Abstract
Cellular triglycerides (TG) are stored in cytosolic lipid droplets (LDs). Perilipins (PLIN) are a group of LD-proteins that play important roles in the assembly and transport of LDs and in TG metabolism. Two members of the PLIN family are found in insects (PLIN1 & 2 or Lsd1 & 2). We have cloned and expressed Manduca sexta PLIN2 (MsPLIN2), and studied developmental and nutritional changes in the expression of PLIN2. Nutritional changes induced fast alterations in PLIN2 mRNA and protein levels in fat body and midgut of the feeding larvae. The relationship observed between PLIN2 expression and TG synthesis in both larval fat body and midgut suggests that PLIN2 is needed when tissues are accumulating TG. However, when the fat body was storing TG at maximal capacity, MsPLIN2 levels declined. This unexpected finding suggests the occurrence of alternative mechanism/s to shield TG from the action of lipases in M. sexta LDs. In addition, it implies that the cellular level of lipid storage could be modulating MsPLIN2 expression and/or degradation. The study also confirmed that MsPLIN2 was most abundant in the adult fat body, which is characterized by a high rate TG hydrolysis and lipid mobilization. Whether MsPLIN2 is directly involved in lipolysis and/or the secretion of lipids in the fat body of adult of M. sexta is unknown at this time. Nonetheless, the coexistence of high PLIN2 level and lipolysis suggests a complex role for MsPLIN2. Altogether, we found that MsPLIN2 is needed when the synthesis of glycerides, DG and TG, is active even if the insect is accumulating or consuming TG.
Keywords: PLIN2, LSD2, lipid droplets, triacylglycerol, triglycerides, fat body, Manduca sexta
1. INTRODUCTION
The fat body is the main tissue for storage of fatty acids (FA) in insects. As in other organisms, large FA reserves are needed to ensure energy production. However, in insects, FA also play an essential role in reproduction and as precursors of cuticular lipids, waxes, and pheromones (Downer and Matthews, 1976). Fat body adipocytes are characterized by the presence of large spherical lipid droplets (LDs), which store FA in the form of triglycerides (TG). The structure of LDs is commonly described as formed by a core of neutral lipids, predominantly TG and sterol esters, surrounded by a monolayer of phospholipids and a variety of proteins (Penno et al., 2013; Storey et al., 2011). The phospholipid monolayer and the protein coat constitute the boundary that separates the hydrophobic core of LDs from the aqueous environment. Proteomic studies of isolated LDs have shown that the protein coat is composed by a large number of proteins (Beilstein et al., 2013; Beller et al., 2006; Brasaemle et al., 2004; Cermelli et al., 2006; Hodges and Wu, 2010; Soulages et al., 2012). Perilipins, or PAT proteins, are a small group of LD-proteins that share a common domain, the PAT domain (Pfam03036) and the fact that they are normally associated with LDs. This group is composed by perilipin (PLIN1), ADRP (PLIN2), TIP47 (PLIN3), S3-12 (PLIN4) and OXPAT (PLIN5) (Brasaemle, 2007; Kimmel et al., 2010). Two PLIN genes are found in insects. The products of these genes were originally named Lsd1 and Lsd2 (Miura et al., 2002), but from now on we will refer to them as PLIN1 and PLIN2, respectively, to accommodate the recently proposed change in nomenclature (Kimmel et al., 2010). PLIN proteins have in common a conserved region of ~100 amino acids towards the N-terminal called the PAT domain (Lu et al., 2001). The function of this region remains unknown but it is not required for binding to LDs (McManaman et al., 2003). PLIN proteins play important roles in the processes of lipid storage and mobilization (Arrese et al., 2014), however their mechanisms of function are not completely known in any system, yet.
PLIN1 is the best characterized lipid droplet protein in vertebrates and in insects. PLIN1 is a major regulator of lipolysis. Studies in vertebrate systems have suggested that unphosphorylated PLIN1 behaves as a barrier that prevents lipases from gaining access to neutral lipids in the droplet core (Brasaemle et al., 2000; Souza et al., 2002; Tansey et al., 2004). In contrast, phosphorylated PLIN1 actively facilitates lipase action, in part by recruiting lipases to the droplet surface (Brasaemle, 2007; Ducharme and Bickel, 2008). Studies in insects have also shown that PLIN1 plays major roles in the storage and degradation of TG (Beller et al., 2010; Bickel et al., 2009). Hormonal stimulation of the lipolytic response in Manduca sexta involves the acute phosphorylation of PLIN1 (Patel et al., 2005) presumably by PKA (Arrese et al., 2008a). Phosphorylation of PLIN1 by Ca2+/Calmodulin-dependent protein kinase-II was linked to activation of lipolysis and pheromone synthesis in pheromone glands of Bombyx mori (Ohnishi et al., 2011).
The function of insect PLIN2 has been investigated mostly through genetic studies in Drosophila. Overexpression of PLIN2 promoted lipid storage in flies, whereas loss-of-function mutants were leaner than wild type flies (Gronke et al., 2003). PLIN2 mutant females were shown to have altered lipid accumulation and lower lipid levels in eggs (Teixeira et al., 2003). Moreover, the lack of PLIN2 has been associated with defects in the transport of LDs during embryonic development (Welte et al., 2005). More recently, it was shown that Drosophila PLIN2 associates with small LDs in close proximity to plasma membrane or the nuclear envelope of fat body adipocytes (Bi et al., 2012; Diaconeasa et al., 2013). Drosophila PLIN2 is mostly expressed in tissues directly engaged in lipid metabolism, such as larval fat body and ovary, but it was also found to play a role in lipid accumulation in wing imaginal discs (Fauny et al., 2005). Drosophila PLIN2 protein was found to be highly helical protein, soluble in aqueous medium but also able to tightly bind and form stable complexes with phospholipid (Arrese et al., 2008b). Two putative amphipathic helices could mediate lipid binding. The role of PLIN2 seems to be complex. It is important for lipid storage (Gronke et al., 2003) and it also plays a role in the mobilization of fat from the fat body (Diaconeasa et al., 2013). Although mutants affecting PLIN2 expression have global effects on the accumulation of lipid in Drosophila, the precise role of PLIN2 in the metabolism of TG is not known. It has been speculated that PLIN2 prevents the hydrolysis of TG located in LDs. M. sexta is not suitable for genetic studies but it is an important model insect to the study of lipid metabolism from a physiological and biochemical perspective. Numerous studies have focused in the metabolism, mobilization and transport of fat in M. sexta (Arrese and Soulages, 2010). Although fat is always a prominent energy reserve in Manduca, there are major physiological differences between larva and adult that can be exploited to benefit the study of lipid storage and mobilization in insects. For example, larvae have gluttonous appetite feeding almost constantly while adults feed infrequently (Ziegler, 1991). By the end of the larval stage, substantial energy reserves –glycogen, protein and lipids- are accumulated in the fat body. These reserves will be utilized to support life and development in the subsequent non-feeding periods that in the lab includes adult life (Ziegler, 1991). Glycogen reserves are used first and the adult of M. sexta emerges with a very low content of glycogen in the fat body. Adult insects rely on lipid reserves to support life and fat body lipid, which is the exclusive source of hemolymph lipids in adults, is mobilized to the hemolymph (Ziegler, 1991). Given these striking differences, the fat body of these insects is a good model system to study fat storage in the 5th instar larvae, and fat mobilization in the adults. To gain information on the function of PLIN2 in M. sexta, we have studied transcript and protein expression levels under different developmental and metabolic conditions in the fat body -the central organ for energy storage (Fernando-Warnakulasuriya et al., 1988)- but also in the midgut, which have a significant capability to synthesize triglycerides (Canavoso and Wells, 2000). The results suggest a role of PLIN2 in the synthesis of TG disregard the insect was accumulating or mobilizing TG. However, the expression of PLIN2 in the larval fat body was complex. PLIN2 followed the accumulation of lipids only to a certain lipid level. Maximal lipid storage capacity was accompanied by a decrease in PLIN2 level suggesting the occurrence of alternative mechanism/s to shield TG from the action of lipases in M. sexta LDs but also that the level of cellular lipids could be modulating PLIN2 expression and/or degradation.
