotype x toxin ANOVA, non-significant).
Fig. 5.
Protein (A, DAT; B, TH) quantification via immunohistochemistry in the striatum of 11 months-old WT or DAT-KD mice injected with saline (WT_SAL n=5, DAT_SAL n=7) or PQ/MB (WT_PQ/MB n=6, DAT_PQ/MB n=9). Data are shown as the mean + SEM, 2×2 genotype x toxin ANOVA, Holm-Sidak, ^p≤0.05, ^^p<0.01 compared to respective WT).
Similar to pups and aged DAT-KD mice there were no genotype differences in TH immunoreactivity in saline treated mice across all subregions (2×3 genotype x subregion ANOVA, no effect of genotype). However, a trend to reduction became significant after PQ/MB injection as shown in Fig. 5B for the medial subregion (2×2 genotype x toxin ANOVA, medial, effect of genotype: F(1,16)=8.9, p=0.006, Holm-Sidak WT toxin vs DAT-KD toxin p=0.037, WT saline vs DAT-KD saline p=0.053; caudal, effect of genotype: F(1,27)=8.8, p=0.006, WT toxin vs DAT-KD toxin p=0.005, WT saline vs DAT-KD saline p=0.272). There was no main effect of toxin in any of the subregions supporting a general absence of effects of PQ/MB in 11 months old mice. Accordingly there was no rationale for injecting further groups of animals at this age for neurochemical and behavioral analyses.
4. Discussion
Neurodegeneration in sporadic PD results from the combination of genetic predisposition with environmental factors and aging, the main risk factor for PD (Ritz, et al., 2016). However, predicting the effects of genetic variants on disease risk in the presence of environmental toxicants is not straightforward. For example, genetic factors could contribute to the risk of PD by decreasing the number of dopamine neurons at birth, such that neuronal loss due to aging could reach the critical threshold that leads to the symptoms of PD earlier than in individuals born with more neurons, and this effect could be compounded by toxic insults occurring later in life (Calne and Langston, 1983, Langston, 1990, McGeer, et al., 1988). Conversely, genetic variation may also protect neurons from toxic exposure by reducing toxin uptake or increasing defenses (Rappold, et al., 2011, Ritz, et al., 2016). Here we assessed the interaction of genetic variation, aging and toxin exposure on the integrity of the nigrostriatal system. We show that the susceptibility of dopamine neurons to the genetic variation in DAT levels, aging, and paraquat/maneb exposure differ based on their subregional location, suggesting that the heterogeneity of the SNc contributes to the final outcome on disease risk. Specifically, low DAT expression reduced the number of dopaminergic neurons preferably in the ventral and dorsal SNc from birth, while the remaining dopaminergic neurons in the dorsal subregion are resistant to aging and paraquat/maneb. In contrast, aging reduces preferably dopamine neurons in the lateral SNc in both genotypes, and in WT additionally in the medial and ventral subregion. Finally the medial and ventral tier of SNc dopamine neurons was most vulnerable to the effects of paraquat/maneb in both genotypes. This is in line with previous studies in mice demonstrating higher susceptibility of ventral tier SNc neurons to paraquat (Fernagut, et al., 2007). Although there are anatomical variations, similar subgroups of dopamine neurons can be identified in the SNc of man and mice (Fu, et al., 2016). In humans aging affects preferably the dorsal (−6.9%) and medial ventral tier (−5.4%) whereas PD cases exhibited the greatest loss in the lateral ventral tier (average loss 91%), and smaller losses in the medial ventral tier (−71%) and, particularly, the dorsal tier (−56%) (Fearnley and Lees, 1991). Many potential reasons for these differences in vulnerability have been proposed, and their identification could reveal novel therapeutic strategies (Fu, et al., 2016). Specific protein expression profiles may contribute to disease vulnerability, but this conclusion warrants more research on isolated neuronal populations (Brichta and Greengard, 2014). Our data suggest that genetic variances, such as DAT/SLC6A3 variations, may contribute to SNc regional vulnerability, especially in the context of environmental exposures and aging.
