Abstract
Objective
To determine factors that influence sperm recovery after testosterone-associated infertility.
Design
Clinical retrospective study.
Setting
Academic male-infertility urology clinic.
Patient(s)
Sixty-six men who presented with infertility after testosterone use.
Intervention(s)
Testosterone (T) cessation and combination high-dose human chorionic gonadotropin (hCG) and selective estrogen modulator (SERM) therapy.
Main Outcome Measure(s)
Whether patients successfully achieved or failed to achieve a total motile count (TMC) of greater than 5 million sperm within 12 months of T cessation and initiation of therapy.
Result(s)
A TMC of greater than 5 million sperm was achieved by 46 men (70%). Both increased age and duration of testosterone use directly correlated with time to sperm recovery at both 6 and 12 months of hCG/SERM therapy. Age more consistently limited sperm recovery, while duration of testosterone use had less influence at 12 months than at 6 months. Only 64.8% of azoospermic men achieved a TMC greater than 5 million sperm at 12 months, compared with 91.7% of cryptozoospermic men, yet this did not predict a failure of sperm recovery.
Conclusion(s)
Increasing age and duration of testosterone use significantly reduce the likelihood of recovery of sperm in the ejaculate, based on a criterion of a TMC of 5 million sperm, at 6 and 12 months. Physicians should be cautious in pursuing long-term testosterone therapy, particularly in men who still desire fertility. Using these findings, physicians can counsel men regarding the likelihood of recovery of sperm at 6 and 12 months.
Keywords: Infertility, Testosterone, Sperm, Azoospermia, Human chorionic gonadotropin, Spermatogenesis-Blocking Agents
CAPSULE
In this retrospective analysis of 66 men with testosterone-associated infertility, we found that duration of testosterone therapy and age at presentation are directly correlated with time to sperm recovery.
INTRODUCTION
The use of exogenous testosterone (T) in the treatment of hypogonadism has known risks with regards to male infertility. Serum testosterone levels in men begin to decrease in an age-dependent manner starting in the late 30’s (1–3), and the number of testosterone prescriptions has drastically increased in recent years, from 1.2 million patients in 2010 to 2.2 million patients in 2013 (4). Of men receiving testosterone therapy (TTh), 12.4% were younger than 39 years old, indicating that a large number of men seek TTh during the reproductive years (5). One study found that 7% of male patients seeking care for infertility were on TTh at the time of their visit, and concomitant TTh was the fourth most common etiology of male infertility in the two large infertility practices in the study (6). Coupled with the increase in T prescriptions, physicians are often failing to inform patients of the risk of testosterone-induced infertility, in part due to a lack of knowledge of the fertility-related adverse effects of TTh. In a 2010 survey of urologist members of the American Urological Association, 25% incorrectly believed that TTh would improve a male’s fertility (7); such beliefs likely contribute to the growing number of men with T-induced infertility (8).
Exogenous T inhibits spermatogenesis by suppressing secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland, limiting the signals required for endogenous testosterone production and spermatogenesis (9). Thus, the use of testosterone by younger men increasingly intersects with their reproductive potential for many men, and approaches to predicting and mitigating the negative effects of testosterone on fertility are needed.
Several studies have demonstrated that cessation of TTh in men seeking fertility treatment can lead to a return to baseline sperm concentrations (6, 10–12). However, the time for return of sperm to the ejaculate in quantities sufficient for fertility remains unclear. In a pooled analysis of 30 studies using testosterone as a short-term hormonal contraceptive in eugonadal men, Liu et al. demonstrated that the average probability of sperm recovery to 20 million sperm / mL was 67% within 6 months, 90% within 12 months, 96% within 16 months, and 100% within 24 months, but suggested that men who started with a low-normal sperm count and who were older required more time to recover (10). Another study examining more than 14,000 semen samples from World Health Organization (WHO) studies in which androgens were evaluated as a potential male contraceptive found that sperm production after therapy was only approximately 85% of pre-treatment concentrations (11). The literature also suggests that men who have been on high-dose TTh for longer periods will require longer to recover normal sperm production (12, 13).