2. MATERIALS AND METHODS
2.1. Cloning and sequencing of M. sexta PLIN2 (Lsd2)
Four M. sexta peptides sequences matching the predicted B. mori PLIN2 were obtained by the MS/MS analysis of lipid droplet-associated proteins (Soulages et al., 2012). The four peptides were: RVPLVTEQPKVIVETT, SPHVNKVYGAR, LKELSWAKAN, RVWANLAYK. MsPLIN2 cDNA was cloned by 5′- and 3′-RACE reactions using a primer designed on the basis of the peptide sequence LKELSWAKAN. The primer sequences were obtained from the coding DBNA sequence of B. mori PLIN2 (accession number: NP_001138804) in which the peptide LKELSWAKAN is conserved. Total RNA was isolated from the fat bodies of M. sexta larva using Trizol reagent (Invitrogen). From total RNA, poly(A)+ RNA was subsequently isolated using Poly(A) Purist MAG (Ambion). mRNA was reverse transcribed using oligod(T)18-perimer. The resulting cDNA was used as template in 5′ and 3′-RACE. The SMART RACE cDNA Amplification kit (BD Biosciences) was used according to manufacturer’s instructions. 3′-RACE was performed using the forward primer PLIN2-F: 5′-TTGAAGGAGCTCTCGTGGGCCAAAGCGAAC-3′ with reverse primers provided with the kit. The product from 3′-RACE reaction was a single band (~800bp) and was cloned into pGEM Easy Vector (Promega). For the 5′-RACE the reverse primer PLIN2-R: 5′-GTTCGCTTTGGCCCACGAGAGCTCCTTCAA-3′ and the forward primer supplied with the kit was used. The 5′-RACE reaction produced a major product of ~650bp that was gel purified and cloned into pGEM vector. The 800bp and 650bp clones were sequenced in both directions. The sequences were used to design two new primers (5′-CGCAGAGGACCTGGAGTGTTCTGTTA-3′, and 5′-TTGCTGTATAAGTCCATATAAAATAC-3′) to clone the full-length MsPLIN2. The PCR reaction produced a single band (~1.4kb) and was cloned into pGEM vector and sequenced. MsPLIN2 cDNA sequence was deposited in Genebank (accession number: JF809664). The M. sexta genome has been recently sequenced and assembled (Kanost et al., 2016) (http://www.ncbi.nlm.nih.gov/assembly/GCA_000262585.1). The PLIN2 gene was found in scaffold00010 (Msex2.00759) and this information was used to extract the gene structure of M. sexta PLIN2. Protein sequences were aligned using the Alignment Explorer/Muscle (Edgar, 2004) implemented in Molecular Evolutionary Genetics Analysis (MEGA version 5.10) (Tamura et al., 2011). The default presets for gap penalties and iterations were used for aligning the sequences.
2.2. Expression and purification of recombinant PLIN2/LSD2 and antibody preparation
The coding region of M. sexta PLIN2 was amplified by PCR using the forward primer 5′-GACGACGACAAGATGGCAACAGAAGTGAGTCAAGCACCGGCA-3′ and the reverse primer 5′-GAGGAGAAGCCCGGTCTAATTTTGCGAATGCTCCGCGGATTTCGCCT-3′. The 942bp PCR product was cloned into pET32-Ek/LIC vector (Novagen) and the sequence was confirmed by sequencing in both directions.
E. coli strain NovaBlue GigaSingles cells (Novagen) were transformed with the recombinant plasmid. Positive clones were confirmed by DNA sequencing. E coli Rosetta 2 cells (Novagen) were transformed for protein expression. Expression of the recombinant protein was induced with 1mM IPTG. After 2.5h, bacteria were collected and resuspended in lysis buffer (50mM Tris pH8, 1mM EDTA, 100mM NaCl, 1mM PMSF) containing 0.3 mg/ml of lysozyme. After 30min incubation, the preparation was centrifuged at 160,000xg for 30min. The fusion protein was found in the pellet, which was resuspended in 20mM Tris pH8, 6M urea, 500mM by sonication. After centrifugation (160,000xg, 15 min), recombinant protein was purified by Ni-affinity chromatography. The fusion protein was cleaved by thrombin to remove the thioredoxin/His-tag portion. Purified PLIN2 was used to generate polyclonal antibodies.
2.3. Insect rearing and feeding
M. sexta eggs were purchased from Carolina Biological Supplies. Larvae were reared at 25°C on artificial diet (Bell and Joachim, 1976) unless otherwise it is indicated. The end of the 4th-instar was identified by the appearance of head capsule (HC) slippage. Tissues were dissected from 5th-instar larvae and sorted by days from the HC slippage (day 0). Wanderer insects were identified by initiation of wandering behavior and the exposure of the dorsal aorta. Adult insects were maintained at room temperature without food since adults tend not to feed under lab conditions. When 5th instar larvae were reared on tomato leaves, each animal was maintained in an individual cup and provided with increasing amounts of leaves from 0.3 g (day 1) to 2.4 g (day 9). These animals were reared from hatching to the 4th instar on the artificial diet.
For starvation and refeeding experiments, 5th instar larvae (day 2) were kept with or without artificial diet for 24h (“Fed” and “Starved”, respectively). On day 3, starved insects were re-fed with artificial diet (ad libitum). Tissues were collected at different periods of time post-refeeding. Starved adult insects (day 2) were provided with trehalose by injection (Arrese et al., 1996) and control animals were injected with water. Fat bodies were collected 24h post-injection.