4.1 Impact of low DAT expression on nigrostriatal dopaminergic neurons
DAT-KD mice with 10% remaining DAT expression have been previously generated as a model for attention deficit hyperactivity disorders, and were shown to exhibit increased extracellular dopamine, decrease in tissue dopamine and a hyperactive phenotype (Zhuang, et al., 2001). Recent epidemiological evidence that DAT/SLC6A3 variations significantly contribute to PD risk (Kelada, et al., 2005, Ritz, et al., 2009) prompted us to re-evaluate these mice in the context of PD. We extend the previous characterization of these mice by providing extensive morphological and neurochemical measures as well as behavioral data, at different ages, in the absence and presence of exposure to toxins known to increase the risk of PD. Importantly, we confirmed with immunohistochemistry the expected low striatal DAT levels with low inter-individual variation in all DAT-KD mice used in our study. We found that low expression of DAT resulted in 30% less TH-positive neurons in the SNc of pups, an effect sustained up to 18 months of age in subregions of the SNc. This differed from another genetically engineered mouse with less than 5% DAT expression that did not exhibit a loss of midbrain dopaminergic neurons, but severe losses of striatal TH and dopamine (Rappold, et al., 2011). Different mouse or rat strains vary in the response of their nigrostriatal system to insults or alterations, as shown repeatedly for several neurotoxins (Gerlach and Riederer, 1996, Sedelis, et al., 2000, Sherer, et al., 2003) or genetic modifications (Chesselet and Richter, 2011, Chesselet, et al., 2012). Our DAT-KD mice (Zhuang, et al., 2001) are on the 129 SvJ background, whereas the line used by Rappold, et al. (2011) was backcrossed into C57Bl/6. The 129 SvJ mice have less hippocampal neurogenesis (Kempermann, et al., 1998), exhibit differences in inflammatory cell recruitment (Hoover-Plow, et al., 2008) and oxidative stress response (Syn, et al., 2009) and show different pharmacological and behavioral responses compared to C57Bl/6 (Homanics, et al., 1999). Therefore, it is not surprising that the nigrostriatal system in these mice responds differently to a low level of DAT expression, further illustrating the importance of multiple genetic factors on the number and health of dopaminergic neurons.
The loss of dopaminergic neurons observed in our line of DAT-KD is in agreement with data from adult DAT knock-out mice on the C57BL6 background (28% reduction in TH-positive neurons in the midbrain) (Jaber, et al., 1999), although Jaber et al (1999) did not perform a counterstain to distinguish a loss of phenotype from a true loss of neurons, as observed in our study. Interestingly, loss-of-function mutations in DAT are associated with infantile parkinsonism-dystonia syndrome (Kurian, et al., 2011), supporting a role of DAT in the development of nigrostriatal dopamine neurons. In contrast to DAT-KD, DAT knock-out mice displayed a growth retardation phenotype and a subset of animals die before reaching adulthood (Bosse, et al., 1997). A 90% loss of TH protein in the striatum in DAT knock-out with much milder loss of the vesicular monoamine transporter, suggested a partial preservation of TH terminals (Jaber, et al., 1999). In contrast, we found only a slight reduction of striatal TH at an older age in DAT-KD mice, implying that the remaining 10% of DAT expression in DAT-KD mice is sufficient to preserve TH expression in the striatum. These subtle differences in TH expression support the notion that the lack of dopamine recycling is sufficient to reduce the level of tissue dopamine without altering the integrity of the nigrostriatal system. Our data suggest that individuals carrying genetic variant of DAT may be born with a compromised nigrostriatal system. Thus, even though current data favor the hypothesis that excess DAT is the major factor associated with increased PD risk (Faraone, et al., 2014, Masoud, et al., 2015, Ritz, et al., 2016), our results extend this risk to genetic variants that might decrease DAT expression and suggest that this possibility deserves further study.