Human chorionic gonadotropin (hCG) and selective estrogen receptor modulators (SERMs) are effective at restoring spermatogenesis alone and in combination (12, 14–17). The efficacy of hCG is attributed to its structural similarity to LH. SERMs potentiate spermatogenesis by inhibiting negative feedback by estrogen, thereby raising GnRH and gonadotropin levels and increasing downstream testosterone production. Numerous protocols combining hCG and SERMs are available for the restoration endogenous testosterone in testosterone-suppressed men. Ishikawa et al. used 5000 IU of hCG three times a week for 3–6 months, in combination with recombinant FSH supplementation, with recovery of spermatogenesis observed in 44–100% of patients (18). hCG doses described in the literature range from 3,000 – 10,000 IU, administered 2–3 times per week (8, 13, 15, 18, 19). In a retrospective chart review of azoospermic or severely oligospermic men, Wenkler et al. observed return of spermatogenesis in a mean of 4.6 months with a mean density of 22 million sperm / mL in 95.9% of subjects receiving hCG 3000 IU every other day, along with either FSH, clomiphene citrate, tamoxifen, or anastrozole (12). In another retrospective review by Coward et al., men previously on TTh and seeking vasectomy reversal were treated with high-dose hCG (3000 IU every other day) and clomiphene citrate, with 83% having normalization of LH, FSH and testosterone levels (15).
Previous studies analyzed only patients who had been on testosterone for a short duration, for contraception purposes, or who were eugonadal at the time if TTh initiation; our study analyzes men with a prolonged duration of TTh use and focuses on men who were cryptozoospermic or azoospermic at cessation of TTh. The primary objective of the present study is to determine the factors that influence sperm recovery after presumed testosterone-associated infertility.
MATERIAL AND METHODS
Patient Selection
After institutional review board approval, we retrospectively reviewed the records of 66 men with testosterone-associated infertility who were evaluated at a single academic infertility clinic between 2004 and 2015. Men were included if they presented for infertility, were 18 years or older, had been on testosterone for a recorded duration, and were found to be azoospermic or cryptozoospermic (<1 million sperm/mL) at the time of TTh cessation. In addition they must have ceased TTh and began hCG therapy within a single visit, and had a least one follow-up semen analysis. Men were excluded if they had a history of vasectomy, obstructive azoospermia, or a known primary cause of testicular failure such as chromosomal abnormalities, Y-chromosome microdeletions, history of testicular trauma or infection, or history of cryptorchidism. No men included in the analysis were concurrently on recombinant FSH. Age at time of T cessation, total duration of TTh use, route of TTh, duration and dosage of hCG therapy, use and type of selective estrogen receptor modulators (SERM), serum levels of testosterone, FSH, and LH at time of presentation, and sperm concentration at presentation were recorded and compared.
Treatment
At initial presentation, men underwent a physical examination by a urologist with fellowship training in male reproductive medicine, as well as evaluation of serum testosterone, LH, FSH, prolactin, and estradiol levels, and semen analysis. Men were instructed to stop testosterone use and begin a regimen of 3,000 IU of hCG administered subcutaneously three times per week. All men in this study were also prescribed either clomiphene citrate or tamoxifen citrate. Patients were seen in follow-up approximately every 3 months, with semen analyses and hormonal evaluation performed at each visit
Statistical Analysis
The main outcome measure was whether patients achieved a total motile sperm count (TMC) of greater than 5 million sperm during evaluation within 6 months or within 12 months of stopping TTh and beginning hCG therapy. This total motile count reflects the minimum number of sperm used for intrauterine insemination (IUI) at our institution. Two binary variables (TMC >5 million reached within 12 months or within 6 months) were created, which were the dependent variables of interest.
We compared the patient characteristics between those who reached TMC > 5 million within 12 months using the Student t test for normally distributed continuous variables, the Mann-Whitney U test for non-parametric continuous variables, and the chi-square test or Fisher exact test for categorical variables. Only duration of TTh was found to be a nonparametric variable.
We used a multivariate linear probability model to estimate the effects of various factors on successfully reaching a TMC of >5 million sperm. Six independent variables were used in the final model – three continuous variables (duration of TTh, age at TTh cessation, and testosterone level at presentation), and three categorical variables (whether TTh was delivered by intramuscular injection, transdermal application, or pellet insertion; use of clomiphene or tamoxifen citrate; and presence of cryptozoospermia or azoospermia). We then use the results of this regression analysis to calculate the predicted probability of achieving a TMC > 5 million within 12 months and 6 months at different ages and durations of TTh. For example, to calculate the predicted probability of success for a 30 year-old man with a TTh duration of 1 year, we assumed every patient in our sample is 30 years old and was on TTh for 1 year. We then use the coefficients estimated from the regression to calculate each patient’s predicted probability of success. These probabilities were then averaged across the entire sample to calculate the final predicted probability for those parameters. We repeat this process for each combination of age and duration of TTh. All statistical analysis was performed using STATA 14.1, with p<0.05 considered significant.