2.4. Quantitative RT-PCR
Total RNA was extracted from fat bodies or midguts using Trizol reagent (Invitrogen). Tissue from at least two insects was pooled and used for each preparation of total RNA. cDNA was synthesized from 1.0μg of total RNA using iScript cDNA synthesis Kit (Bio-Rad) following manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was carried out using iTaq Universal SYBR Green Supermix (Bio-Rad) and CFX Connect Real-Time PCR Detection System (Bio-Rad) in 10μl reactions. The PCR reaction conditions were initial denaturation at 95°C for 1 min, followed by 45 cycles alternating denaturation at 95 °C for 2s and annealing/extension at 60°C for 45s. PLIN2 (5′-GGGAGTAGTGCTGGCGGCAT-3′ and 5′-CGACGATGACCTTTGGCTGCTC-3′), DGAT (5′-CTTGTTGGCCCCTACTCTATGTTACGA-3′ and 5′-GTCACTGAGGGTATCATCCATTGCTG-3′) and TGL (5′-ATGAACGATAGTACGGAAAGGAAAAGAGATAGCGA-3′ 5′-CCCGCCATATTGATTTATCTTCGACATCCA-3′) specific primers were used in corresponding reactions. Expression of each transcript was normalized using the ribosomal protein S3 (5′-TACAAACTCATTGGAGGTCTGGCCGT-3′ and 5′-ACGAACTTCATGGACTTGGCTCTC-3′) as an internal control. At least two independent sets of total RNA were analyzed in triplicate. Relative values are plotted as the mean ± SD.
2.5. Preparation of Lipid Droplets
Fat body or midgut were dissected and homogenized in homogenization buffer (HB) (20mM Tris, pH 7.4, 0.25M sucrose, 1mM EDTA, 1mM benzamidine, 1mM PMSF, 10 mg/L leupeptine, 1mM DTT) containing 0.25 M sucrose. Homogenates were centrifuged at 1000g for 10 min. The supernatant was overlaid with HB without sucrose and centrifuged at 160,000g for 1h. The distinctive thin white layer floating at the top (LDs) was collected and resuspended in HB buffer. Midguts were dissected in phosphate buffered saline (PBS 50mM Na2HPO4, pH 7.4, 150mM NaCl), washed exhaustively with this buffer and then homogenized as mentioned above for fat body tissue. Purified LDs were resuspended in HB buffer and the protein concentration was determined by Bradford’s method (Sigma). Laemmli buffer (Sigma) was added to LDs samples used for SDS-PAGE.
Subcellular fractionation
Fat body homogenate was centrifuged at 1000g for 10min and the resulting supernatant was adjusted to 1.17M sucrose and transferred to a SW40 centrifuge tube to be subsequently overlaid with 1 ml each of 1.02 M sucrose, 0.87 M sucrose, 0.58 M sucrose, 0.29 M sucrose, 0.15 M sucrose in HB buffer and 1.5 ml of buffer without sucrose. Density gradients were centrifuged at 160,000 g for 4 h and fractionated into seven fractions, which were analyzed by western blot to determine PLIN2.
2.6. Western blot
Anti-PLIN2 polyclonal antibodies were generated in rabbit using purified recombinant MsPLIN2 by Cocalico Biologicals Inc. For western blot, proteins were separated by 4–15% SDS-PAGE (Bio-Rad) and transferred to nitrocellulose membranes using Bio-Rad Trans-Blot Turbo system (Bio-Rad). Immunodetection was performed using horseradish peroxidase-conjugated secondary antibody (Millipore) and ECL chemiluminescence reagents (Amershan Pharmacia). PLIN2 levels were estimated by measuring the net band density on the X-ray films by AlphaEaseFC software (Innotech). Ponceau S staining of the membrane followed by densitometry was used to estimate the total amount of protein in each lane (Romero-Calvo et al., 2010).
2.7. Lipid analysis
Total lipid was extracted from M. sexta fat body with chloroform-methanol (Folch et al., 1957). TG was determined using the Infinity Triglycerides Reagent (Thermo) according to manufacturer’s instructions. Triolein was used as standard for the calibration curve. TG content of fat body was expressed as μmol TG/ fat body.
2.8. Lipid droplets size distribution
Fat bodies dissected from 5thinstar larvae fed on artificial diet (day 2.5 and day 5), and on leaves (day 7) were fixed in 2% glutaraldyhyde (in 0.1M sodium cacodylate plus 1 mg/ml CaCl2) for 2 hours. Briefly, samples were embedded in 100% poly/bed resin, semi-thin sections (700nm) were obtained and stained with Mallory’s Azure II Methylene Blue (Richardson et al., 1960). Sections were imaged using transmitted light detector in a Leica SP2 laser scanning confocal microscope. LD sizes were determined using 4 to 6 different fields per sample. In total the sizes of 489, 188 and 407 LDs were determined for fat body samples of 5th instar day 2.5 and day 5 that were fed on regular diet, and day 7 of 5th instar fed on leaves. The average percentage of LDs belonging to four group sizes (<1.5μm, 1.5–5μm, 5–9μm and >9μm) and their corresponding SD were estimated. The means were compared by ANOVA followed by Tukey’s multiple comparisons test.
3. RESULTS AND DISCUSSION
3.1. Cloning and analysis of M. sexta PLIN2/Lsd2 cDNA sequence
Using mRNA from M. sexta larval fat body, sequence information from B. mori genome and peptide sequence information obtained from MS/MS analysis of M. sexta LDs (Soulages et al., 2012), we obtained a PLIN2 clone (MsPLIN2). The complete MsPLIN2 cDNA sequence (1350bp, GenBank: JF809664.1) comprises a 104 nucleotide 5′non-coding region, an open reading frame (ORF) of 915bp (positions 105–1019) and a 3′ non-coding region of 331 nucleotides followed by a poly(A) tail of 14nt. A predicted poly-adenylation signaling site AATAA was found at position 14 upstream of the poly(A)-tail. The ORF encodes a 304 amino acid protein with a theoretical mass of 32.4kDa and isoelectric point of 8.65 (Fig S1). The alignment of MsPLIN2 deduced protein sequence with the sequences of the vertebrate proteins of the PAT family, PLIN1-PLIN5, indicated that MsPLIN2 is more similar to the vertebrates’ PLIN2 (22–24.6% identity), which is also known as adipose differentiation related protein (ADRP) (Fig S2).
Alignment of thirteen PLIN2 protein sequences covering three insect orders showed a low overall conservation. The N-terminal region, which contains the PAT domain (pfam03036) and it is characteristic of PLIN proteins (Lu et al., 2001; Miura et al., 2002), is the most conserved region of insect PLIN2 (Fig S3). The N-terminal region (1–115) also contains eleven amino acids that are conserved among the PLIN2 protein sequences of three vertebrates (Fig S2). Among insects MsPLIN2 protein sequence is very similar to that of B. mori (~80% identity). However, the level of identity decreases when MsPLIN2 is compared with the corresponding proteins from insects of different orders, such as Diptera (33–38% identity) and Hymenoptera (20–24% identity) (Fig S4). A comparative sequence analysis of insect PLIN proteins (thirteen PLIN2 and three PLIN1) using the neighbor joining method shows that PLIN1 and PLIN2 proteins cluster in separate clades and also shows a clear divergence among PLIN2 proteins from different insect orders (Fig 1).