4.2 Age-dependent susceptibility to toxins in WT mice
As originally proposed by Barbeau, et al. (1987), multiple epidemiological studies have pointed to a role for environmental toxins, including a combination of paraquat and maneb, in increasing the risk of PD (Costello, et al., 2009). The route of environmental exposure in humans is likely a combination of oral intake, subcutaneous absorption and inhalation of varied amounts of toxins. In rodents i.p. injections which allow administration of a controlled amount of toxins were found appropriate for brain penetration and neurotoxicity while avoiding acute toxicity to sensitive tissues such as lung tissue (Barlow, et al., 2003, Rojo, et al., 2007). The dopamine neuron loss caused by 3 injections of paraquat alone reaches approximately 25% of nigral neurons (Fernagut, et al., 2007, McCormack, et al., 2002), depending on the genetic background of mice (Yin, et al., 2011). Combining paraquat with maneb increased this loss to approximately 40% (Kachroo, et al., 2010), which could be due to increased oxidative stress (Patel, et al., 2006) or to enhanced brain accumulation of paraquat in the presence of maneb with a peak concentration at 6h and still measurable paraquat concentrations at 12h after injection (Barlow, et al., 2003). These previous studies with paraquat/maneb have used multiple injections and several weeks of exposure, leading to substantial mortality (Kachroo, et al., 2010).
Here we found that a single injection of the standard doses of paraquat and maneb (10mg/kg and 30mg/kg, respectively) is sufficient to cause a 37% loss in TH-positive neurons in 5 weeks old 129SvJ WT mice 7 days post-injection. In contrast, the same toxin regimen administered to 11 months old mice did not result in significant further loss of dopaminergic neurons compared to vehicle treated mice, which have already lost a substantial number of neurons due to aging. This suggests that the neurons sensitive to the toxin exposure in young mice were lost during aging in the absence of environmental insult. In accordance with our results, epidemiological studies in humans showed that exposure to a combination of maneb and paraquat increases PD risk particularly in younger subjects and/or when exposure occurs at younger ages (Costello, et al., 2009). Thus, both the age (5 weeks) and genetic background (129 SvJ) of the mice could account for the observed loss of neurons after a single injection of paraquat/maneb in our study, emphasizing the importance of both genetic factors and age at toxin exposure for the vulnerability of the nigrostriatal dopaminergic system.
4.3 Low DAT partially but incompletely protected dopamine neurons from paraquat/maneb toxicity
We next aimed to investigate if the remaining 70% of TH-positive SNc neurons and the preserved striatal TH-positive terminals were either protected or more vulnerable to a single administration of paraquat/maneb. The overall loss of SNc TH-positive neurons after toxin exposure was lower in DAT-KD than WT mice and, in contrast to WT, total neuronal loss was not significant in the DAT-KD, suggesting a loss of TH expression rather than cell death. The genotypic difference was particularly clear in the dorsal tier of the SNc, which was completely spared from toxicity in DAT-KD mice. Therefore, in agreement with previous studies in another line of mice (Rappold, et al., 2011), low DAT increased resistance of subgroups of dopamine neurons to paraquat/maneb toxicity, an effect attributed to a decreased uptake of reduced paraquat. Another potential scenario could be that lower DAT levels during ontogeny induced adaptive responses in at least a subgroup of neurons making them less vulnerable to toxicity. However, such compensation would likely include increasing demands on the remaining dopamine neurons to uphold function. Given that dopamine itself is thought to contribute to neurodegeneration (Chesselet, 2003), it seems unlikely that this would provide neuroprotection. Indeed, our detailed regional analysis showed that this partial protection did not extend to the entire SNc. Toxin exposure decreased TH-positive and total neuron number in the medial SNc, a particularly vulnerable region in PD, even in DAT-KD mice. Therefore, the apparent protection of low DAT levels against toxin exposure is regionally limited.
Toxin-induced changes in striatal TH and DAT in young DAT-KD mice also differed from WT mice. WT mice, but not DAT-KD mice, showed a strong increase in DAT and TH protein expression 7 days after a single toxin exposure. Young-adult DAT-KD mice showed a slight reduction in TH in the striatum after toxin exposure. It is unclear if the WT response is a compensatory effect or a reduction in protein degradation due to an inhibition of the proteasome by the toxins (Wills, et al., 2012, Yang, et al., 2007, Zhou, et al., 2004). Low DAT levels therefore either directly decrease compensatory mechanisms triggered by toxin exposure and/or protect from striatal toxicity. Increased striatal TH and DAT in toxin exposed WT mice did not lead to increased dopamine levels; however, this does not preclude a toxin-mediated increase in extracellular dopamine (dopamine overflow) as previously observed (Shimizu, et al., 2003, Zhang, et al., 2003). Contrary to our expectation, toxins did not increase the toxic metabolite DOPAL in either genotype, even though the ratio DOPAL/DA was higher in DAT-KD than in WT mice.