RESULTS
Sixty-six men met criteria and were included in this analysis at 12 months. Table 1 shows the baseline characteristics of all men and is further stratified by those who successfully achieved a TMC >5 million sperm within 12 months and those who did not. The mean±SD age of the cohort was 40.2±8.7 years, and the median duration of TTh was 2 years (range 0.17 – 25) years. Thirty-five men used intramuscular injections of testosterone, twenty-two topical testosterone, and nine used pellets. Forty-six men (69.7%) successfully achieved a TMC of 5 million or greater within 12 months. For men with successful recovery of spermatogenesis, the mean±SD age was 38.3±7.0 years and duration of TTh use was a median of 1.67 (range 0.17 – 15) years. For men without successful recovery of spermatogenesis, the mean±SD age was 44.0±10.7 years and median duration of TTh was 4.0 (range 0.25 – 25) years. The average TMC for men with a TMC > 5 million sperm within 12 months was 40.0±44.6 million sperm, while the average for those men with a TMC < 5 million sperm was 1.8±1.6 million sperm. The average sperm density for men who achieved a TMC > 5 million sperm within 12 months was 33.9±36.8 million sperm/mL while the average sperm density for those with a TMC < 5 million sperm was 4.7±7.1 million sperm/mL. Table 2 denotes semen parameters, FSH, LH, and testosterone levels for each formulation of testosterone at baseline, at 6 months, and at 12 months. Semen density and TMC increases at each evaluation, from baseline to 6 months to 12 months, demonstrating the waning effect of testosterone over time.
Table 1.
Baseline characteristics and comparison of men with successful and unsuccessful sperm recovery.
| Biometric Features | All Men (n = 66) |
Success (n = 46) |
Failure (n = 20) |
P-value |
|---|---|---|---|---|
| Age of men (y) | 40.2 (±8.7) | 38.3 (±7.0) | 44.8 (±10.5) | 0.018 |
| Time on TTh (y) | 2.0 (0.17–25) | 1.67 (0.17–15) | 4.0 (0.25–25) | 0.001 |
| Initial Hormone Analysis | ||||
| Initial T (ng/dl) | 632 (±369) | 656 (±387) | 577 (±327) | 0.654 |
| Initial FSH (mIU/mL) * | 1.3 (±3.0) | 1.2 (±3.1) | 1.6 (±2.5) | 0.372 |
| Initial LH (mIU/mL) * | 0.9 (±2.5) | 0.9 (±2.8) | 0.9 (±1.6) | 0.725 |
| Route of Testosterone | ||||
| Injection | 35 (53.0) | 23 (50.0) | 12 (60.0) | 0.654 |
| Topical | 22 (33.3) | 17 (37.0) | 5 (25.0) | |
| Pellets | 9 (13.6) | 6 (13.0) | 3 (15.0) | |
| SERM | ||||
| Clomiphene Citrate | 45 (68.2) | 32 (69.6) | 13 (65.0) | 0.859 |
| Tamoxifen Citrate | 21 (31.8) | 14 (30.4) | 7 (35.0) | |
| Fertility Diagnosis | ||||
| Azoospermia | 54 (81.8) | 34 (76.1) | 19 (95.0) | 0.055 |
| Cryptozoospermia | 12 (18.2) | 11 (23.9) | 1 (5.0) |
Note: Data are presented as mean (±SD), median (range), or n (%)
Missing information in some subjects
Table 2.