Figure 1. Phylogeny of insect PLIN proteins.
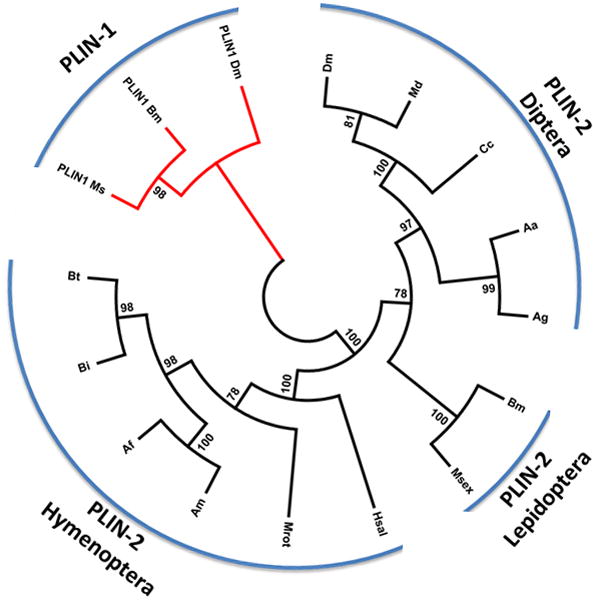
Alignments were produced with Clustal Omega and the phylogeny was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analyzed (Felsenstein, 1985). Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (Felsenstein, 1985). The evolutionary distances were computed using the JTT matrix-based method (Jones et al., 1992) and are in the units of the number of amino acid substitutions per site. The analysis involved 16 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 228 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011). The PLIN2 sequences identifiers and abbreviations used are: Hsal: Harpegnathos saltator (EFN86125.1); Mr: Megachile rotundata (XP_003703225.1); Bi: Bombus impatiens (XP_003485735.1); Bt: Bombus terrestris (XP_003397531.1); Am: Apis mellifera (XP_003249235.1); Af: Apis florea (XP_003697663.1); Bm: Bombyx mori (NP_001138804.1); Msex: Manduca sexta (AEJ33049.1); Cc: Ceratitis capitata (XP_004524967.1); Dm: Drosophila melanogaster (NP_001036276.1); Md: Musca domestica (XP_005188653.1); Aa: Aedes aegypti (XP_001658058.1); Ag: Anopheles gambiae (XP_310971.5).
PLIN1 sequence identifiers are: Ms: Manduca sexta Perilipin 1 (AIE17454.1), Bm: Bombyx mori Perilipin 1 (NP_001040143.1|) and Dm: Drosophila melanogaster Perilipin 1 (NP_732904.2).
The structure of MsPLIN2 gene was predicted from the information provided by the Manduca genome (Fig 2). MsPLIN2 gene is found in scaffold 10 of the M. sexta genome (Kanost et al, 2016). The gene spans ~11 kb and contains 6 exons (5 coding and a 5′UTR exon). The five predicted coding exons encode a protein of 331 amino acids nearly identical to the protein predicted by the cDNA cloned in this study. The only difference between the protein predicted by the genome and our clone is at position 183 (Q in genome vs R in the cDNA). The reported gene structures of mouse MmPLIN2/ADRP and Drosophila DmPLIN-2 (CG9057) were also included in the Fig 2. The exons of MmPLIN2 were aligned with MsPLIN2 and DmPLIN2-A according the alignments of their amino acid sequences. Manduca and Drosophila genes share splicing sites. However, overall the gene structures are significantly different. The most conserved region in this protein family, the PAT domain, comprises two exons in Manduca and Drosophila but three exons in mouse.
Figure 2. Comparison of M. sexta, D. melanogaster, and Mus musculus plin2 gene structures.
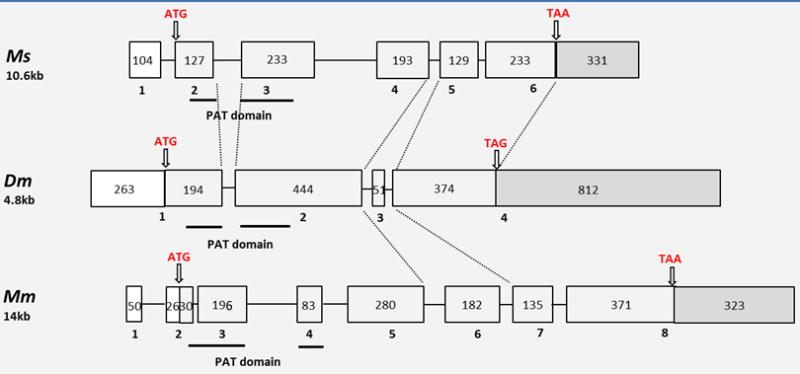
M. sexta PLIN2 genomic information was obtained from the Manduca genome sequence data available at http://www.ncbi.nlm.nih.gov/assembly/GCA_000262585.1, Dm (D. melanogaster PLIN2-B, accession number: AAF48419.1), and MmPLIN2 (NP_031434.3). The size of these genes is shown below the name of species. Exons, introns, 5′-UTR and 3′-UTR are shown with light grey boxes, lines, white boxes and dark grey boxes, respectively. Exon number is shown beneath the corresponding box. The initiation and termination codons are pointed with arrows. The number inside the exon box indicates the exon’s length (nt).
3.2. MsPLIN2 characterization: recombinant protein and subcellular localization
Recombinant MsPLIN2 was produced in E. coli as a fusion protein with thioredoxin (Trx), Trx-[His]6-Stag-PLIN2 (49.4 kDa) as we previously described for Drosophila PLIN2 (DmPLIN2) (Arrese et al., 2008b). The fusion protein contain a cleavage site for removal of the Stag-PLIN2 portion. The fusion protein was extracted from the inclusion bodies with 6 M urea. After purification to homogeneity by Ni-affinity chromatography (Fig 3, lane 1), the fusion protein was cleaved to release Stagged PLIN2. Both of these proteins, full length fusion protein and Stagged PLIN2 had an electrophoretic mobility in SDS-PAGE that was lower than that expected from their sizes. The molecular mass of the fusion protein is 49.4 kDa but the apparent molecular mass in SDS-PAGE was 56 kDa (Fig 3, lane 1). Likewise, Stag-PLIN2 has a molecular mass of 35.5 kDa but the electrophoretic mobility corresponded to an apparent mass of ~45 kDa (Fig 3, lane 2). The identity of this band was confirmed by mass spectrometry. A similar situation was reported for recombinant DmPLIN2 (41.1 kDa) whose observed electrophoretic mobility corresponded to 55 kDa (Arrese et al., 2008b).