Paraquat, with or without co-administration of maneb, can induce alpha-synuclein accumulation over several weeks in mice (Wills, et al., 2012). Here, we show that a single injection of paraquat/maneb was not sufficient to induce alpha-synuclein pathology 7 days after injection. Importantly, only DAT-KD mice developed an increased number of iron deposits in the SN in response to the single paraquat/maneb injection. Iron accumulates in the midbrain of PD patients and with age (Gerlach, et al., 1994). Data from animal models and epidemiological studies in humans suggest the involvement of iron in the pathophysiology of PD (Rhodes and Ritz, 2008), and protective haplotypes of the genes for transferrin and the transferrin receptor, which are major regulators of iron homeostasis, modulate PD risk (Rhodes, et al., 2014). Iron accumulation is likely not simply a result of cell death (He, et al., 2003) since it was not observed in toxin-exposed WT or non-toxin exposed DAT-KD mice, which all show neuronal loss. Rather, the accumulation of iron in DAT-KD mice exposed to toxins appears to result from a gene-environment interaction that could cause further damage to the nigrostriatal system over time, similar to the long term effects of traumatic brain injury in rats (Hutson, et al., 2011). Interestingly, paraquat injection exacerbated neurodegeneration caused by traumatic brain injury only if applied in a specific time frame close to the impact (Hutson, et al., 2011). It will be important to further explore the long term effects of toxin exposure at young age in DAT-KD and WT mice, including multiple exposure protocols.
5. Conclusions
Our data indicate that even though low DAT function may be partially protective against some environmental toxins, it may also lead to a lower number of dopamine neurons thus predisposing an individual to develop PD. Furthermore, the observation that low DAT function prevented toxin-induced increases in TH and DAT, and promoted toxin-induced iron accumulation, points to additional mechanisms that may influence the risk of PD. Our data also suggest that the impact of toxin exposure could be greater early in life (Calne and Langston, 1983, Costello, et al., 2009). Only the combined impact of genetics, environmental toxins and aging reduced all subsets of nigrostriatal dopaminergic neurons, which provides a rationale for the observation that specific combinations of factors are required for an increased risk to develop Parkinson’s disease.
Supplementary Material
Dopamine transporter knock-down mice (DAT-KD) have less dopamine neurons from birth
DAT-KD and aging reduced different subsets of nigral dopamine neurons
Paraquat/Maneb decreased dopamine neurons at 5 weeks but not 11 months of age
In DAT-KD mice only subsets, not all dopamine neurons were resistant to the toxins
Effects on different dopamine neurons may increase the risk of Parkinson’s disease
Acknowledgments
Supported by NIEHS Grant P01-ES016732 and the American Parkinson Disease Association Center for Advanced Research at UCLA. We thank Dr. Beate Ritz for her valuable feedback on the manuscript.