Median hormone levels and semen parameters for patients receiving testosterone via intramuscular injection, transdermal, and pellets at baseline, 6 months, and 12 months.
| Initial Evaluation | Evaluation within 6 months | Evaluation within 12 months | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testosterone Type |
Subgroups | FSH | LH | Testosterone | Semen Volume |
Semen Density |
Semen Mobility |
Total Motile Count |
FSH | LH | Testosterone | Semen Volume |
Semen Density |
Mobility | Total Motile Count |
FSH | LH | Testosterone | Semen Volume |
Semen Density |
Mobility | Total Motile Count |
|
Injection Testosterone (n = 35) |
All Patients | 0.4 (0.1–2.0) |
0.2 (0.02–0.5) |
630 (395–1030) |
2.5 (1.5–3.5) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
1.35 (0.8–4.0) |
2.4 (0.3–4.8) |
536 (245–585) |
2.5 (1.5–3.5) |
10.0 (5.3–20.0) |
50 (33–65) |
11.4 (3.9–25.6) |
3.0 (1.0–5.0) |
2.0 (0.3–4.0) |
490 (275–662) |
2.5 (1.5–3.0) |
11.6 (5.1–24.9) |
45 (30–64) |
15.8 (3.9–36.5) |
|
<5 TMC (n = 13) |
0.70 (0.1–2.0) |
0.3 (0.08–0.6) |
509 (431–638) |
2.8 (1.5–3.3) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
2.0 (0.2–3.5) |
0.6 (0.07–3.3) |
585 (561–612) |
1.8 (1.4–2.6) |
4.6 (1.8–6.4) |
28 (25–40) |
2.2 (1.3– 3.6) |
2.0 (0.2–3.5) |
0.5 (0.06–3.3) |
578 (612–473) |
1.5 (1.0–2.4) |
3.6 (1.3–5.1) |
30 (25–40) |
2.1 (0.9–3.3) |
|
|
>5 TMC (n = 22) |
0.4 (0.1–0.7) |
0.1 (0.01–0.4) |
772 (316 –1059) |
2.25 (1.5–3.5) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
1.0 (0.9–5.0) |
2.8 (1.6–5.3) |
386 (233–561) |
2.5 (2.0–3.6) |
16.0 (21.7–9.1) |
60 (48–65) |
21.2 (10.9– 36.4) |
3.0 (1.0–6.2) |
2.5 (1.2–3.8) |
394 (257– 639) |
3.0 (2.0–3.5) |
20.4 (9.2–32.1) |
58 (44–65) |
29.8 (46.9–14.9) |
|
|
Topical Testosterone (n = 22) |
All Patients | 0.3 (0.07–0.6) |
0.1 (0.04–0.4) |
532 (371–774) |
2.4 (1.5–3.0) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
1.4 (0.7–2.6) |
0.9 (0.3–1.9) |
418 (291–564) |
2.0 (1.1–2.4) |
17.2 (7.2–41.2) |
53 (43–60) |
9.7 (4.4–21.4) |
2.8 (0.9–4.8) |
1.7 (0.3–3.0) |
386 (265–551) |
2.0 (2.0–2.7) |
18.0 (6.7–47.6). |
50 (42–60) |
18.0 (5.6–61.8) |
|
<5 TMC (n = 6) |
0.4 (0.0–0.7) |
0.07 (0.03–0.3) |
657 (512–816) |
3.25 (1.9–3.9) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
1.3 (1.0–3.7) |
1.3 (0.7–1.7) |
499 (370–535) |
3.3 (2.0–4.0) |
1.6 (0.5–4.6) |
44 (15–55) |
1.95 (0.3–3.7) |
5.5 (4.1–9.8) |
2.5 (1.8–5.0) |
370 (221–499) |
2.7 (2.0–3.8) |
1.3 (0.6–2.4) |
40 (16–52) |
1.2 (0.1–3.2) |
|
|
>5 TMC (n = 16) |
0.4 (0.09–0.7) |
0.1 (0.1–0.6) |
527 (312–721) |
2.0 (1.5–3.0) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
1.5 (0.7–2.5) |
0.8 (0.3–2.5) |
402 (300–567) |
2.0 (1.0–2.0) |
20.4 (16.3–47.6) |
55 (45–60) |
18.0 (8.8–27.7) |
2.0 (0.7–3.0) |
0.7 (0.3–3.0) |
386 (282–588) |
2.0 (2.0–2.1) |
20.5 (16.3–57.2) |
53 (46–60) |
22.9 (9.2–71.7) |
|
|
Pellet Testosterone (n=9) |
All Patients | 0.7 (0.3–3.2) |
0.3 (0.2–1.4) |
559 (208–703) |
1.8 (1.1–2.0) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
2.0 (0.2–5.6) |
0.2 (0.1–2.6) |
566 (278–777) |
1.5 (0.9–2.0) |
14.5 (2.2–23.5) |
45 (31–52) |
4.48 (0.9–16.1) |
2.0 (0.2–5.6) |
0.24 (0.2–3.7) |
542 (367–707) |
2.0 (1.0–2.5) |
24.0 (32.7–12.8) |