Figure 3. Recombinant MsPLIN2, polyclonal antibodies and subcellular localization of MsPLIN2.
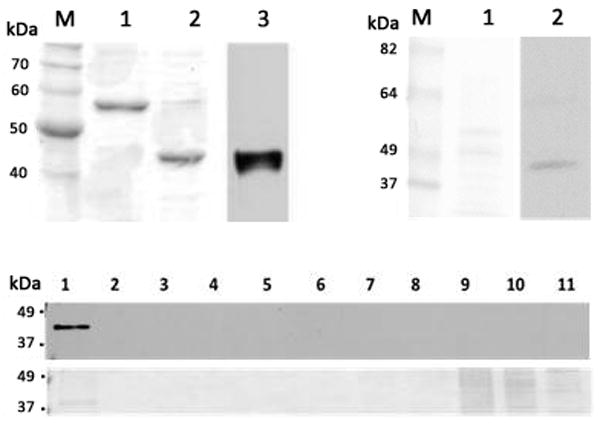
Top left panel: recTrx-PLIN2 uncut (lane 1), and after thrombin cleavage (lane 2) were separated by SDS-PAGE and stained with Coomassie. Western blot analysis of sample shown in lane 2 using anti-MsPLIN2 antibodies; Top right panel: LDs (~10 μg) isolated from the fat bodies of adult of M. sexta (lane 1, Ponceau staining) were analyzed by western blot (lane 2); Bottom panel: Subcellular localization of MsPLIN2 was carried out using fat body homogenate from adult male insects that was subjected to ultracentrifugation in a sucrose gradient. The gradient was fractionated into eleven fractions and each fractions was analyzed by western blot using MsPLIN2 antibodies (top), the corresponding Ponceau S staining is shown in the bottom panel.
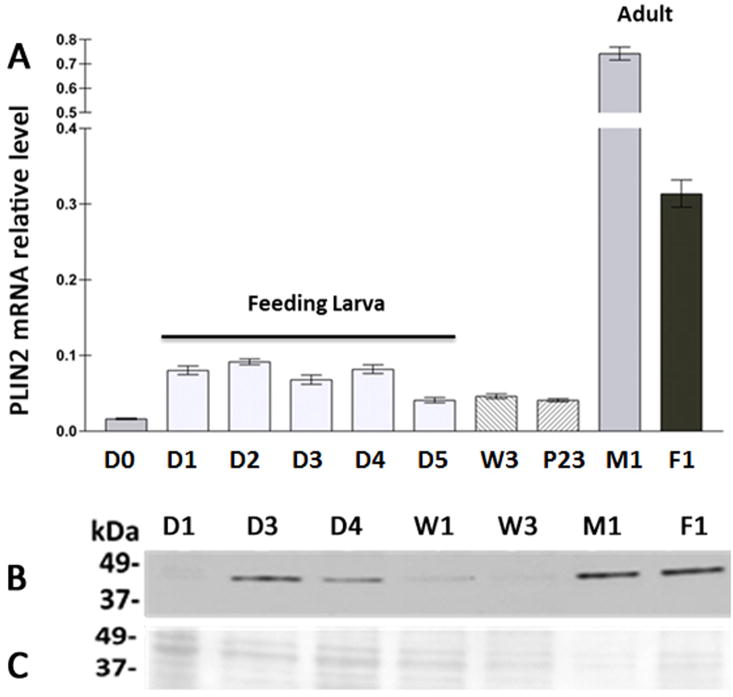
Manduca Stag-PLIN2 was used to generate polyclonal antibodies (Fig 3, lane 3). Immunoblot analysis of lipid droplet-associated proteins isolated from the fat body of adult insects identified a band of ~40 kDa (Fig 3, right panel, lane 2). Consistently with the electrophoretic properties of recombinant PLIN2, the band identified in the immunoblot had a larger apparent mass than the actual molecular mass (32.4kDa). The apparent size of DmPLIN2 in tissue samples analyzed by western blot was 44–46kDa (Gronke et al., 2003). However, the molecular mass for DmPLIN2 is 36–38 kDa (Attrill et al., 2016). Altogether, MsPLIN2 showed a lower electrophoretic mobility on SDS-PAGE and this fact was consistent with all the data available for other PLIN2 proteins. The immunoblot results are consistent with the electrophoretic mobility of PLIN2 and we concluded that the antibody recognized MsPLIN2 as a band of ~40 kDa in western blot. The lack of MsPLIN2 detection in the young 5th instar larva, as seen in Fig 5-B lane D1, coincided with the mass spectrometry analysis of LDs (Soulages et al., 2012).
Figure 5. Developmental changes in fat body MsPLIN2 mRNA levels.
A) PLIN2 mRNA level was determined by qRT–PCR using mRNA RpS3 as reference. At least two independent sets of total RNA were analyzed in triplicate and relative values are plotted as the mean ± SD. D0, D1, D2, D3, D4 and D5 refers to days of 5th-instar larva, W3: wanderer day 3, P23: pupa day 23; M1 and F1: 1-day-old male and female adults, respectively. B) PLIN2 western blot: each gel lane was loaded with 10–20μg of LDs proteins. C) Ponceau S staining of membrane used in western blot shown in panel B.
To study the subcellular localization of PLIN2 in the fat body of M. sexta, the tissue was homogenized and fractionated in a sucrose density gradient by centrifugation. Each fraction was analyzed by western blot using MsPLIN2 antibodies. PLIN2- a band ~40kDa- was only associated with the lipid droplet fraction that floated at the top of the gradient (Fig 3, bottom panel) implying a role for PLIN2 in M. sexta lipid metabolism.
3.3. Tissue expression of MsPLIN2
Since the occurrence of PLIN2 in Drosophila correlates with the ability of the fly to accumulate lipids (Fauny et al., 2005; Gronke et al., 2003; Teixeira et al., 2003), we assessed first the expression of PLIN2 in the midgut and fat body of 5th instar larva and in the fat body of adult insects. PCR and western blot studies identified both mRNA (Fig 4A) and PLIN2 protein (Fig 4B) in all the tissues. Interestingly, PLIN2 seemed to be less abundant in the fat body of the feeding larva, which is accumulating lipids, than in the fat body of adults, which mobilizes lipids to the hemolymph (Fig 4B). This result that is in agreement with a previous proteomic study of LDs (Soulages et al., 2012) is intriguing given that PLIN2 in other systems has been involved in TG storage.
Figure 4. PLIN2 levels in fat body (FB) and midgut (MG) of 5th instar larva (day2) and FB of adult insects.