Abbreviations
- PD
Parkinson’s disease
- DAT
dopamine transporter
- DAT-KD
dopamine transporter knock-down
- SNc
Substantia nigra pars compacta
- SNr
Substantia nigra pars reticulata
- SN
Substantia nigra
- TH
Tyrosine-hydroxylase
- WT
wild-type
Footnotes
Appendices: Supplementary material (Fig. S1–4, Table S1–2, supplementary methods).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbeau A, Roy M, Bernier G, Campanella G, Paris S. Ecogenetics of Parkinson’s disease: prevalence and environmental aspects in rural areas. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1987;14(1):36–41. doi: 10.1017/s0317167100026147. [DOI] [PubMed] [Google Scholar]
- Barlow BK, Thiruchelvam MJ, Bennice L, Cory-Slechta DA, Ballatori N, Richfield EK. Increased synaptosomal dopamine content and brain concentration of paraquat produced by selective dithiocarbamates. J Neurochem. 2003;85(4):1075–86. doi: 10.1046/j.1471-4159.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19(1):127–38. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Frontiers in neuroanatomy. 2014;8:152. doi: 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res. 2003;989(2):205–13. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW. Aetiology of Parkinson’s disease. Lancet. 1983;2(8365–66):1457–9. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. Dopamine and Parkinson’s disease: is the killer in the house? Mol Psychiatry. 2003;8(4):369–70. doi: 10.1038/sj.mp.4001289. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011;10(12):1108–18. doi: 10.1016/S1474-4422(11)70227-7. S1474-4422(11)70227-7 [pii] [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics. 2012;9(2):297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Muller U, Moller HJ, Ettinger U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse. 2011;65(10):998–1005. doi: 10.1002/syn.20927. [DOI] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. American journal of epidemiology. 2009;169(8):919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord. 2012;27(5):597–607. doi: 10.1002/mds.24921. [DOI] [PubMed] [Google Scholar]
- Drgon T, Lin Z, Wang GJ, Fowler J, Pablo J, Mash DC, Volkow N, Uhl GR. Common human 5′ dopamine transporter (SLC6A3) haplotypes yield varying expression levels in vivo. Cellular and molecular neurobiology. 2006;26(4–6):875–89. doi: 10.1007/s10571-006-9014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol Psychiatry. 2014;19(8):880–9. doi: 10.1038/mp.2013.126. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61(12):991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(2):636–41. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24(42):9434–40. doi: 10.1523/JNEUROSCI.3080-04.2004. 24/42/9434 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Paxinos G, Watson C, Halliday GM. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J Chem Neuroanat. 2016 doi: 10.1016/j.jchemneu.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63(3):793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Riederer P. Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm. 1996;103(8–9):987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci U S A. 1996;93(5):1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic Biol Med. 2003;35(5):540–7. doi: 10.1016/s0891-5849(03)00385-x. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Quinlan JJ, Firestone LL. Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav. 1999;63(1):21–6. doi: 10.1016/s0091-3057(98)00232-9. [DOI] [PubMed] [Google Scholar]
- Hoover-Plow JL, Gong Y, Shchurin A, Busuttil SJ, Schneeman TA, Hart E. Strain and model dependent differences in inflammatory cell recruitment in mice. Inflamm Res. 2008;57(10):457–63. doi: 10.1007/s00011-008-7062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma. 2011;28(9):1783–801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagne C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11(10):3499–511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Irizarry MC, Schwarzschild MA. Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol. 2010;223(2):657–61. doi: 10.1016/j.expneurol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelada SN, Costa-Mallen P, Checkoway H, Carlson CS, Weller TS, Swanson PD, Franklin GM, Longstreth WT, Jr, Afsharinejad Z, Costa LG. Dopamine transporter (SLC6A3) 5′ region haplotypes significantly affect transcriptional activity in vitro but are not associated with Parkinson’s disease. Pharmacogenetics and genomics. 2005;15(9):659–68. doi: 10.1097/01.fpc.0000170917.04275.d6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8(16):939–42. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136(Pt 8):2419–31. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S, Jr, Sanger T, Gissen P, Assmann BE, Reith ME, Maher ER. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011;10(1):54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, Magen I, Cepeda C, Ackerson LC, Walwyn W, Masliah E, Chesselet MF, Levine MS, Maidment NT. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J Neurosci Res. 2011;89(7):1091–102. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW. Predicting Parkinson’s disease. Neurology. 1990;40(10 Suppl 3):suppl 70–4. discussion 5–6. [PubMed] [Google Scholar]