40 (34–55) |
9.6 (1.3–45.0) |
|
<5 TMC (n = 3) |
0.8 (0.5–5.4) |
0.4 (0.3–2.2) |
460 (334–587) |
1.0 (0.6–1.5) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
2.0 (0.7–3.6) |
0.7 (0.2–3.8) |
422 (216–716) |
2.0 (1.0–2.0) |
1.5 (0.8–4.5) |
30 (25–50) |
0.75 (0.4–1.2) |
2.0 (1.1–10) |
0.24 (0.2–6.1) |
566 (422–866) |
1.0 (0.7–2.0) |
4.5 (2.6–8.3) |
30 (15–40) |
1.2 (0.6–1.3) |
|
|
>5 TMC (n = 6) |
0.7 (0.5–4.0) |
0.4 (0.2–1.6) |
459 (207–673) |
1.5 (1.0–2.0) |
0.0 (0-0) |
0.0 (0-0) |
0.0 (0-0) |
2.9 (0.2–6.9) |
0.2 (0.1–2.1) |
707 (492–777) |
1.0 (0.8–2.0) |
24.0 (21.9–27.2) |
53.0 (40–54) |
18.3 (9.6–23.5) |
2.6 (0.4–5.3) |
1.8 (0.2–3.5) |
500 (390–666) |
2.0 (1.3–2.4) |
30.0 (24.8–52.1) |
48 (36–67) |
39.4 (15.7–78.4) |
|
Note: Data are presented as median (interquartile range)
When comparing the differences between men who did and did not have successful recovery of spermatogenesis, age (p=0.018) and duration of TTh (p=0.006) were identified as significant predisposing factors. Route of testosterone administration, initial serum testosterone level, type of SERM used and initial sperm concentration were not found to be significant predictors of sperm recovery. Multivariate linear regressions were performed in order to determine the magnitude of effect on the likelihood of success at both 6 and 12 months; correlation coefficients are reported in Table 3. Supplemental Table 1 compares the result of a multivariate linear regression including limited predictors with a multivariate linear regression with an expanded number of predictors, demonstrating that the expanded model accounts for a larger proportion of the variance in sperm recovery. Results presented here use the results of the expanded regression.
Table 3.
Multivariate linear regression.
| 6 Month Analysis | 12 Month Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient (SE) |
95% CI | P-value | Coefficient (SE) |
95% CI | P-value |
| Time on TTh (years) | −0.0555 | (−0.0771, −0.0339) | <0.001 | −0.0306 | (−0.0555, −0.0057) | 0.017 |
| Age (years) | −0.0163 | (−0.0298, −0.0028) | 0.019 | −0.0171 | (−0.0308, −0.0034) | 0.015 |
| Initial T | 0.0002 | (−0.0001, 0.0005) | 0.176 | 0.00002 | (−0.0002, 0.0002) | 0.866 |
| Clomiphene citrate | −0.0641 | (−0.3045, 0.1763) | 0.595 | 0.0094 | (−0.2179, 0.2366) | 0.935 |
| Cryptospermic | 0.184 | (0.0700, 0.4390) | 0.152 | 0.2180 | (−0.006, 0.4410) | 0.056 |
| Type of Testosterone (Reference: Injection) | ||||||
| Transdermal | 0.0451 | (−0.2154, 0.3055) | 0.730 | 0.124 | (−0.1380, −0.3870) | 0.347 |
| Pellet | 0.177 | (−0.076, 0.430) | 0.166 | 0.163 | (−0.1100, 0.4360) | 0.237 |
Duration of TTh, age at TTh cessation, and initial sperm concentration were significant predictors for successfully reaching a TMC of 5 million within 12 months. Duration of TTh has a correlation coefficient of −0.0306 (ρ = 0.017; 95% CI −0.0555, −0.0057), suggesting that the probability of reaching a TMC of 5 million sperm decreases by 3.06% for each additional year of TTh. Age has a correlation coefficient of −0.0171 (ρ = 0.015; 95% CI −0.0308, −0.0034), which suggests that the probability of reaching a TMC of 5 million decreases by 1.71% for every year of age.