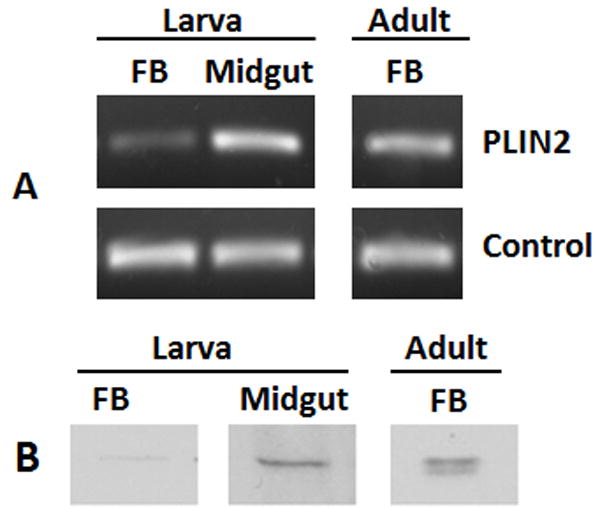
A) Determined by RT-PCR using the amplification of the ribosomal S3 transcript (RpS3) as control; B) PLIN2 proteins from LDs preparations (~10μg) were determined by western blot analysis as described in the Materials and Methods.
3.4. Expression of PLIN2 in fat body during development
About 90 % of the growth of a Manduca larva takes place during the last (5th) larval stage, which is characterized by a very intense feeding behavior resulting in a massive accumulation of lipids in the fat body (Nijhout et al., 2006). Fat body triglycerides (TG) peak at the end of this period (day 5), just before the beginning of the non-feeding phase that, in the lab, extends through the adult life (Fernando-Warnakulasuriya et al., 1988). During the wandering period (the days prior to pupation) there is a small decline in the fat body lipid content, which will remain practically constant through pupal stage. During adult life, which in the lab extends for about ten days, fat body lipids are consumed (Ziegler, 1991). Unlike larva, adults can mobilize great amount of lipids from the fat body to the hemolymph and the fat body is the only tissue supplying those lipids. The hemolymph lipid concentration in adults is higher (~50 mg/ml) than in larval insects (~ 1 mg/ml) (Tsuchida and Wells, 1988; Ziegler et al., 1995). Consistently, the fat body of adults is under a higher lipolytic condition (Arrese et al., 2010). Lipase activity increased rapidly in adults peaking around the second day of life. At this point, the fat body lipase activity is ~ 9-fold higher than in the larva of M. sexta (Arrese et al., 2010). Thus, larva and adult of M. sexta not only have different life styles but also those differences impose a change in the flow of lipid metabolism as well. While 5th instar larvae store lipids in the fat body, adults consume those lipids to support life. The latter is even more pronounced in starving adults, in which glycogen storage is rapidly depleted and these animals have a very low hemolymph trehalose concentration and a much higher hemolymph lipid concentration (Ziegler, 1991). Notoriously, the adult of M. sexta does not accumulate lipids. Even feeding adults use excess of sugar to restore hemolymph trehalose and glycogen storage in the fat body while the lipid storage remains unchanged (Ziegler, 1991).
As a first step to examine the connection between PLIN2 expression and lipid storage in the fat body of M. sexta, PLIN2 mRNA levels were determined through development including each day of the 5th larva (days 1 to 5), two molting (non-feeding) larva (5th-day 0 and wandering-day 3), pupa (day 23), and male and female adult (day 1). A moderate increase in PLIN2 mRNA was observed at the beginning of 5th instar followed by a decline towards the end (day 5). The levels that remained low from the beginning of the non-feeding period extending to the pupal stage (W3 and P23) increased in the adult fat body, which showed the highest PLIN2 mRNA levels. The low levels of PLIN2 mRNA in W3 and P23 seemed consistent with the notion that PLIN2 is required for TG accumulation. Adults, however, have 8–14-fold higher PLIN2 levels than larval fat body (Fig 5A). Therefore, the finding that MsPLIN2 was more abundant in the highly lipolytic fat body of adult rather than in the feeding larva was puzzling. Is PLIN2 needed for lipolysis? Given that PAT proteins are evolutionary conserved (Miura et al., 2002) and that DmPLIN2 expression correlates with fat accumulation (Gronke et al., 2003), it seems unlikely that MsPLIN2 could be directly involved in lipolysis activation. However, MsPLIN2 could be indirectly linked to lipolysis and directly linked to lipid storage through its involvement in the storage of glycerides produced via re-esterification of the excess of FAs generated during lipolysis. It can be hypothesized (Wolins et al., 2006) that newly synthesized TG would be packed in nascent droplets whose assembly would require PLIN2. If one considers that Manduca mobilizes fat body TG as 1,2-DG, then, under high lipolysis, the fat body is producing significant amount of extra FAs (Arrese and Wells, 1997). FAs can be re-esterified back to glycerides by monoacylglycerol-acyltransferase (MGAT), which catalyzes the synthesis of DG, and by diacylglycerol-acyltransferase (DGAT), which catalyzes the last step of TG synthesis. Therefore, high lipolysis would also be accompanied by high rate of FAs re-esterification and re-synthesis of glycerides. To test this possibility, we measured MGAT, DGAT mRNA levels and PLIN2 mRNA levels in the fat body of adults under high (control) and low (trehalose) lipolytic conditions. MGAT and DGAT genes in Manduca were recently identified (Soulages et al., 2015). DGAT is highly expressed in the fat body of the feeding larva whereas in adults, mRNA MGAT level is slightly higher than DGAT levels (Soulages et al., 2015). To obtain a condition of low lipolysis in the fat body of adults, the trehalose levels of the hemolymph were increased by administering an intra-hemocoel trehalose solution, as previously described (Arrese et al., 1996). In M. sexta adults, the levels of hemolymph trehalose decline over time whereas there is a concomitant increase in the lipid levels (Ziegler, 1991). This tendency can be reverted by injection of trehalose, which induces a decline in hemolymph lipid concentration (Ziegler, 1991) as well as fat body lipase activity (Arrese et al., 1996). Thus, we compared mRNA levels in adults injected with trehalose (low lipolysis) or water (control, high lipolysis). The trehalose effect on lipolysis was confirmed by the decrease in the main fat body lipase mRNA level (TGL), as shown in Fig 6. PLIN2 mRNA levels also decreased in trehalose treated insects (Fig 6) suggesting that PLIN2 could be indeed linked to the re-esterification of FA produced during lipolysis. Consistent with this interpretation, the mRNA levels of MGAT and DGAT also decreased under low lipolysis condition (Fig 6). The fat body of adults is not accumulating fat but rather losing it through FA oxidation and secretion to the hemolymph. Therefore, PLIN2 expression does not correlate with fat accumulation. However, the correlation between PLIN2 levels and lipolysis suggests that a likely role of MsPLIN2 would be to allow the packing of glycerides whose rate of synthesis increases dramatically in starved adult insects due to the increase in FA re-esterification.
Figure 6. MsPLIN2 mRNA levels in the fat body of adult of M. sexta.