- Masoud ST, Vecchio LM, Bergeron Y, Hossain MM, Nguyen LT, Bermejo MK, Kile B, Sotnikova TD, Siesser WB, Gainetdinov RR, Wightman RM, Caron MG, Richardson JR, Miller GW, Ramsey AJ, Cyr M, Salahpour A. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol Dis. 2015;74:66–75. doi: 10.1016/j.nbd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10(2):119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24(4):574–6. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- Morris CM, Candy JM, Oakley AE, Bloxham CA, Edwardson JA. Histochemical distribution of non-haem iron in the human brain. Acta Anat (Basel) 1992;144(3):235–57. doi: 10.1159/000147312. [DOI] [PubMed] [Google Scholar]
- Patel S, Singh V, Kumar A, Gupta YK, Singh MP. Status of antioxidant defense system and expression of toxicant responsive genes in striatum of maneb- and paraquat-induced Parkinson’s disease phenotype in mouse: mechanism of neurodegeneration. Brain Res. 2006;1081(1):9–18. doi: 10.1016/j.brainres.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23(28):9395–402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. 23/28/9395 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, Sen N, Javitch JA, Tieu K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci U S A. 2011;108(51):20766–71. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SL, Buchanan DD, Ahmed I, Taylor KD, Loriot MA, Sinsheimer JS, Bronstein JM, Elbaz A, Mellick GD, Rotter JI, Ritz B. Pooled analysis of iron-related genes in Parkinson’s disease: association with transferrin. Neurobiol Dis. 2014;62:172–8. doi: 10.1016/j.nbd.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis. 2008;32(2):183–95. doi: 10.1016/j.nbd.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter F, Fleming SM, Watson M, Lemesre V, Pellegrino L, Ranes B, Zhu C, Mortazavi F, Mulligan CK, Sioshansi PC, Hean S, De La Rosa K, Khanna R, Flanagan J, Lockhart DJ, Wustman BA, Clark SW, Chesselet MF. A GCase Chaperone Improves Motor Function in a Mouse Model of Synucleinopathy. Neurotherapeutics. 2014;11(4):840–56. doi: 10.1007/s13311-014-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117(6):964–9. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Paul KC, Bronstein JM. Of Pesticides and Men: a California Story of Genes and Environment in Parkinson’s Disease. Curr Environ Health Rep. 2016;3(1):40–52. doi: 10.1007/s40572-016-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo AI, Cavada C, de Sagarra MR, Cuadrado A. Chronic inhalation of rotenone or paraquat does not induce Parkinson’s disease symptoms in mice or rats. Exp Neurol. 2007;208(1):120–6. doi: 10.1016/j.expneurol.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Schallert T. Aging-dependent emergence of sensorimotor dysfunction in rats recovered from dopamine depletion sustained early in life. Ann N Y Acad Sci. 1988;515:108–20. doi: 10.1111/j.1749-6632.1988.tb32972.x. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30(3):171–82. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179(1):9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Matsubara K, Ohtaki K, Fujimaru S, Saito O, Shiono H. Paraquat induces long-lasting dopamine overflow through the excitotoxic pathway in the striatum of freely moving rats. Brain Res. 2003;976(2):243–52. doi: 10.1016/s0006-8993(03)02750-1. [DOI] [PubMed] [Google Scholar]
- Syn WK, Yang L, Chiang DJ, Qian Y, Jung Y, Karaca G, Choi SS, Witek RP, Omenetti A, Pereira TA, Diehl AM. Genetic differences in oxidative stress and inflammatory responses to diet-induced obesity do not alter liver fibrosis in mice. Liver Int. 2009;29(8):1262–72. doi: 10.1111/j.1478-3231.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci. 2003;18(3):589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2009;50(1):45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237(2):318–34. doi: 10.1016/j.expneurol.2012.06.025. S0014-4886(12)00267-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J, Credle J, Oaks AW, Duka V, Lee JH, Jones J, Sidhu A. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One. 2012;7(1):e30745. doi: 10.1371/journal.pone.0030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16(23):2900–10. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- Yin L, Lu L, Prasad K, Richfield EK, Unger EL, Xu J, Jones BC. Genetic-based, differential susceptibility to paraquat neurotoxicity in mice. Neurotoxicol Teratol. 2011;33(3):415–21. doi: 10.1016/j.ntt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V, Montine TJ. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem. 2003;84(2):336–46. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shie FS, Piccardo P, Montine TJ, Zhang J. Proteasomal inhibition induced by manganese ethylene-bis-dithiocarbamate: relevance to Parkinson’s disease. Neuroscience. 2004;128(2):281–91. doi: 10.1016/j.neuroscience.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98(4):1982–7. doi: 10.1073/pnas.98.4.198298/4/1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.