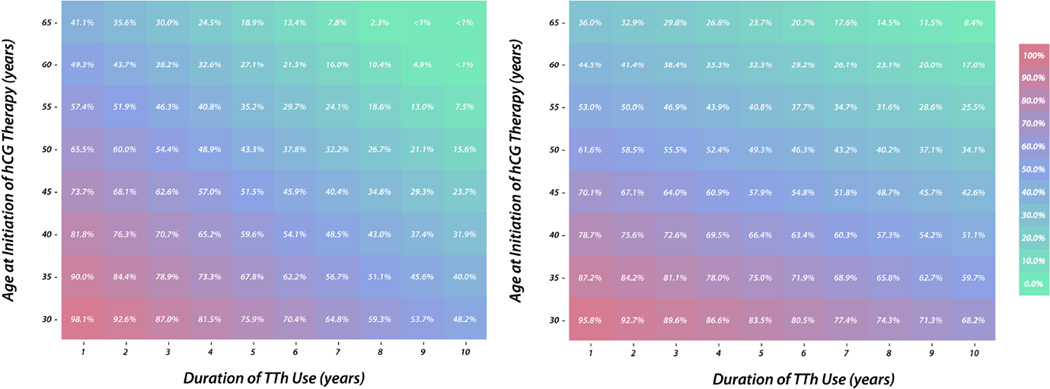
The regression analysis was performed for sperm recovery at 6 months using 59 observations. Similar to the analysis of 12 month data, duration of TTh (ρ = −0.0555, p < 0.001; 95% CI −0.0771, −0.0339) and age (ρ = −0.0163, p = 0.019; 95% CI −0.0298, −0.0028) were identified as significant negative predictors of successful sperm recovery at 6 months. Using the probabilities generated from this linear probability model, the likelihood of sperm recovery at 12 and 6 months based on a man’s age and duration of TTh was calculated (Figures 1a and 1b).
Figure 1.
(A) Probability of achieving a total motile count greater than 5 million sperm within 12 months of TTh cessation. (B) Probability of achieving a total motile count greater than 5 million sperm within 6 months of TTh cessation.
DISCUSSION
Currently, no guidelines are available discussing the management of men presenting with infertility that is presumed to be associated with testosterone use. Furthermore, it is unclear how soon after cessation of testosterone that adequate spermatogenesis should be anticipated. The results of this study facilitate physician counseling of men presenting with azoospermia or cryptozoospermia presumably due to TTh, and provide a means to estimate the likelihood of recovered spermatogenesis at 6 and 12 months after discontinuing TTh. The coefficients for the impact of age are similar at both 6 and 12 months (−0.016 and −0.017), indicating that age has a durable, long-lasting effect on sperm production and recovery of spermatogenesis. In contrast, the contribution of the duration of TTh decreased by approximately 50% between the 6 and 12 month analyses (−0.055 vs −0.030), suggesting that the deleterious impact of TTh on spermatogenesis diminishes with increasing time off of testosterone.
TTh suppresses the hypothalamic-pituitary-gonadal axis and inhibits spermatogenesis within 3.5 months in most men (10, 20–22). The present study examined the successful return of spermatogenesis after discontinuation of TTh and initiation of high-dose hCG and SERM therapy. We found that cryptozoospermic men had a higher likelihood of successful recovery of spermatogenesis when compared with azoospermic men at 12 months but not at 6 months. Of azoospermic men, only 64.8% achieved a TMC >5 million sperm at 12 months, compared with 91.7% of cryptozoospermic men. Thus, sperm recovery progresses at similar rates in both groups during the the first 6 months, but cryptozoospermic men have a higher likelihood of successful recovery of spermatogenesis within 12 months.