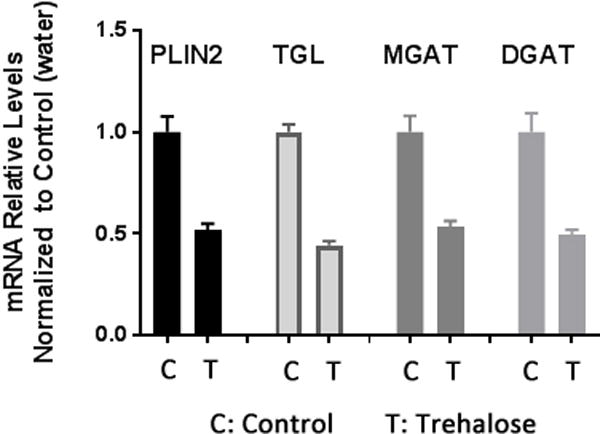
Second day adults in starvation were injected with 20 μl trehalose (0.65 mg/ml) or water (control). Fat body tissue was collected 24h post-injection. The relative levels of each mRNA (mean ± SD) were determined by quantitative RT-PCR using mRNA RpS3 as reference. Two independent sets of RNA were analyzed by triplicate. The relative values, normalized to control (24h post-injection of 20 μl water), are plotted as the mean ± SD.
Finally, it is also worth noting that starving adults injected with trehalose showed a decrease in DGAT mRNA level (Fig 6). This result is consistent with a previous report showing that when adults of M. sexta are sugar fed, the content of lipid stored in the fat body does not increase and the excess of sugar is stored in the fat body as glycogen (Ziegler, 1991). The results highlight the contrasting difference in TG metabolism between the larval and adult stages of M. sexta.
3.5. PLIN2 levels in the larvae of the tobacco hornworm, M. sexta
3.5.1. Fat body
Fat body TG content and PLIN2 protein levels were determined in each day of the feeding period of the 5th instar. Fat body lipids increase nearly 10 fold during the feeding period of the 5th instar (Fig 7). TG accumulation is greater during the last two days (days 4 and 5) (Fig 7). On the other hand, PLIN2 occurrence accompanies the increase in TG in the course of the first three days, when both PLIN2 and TG levels increase 2 folds and 2.6 folds, respectively. However, during days 4 and 5, when the maximum increase in TG mass takes place (4-fold), the content of PLIN2 in the LDs actually decreased by nearly 3-fold (Fig 7). The decrease in PLIN2 at the end of the feeding larval stage could be part of the developmental program. To some extent such decline resembles the situation described for the differentiation of vertebrate adipocyte from fibroblasts where the process start with high expression levels of PLIN2/ADRP but declines towards the end when the accumulation of large amounts of TG is taking place (Brasaemle et al., 1997; Jiang and Serrero, 1992). Fully developed adipocytes have large LDs and no PLIN2. As it will be further supported in section 3.6, a similar situation could be happening in Manduca fat body. Thus, once a critical level of cellular TG is reached, and the number and size of LDs reaches certain level, the expression of PLIN2 may not be required. On the other hand, in the 5th larval instar of Manduca the molting process is triggered when the larvae reach the “critical weight”, which is achieved at day 3 (Nijhout et al., 2006). At this time, juvenile hormone levels start to decrease and a cascade of physiological changes lead to the cessation of feeding by the end of day 5. The fact that the maximal abundance of PLIN2 was observed around the time the critical weight is achieved led us to question whether the decline of PLIN2 at the end of the 5th instar was related to the levels of TG or to the developmental program. Development and growth, which are under complex hormonal regulation, are affected by nutritional changes (Nijhout et al., 2014). To further examine whether the decrease in PLIN2 levels at the end of the 5th instar was part of a developmental program or a consequence of the level of lipid accumulation, we carried out a similar study but feeding the insects on a natural diet consisting of tomato leaves. The rationale was to provide a diet with lower caloric content to slow down development and the process of fat accumulation. The caloric content of the leaf diet was estimated to be ~3.5-fold lower than that of the standard lab diet (Bell and Joachim, 1976). As shown in Fig 8, when insects were fed on leaves the accumulation of lipids occurred at a lower rate than in the animals raised in the standard diet (Fig 7). Under this regime (leaf diet), the 5th instar lasted nine days, four more days than it takes when insects are fed the standard diet. Nevertheless, at the end of the stage, the insects fed on leaves were leaner (5.8 g / insect) than those fed the standard diet (8.8g/insect). As shown in Fig 8, the gradual accumulation of TG observed with the leaf diet was accompanied by a gradual increase in PLIN2 protein levels. Unlike larvae feeding the high calorie diet (Fig 7), in this case the expression of PLIN2 showed a sustained increase through the nine-day period of the 5th instar (Fig 8). The fat body TG content was also significantly lower reaching a maximum value of ~20mg / fat body at the end of development, as compared to ~80mg / fat body in animals fed the standard diet. Animals fed on the leaf diet normally progressed through development (wandering, pupal and adult stages). Thus, these results suggest that the decrease in PLIN2 levels at the end of the 5th-instar in animals fed the standard diet (Fig 7) would be determined to a large extent by the level of fat reserves, rather than by the normal developmental program. Under both conditions, low or high calorie diets (Fig 7 and 8), PLIN2 levels paralleled the increase of TG up to a certain extent (~20 mg TG / fat body).
Figure 7. Protein levels of PLIN2 in 5th-larval fat body.
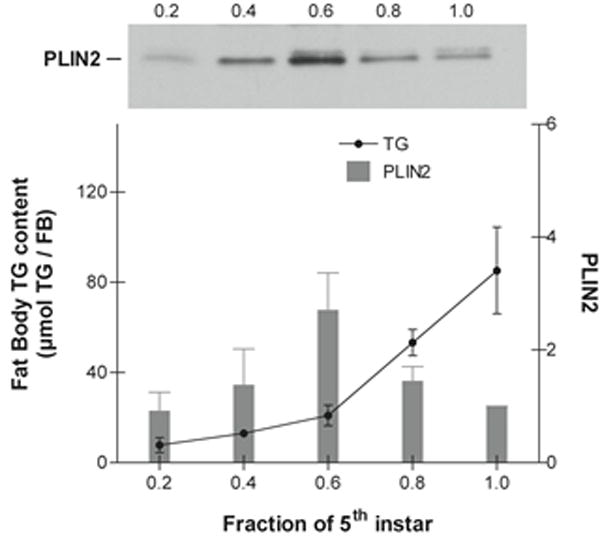
The protein level of lipid droplet-PLIN2 was determined by western blot analysis using ~20 μg of total protein as described in the Materials and Methods. PLIN2 levels from at least three independent sets of LD preparations were obtained. The data in the graph represent the PLIN2 levels (means ± SD) normalized to day 5. The age of the larvae is expressed as a fraction of the total length of the feeding period (5 days). A representative PLIN2 western blot image is shown in the figure. The content of fat body TG (mean ± SD, n=3) is expressed in μmol TG per FB.