In a preliminary study, we demonstrated that hCG therapy promotes sperm recovery after presumed testosterone-associated infertility (12). In the present study, important new findings are observed. Our preliminary work defined recovery of spermatogenesis in azoospermic patients as the presence of any sperm and as any increase in sperm count for oligospermic patients. Our current study sets a more clinically relevant benchmark for sperm recovery – the desirable number of total motile sperm required for IUI. Furthermore, our preliminary work did not evaluate the effects of total duration of TTh, which are more clearly outlined. In the present study, our inclusion criteria are stricter and draw from a larger cohort of men, facilitating a more rigorous set of conclusions, and we concurrently assess factors that influence sperm recovery. Most studies that have examined time to recovery of spermatogenesis after TTh have used testosterone as a male contraceptive in eugonadal men for up to 18 months (20–24). In a pooled analysis of 30 hormone contraception studies encompassing 2,023 men, Liu et al. found age, initial testosterone level, initial LH level, total duration of testosterone, initial semen volume and density, and type of testosterone to be significant predictors for recovery of spermatogenesis (10). However, this study examined men that were in tightly controlled clinical trials, were eugonadal prior to TTh, and were only on testosterone for less than one year on average, limiting the generalizability of the results to hypogonadal men on longer-duration TTh (8). Nevertheless, the probabilities of recovery of spermatogenesis in the present study are comparable to those observed by Liu et al. (10). Liu et al. calculate a 90% chance of recovery to 20 million sperm / ml 12 months after cessation of testosterone with a mean cohort age of 31.8 years and duration of testosterone treatment of 9.45 months. This probability is similar to our calculated probability of a 90.0% chance of achieving a TMC >5 million sperm at 12 months for a 30-year old man with a 1 year duration of TTh yet Liu et al. find a lower average sperm density than our 33.9 million sperm/mL average. When comparing probabilities of sperm recovery at 6 months duration of TTh, those calculated by Liu et al. are lower than those calculated by our model. Our increased probabilities may be attributable to accelerated sperm recovery from hCG and SERM therapy.
While the only significant predictors in our univariate analysis were time on testosterone and age, we included initial T levels, route of T administration, and initial oligospermia as Liu et al. demonstrated in a much larger population that these factors are significant; additionally, the inclusion of these variables strengthened the fit of our model. Yet our model differs from Liu et al. in that our model calculates probabilities for recovery of spermatogenesis for a wider range of ages and longer duration of testosterone in a population of men for whom these data will provide a meaningful basis for patient counseling.
A factor in the growing prevalence of testosterone-associated infertility is the mistaken view that testosterone can improve a man’s fertility (7). As such, is essential that physicians counsel patients that testosterone will reduce their fertility and that longer durations of testosterone or advanced age will prolong time to recovery of spermatogenesis. For men who desire future fertility, physicians should consider a baseline semen analysis prior to initiating TTh. This will ensure that men with underlying testicular dysfunction are identified prior to TTh, which could otherwise confound the interpretation of semen analyses while on post-TTh treatment.
The present study has several strengths and limitations. This is the first study to examine sperm recovery after long-term TTh. We observed that both age and duration of TTh are predictors of recovery of spermatogenesis and present a model that facilitates risk stratification across a broad range of ages and TTh durations. Nevertheless, the retrospective nature of the study limits the impact and generalizability of the data. Importantly, we do not have semen analyses or FSH levels for these men prior to starting testosterone therapy, limiting our ability to discern underlying testicular dysfunction prior to initiation of TTh. While we excluded men with known genetic or other known causes of infertility, we cannot conclude that all men included in this analysis were azoospermic or cryptospermic solely due to testosterone-induced infertility. Our model, however, is still clinically useful as many patients who present with infertility that is presumed to be associated with testosterone use do not have a semen analysis prior to initiating testosterone use. Additionally, our strict inclusion criteria limited the number of men included in this analysis. Finally, the dependent variable in our analysis is whether men successfully achieved a TMC > 5 million sperm within 6 or 12 months; however, we did not observe each subject’s TMC at exactly 6 or 12 months after initiation of HCG/SERM therapy, but rather the semen analysis occurred within or up to 6 and 12 months after therapy initiation. Therefore, it is likely that we underestimate the effects of the predictor variables on TMC recovery.
CONCLUSION
The increased use of TTh in younger men has led to a rise in testosterone-associated infertility. In our retrospective study of 66 men with testosterone-associated infertility who ceased TTh and began high-dose hCG and/or SERM therapy, we identified age and duration of TTh as significant predictors for the recovery of spermatogenesis at 6 and 12 months after TTh cessation. Using our predictive model, physicians can counsel men regarding the likelihood of recovery of spermatogenesis at 6 and 12 months after TTh cessation. Older men on long-term TTh in particular should be counseled regarding the lower probability of successful recovery of spermatogenesis.
Supplementary Material
Acknowledgments
Funding
A.W.P. is a National Institutes of Health K12 Scholar supported by a Male Reproductive Health Research Career Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Program (to Dolores J. Lamb).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. European urology. 2007;52:389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and "Age-Related Hypogonadism"--FDA Concerns. The New England journal of medicine. 2015;373:689–691. doi: 10.1056/NEJMp1506632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layton JB, Li D, Meier CR, Sharpless JL, Sturmer T, Jick SS, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. The Journal of clinical endocrinology and metabolism. 2014;99:835–842. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolettis PN, Purcell ML, Parker W, Poston T, Nangia AK. Medical testosterone: an iatrogenic cause of male infertility and a growing problem. Urology. 2015;85:1068–1072. doi: 10.1016/j.urology.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. The Journal of urology. 2012;187:973–978. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 8.McBride JA, Coward RM. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian journal of andrology. 2016;18:373–380. doi: 10.4103/1008-682X.173938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacIndoe JH, Perry PJ, Yates WR, Holman TL, Ellingrod VL, Scott SD. Testosterone suppression of the HPT axis. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 1997;45:441–447. [PubMed] [Google Scholar]
- 10.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet (London, England) 2006;367:1412–1420. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 11.Ly LP, Liu PY, Handelsman DJ. Rates of suppression and recovery of human sperm output in testosterone-based hormonal contraceptive regimens. Human reproduction (Oxford, England) 2005;20:1733–1740. doi: 10.1093/humrep/deh834. [DOI] [PubMed] [Google Scholar]
- 12.Wenker EP, Dupree JM, Langille GM, Kovac J, Ramasamy R, Lamb D, et al. The Use of HCG-Based Combination Therapy for Recovery of Spermatogenesis after Testosterone Use. The journal of sexual medicine. 2015;12:1334–1337. doi: 10.1111/jsm.12890. [DOI] [PubMed] [Google Scholar]
- 13.Menon DK. Successful treatment of anabolic steroid-induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertility and sterility. 2003;79(Suppl 3):1659–1661. doi: 10.1016/s0015-0282(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu PY, Turner L, Rushford D, McDonald J, Baker HW, Conway AJ, et al. Efficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient men. Human reproduction (Oxford, England) 1999;14:1540–1545. doi: 10.1093/humrep/14.6.1540. [DOI] [PubMed] [Google Scholar]
- 15.Coward RM, Mata DA, Smith RP, Kovac JR, Lipshultz LI. Vasectomy reversal outcomes in men previously on testosterone supplementation therapy. Urology. 2014;84:1335–1340. doi: 10.1016/j.urology.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 16.Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertility and sterility. 2014;101:1271–1279. doi: 10.1016/j.fertnstert.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. The Journal of clinical endocrinology and metabolism. 2005;90:2595–2602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa T, Ooba T, Kondo Y, Yamaguchi K, Fujisawa M. Assessment of gonadotropin therapy in male hypogonadotropic hypogonadism. Fertility and sterility. 2007;88:1697–1699. doi: 10.1016/j.fertnstert.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Gill GV. Anabolic steroid induced hypogonadism treated with human chorionic gonadotropin. Postgraduate medical journal. 1998;74:45–46. doi: 10.1136/pgmj.74.867.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay CJ, Brady BM, Zitzmann M, Osmanagaoglu K, Pollanen P, Apter D, et al. A multicenter phase IIb study of a novel combination of intramuscular androgen (testosterone decanoate) and oral progestogen (etonogestrel) for male hormonal contraception. The Journal of clinical endocrinology and metabolism. 2005;90:2042–2049. doi: 10.1210/jc.2004-0895. [DOI] [PubMed] [Google Scholar]
- 21.Kinniburgh D, Zhu H, Cheng L, Kicman AT, Baird DT, Anderson RA. Oral desogestrel with testosterone pellets induces consistent suppression of spermatogenesis to azoospermia in both Caucasian and Chinese men. Human reproduction (Oxford, England) 2002;17:1490–1501. doi: 10.1093/humrep/17.6.1490. [DOI] [PubMed] [Google Scholar]
- 22.Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM. Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: implications for male contraceptive development. Journal of andrology. 2001;22:1053–1060. doi: 10.1002/j.1939-4640.2001.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 23.Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. The Journal of clinical endocrinology and metabolism. 2003;88:4659–4667. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- 24.Rates of testosterone-induced suppression to severe oligozoospermia or azoospermia in two multinational clinical studies. World Health Organization Task force on Methods for The Regulations of Male Fertility. International journal of andrology. 1995;18:157–165. doi: 10.1111/j.1365-2605.1995.tb00405.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.