Figure 8. Effect of low caloric diet on the lipid accumulation and PLIN2 protein levels in 5th-larval fat body.
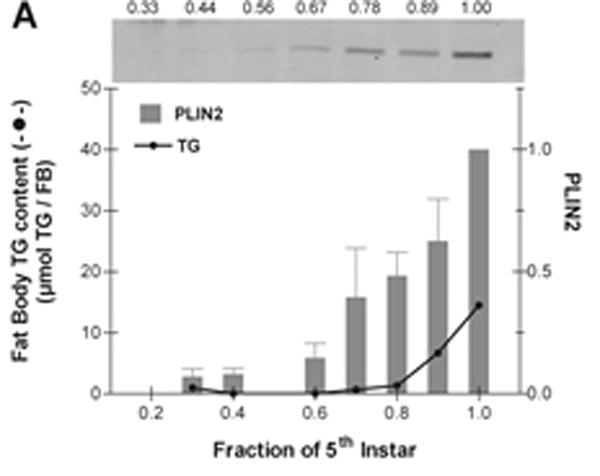
5th-instar larvae were fed on leaf diet. The content of fat body TG (mean ± SD, n=3) is expressed in μmol TG per FB; the error bars are not noticeable because they are shorter than the height of the symbol. The protein level of lipid droplet-PLIN2 was determined by western blot analysis using ~20 μg of total protein as described in the Materials and Methods. The data in the graph are the PLIN2 levels (mean ± SD, n=3) normalized to day 9. A representative western blot image is shown in the figure. The age of the larvae is expressed as a fraction of the total length of the feeding period (9 days).
3.5.1.1. Effect of Starvation and Re-feeding
We also investigated the effect of the nutritional status (starvation and re-feeding) in the expression of PLIN2. To this end, 5th instar larvae (day 2) fed on the standard diet were starved for 24h and subsequently fed standard diet for different periods of time (re-feeding). The levels of PLIN2 mRNA and protein were compared with those observed in larvae of the same age that continued feeding (Fed). DGAT mRNA levels were also determined to assess the ability of the fat body to synthesize TG. Starvation is expected to prevent TG synthesis and thus its accumulation in LDs. As expected, starvation promoted a sharp decrease in DGAT mRNA (Fig 9A) suggesting a decrease in TG synthesis. As shown in the Fig 9A, starvation also promotes a ~ 10-fold decrease in PLIN2 mRNA levels. Conversely, mRNA levels of PLIN2 and DGAT were rapidly restored after re-feeding, as the fat body regained the capacity to synthesize TG. Indicating a relatively fast protein turnover, the changes in PLIN2 mRNA levels were accompanied by similar changes in protein levels; thus, PLIN2 protein decreased to almost undetectable levels after 24h of starvation (Fig 9B) and gradually increased after re-feeding reaching values similar to those of the fed state only 6h after re-feeding (Fig 9B). This result suggests a tight relationship between the availability of nutrients, PLIN2 expression and the TG synthesizing activity of the larval fat body.
Figure 9. Effect of starvation and refeeding in the expression of PLIN2 in the fat body of 5th-instar larvae.
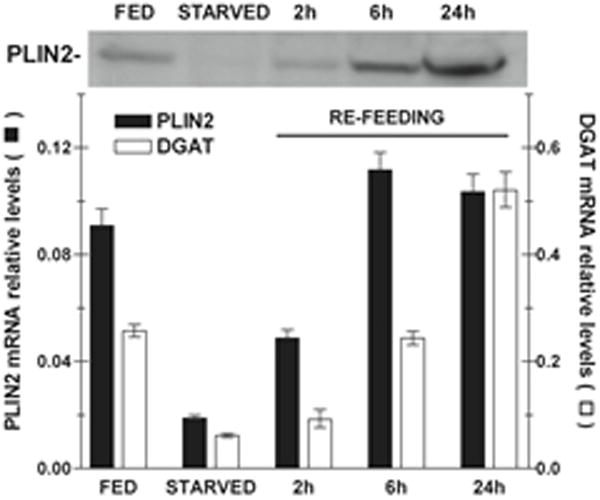
Total RNA and LDs were obtained from the fat body of at least three insects. FED: feeding 5th-instar larvae, day 2; STARVED: 5th-larvae, day 2, subjected to 24h starvation; REFEEDING: 5th-larvae, day 2, starved for 24h followed by refeeding for 2h, 3h and 6h. A) mRNA Levels: The relative levels of PLIN2 and DGAT mRNA were determined by qPCR using mRNA RpS3 as reference. The assay was performed by triplicate and the relative values are plotted as the mean ± SD. B) Representative western blot image obtained with 20μg of LDs proteins for fat bodies of Fed, Starved, and Refed (2h and 6h) insects.
It is worth emphasizing the contrasting difference of the changes in PLIN2 expression induced by starvation in larva (PLIN2 decrease) and adult (PLIN2 increase). The metabolic differences between the larval and adult stages of M. sexta, also translate to the response of the fat body to starvation. Adults never feed constantly, as larvae do, and they do not grow or accumulate reserves of any kind in the fat body. Larvae accumulate reserves but adults use the reserves stored during the larval growth. Larvae of M. sexta under starvation mobilize glycogen whereas adults mobilize lipids (Ziegler et al., 1990). Starvation produces a rapid activation of glycogen phosphorylase in larva (Siegert and Ziegler, 1983) resulting in constant trehalose level in the hemolymph (Gies et al., 1988; Ziegler, 1991). Moreover, there is no difference between the cytosolic lipase activities of fat body from fed or starved (24h) larvae of M. sexta (unpublished results). On the other hand, adults cannot maintain the trehalose levels under starvation and compensate by secreting large amounts of fat body lipids to the hemolymph (Ziegler, 1991). As a result, the fat body is highly lipolytic and the cytosolic lipase activity of adult fat body is high compared to larva (Arrese et al., 2010). The physiological differences between the larval and adult stages of M. sexta parallel the contrasting differences between the metabolisms of TG and glycogen in the response to starvation. Thus, in regard to PLIN2, as the lipolytic state increases in adults we also see an increase in DGAT and PLIN2 expression (Fig 6) even when the fat body is losing TG content. Contrarily, in larva, DGAT expression decreases in starvation -indicating no lipid synthesis- and PLIN2 reaches the lowest, undetectable, levels of expression (Fig 9B). We hypothesized that in the adult fat body under starvation PLIN2 is required for the storage of lipids produced by re-esterification of FAs generated from the high lipolysis, as discussed above.
3.5.2. Midgut
Dietary glycerides are hydrolyzed in the midgut lumen of Manduca larva into FA and glycerol, which are absorbed and rapidly incorporated into DG and TG. TG accounts for 76% of the midgut lipids serving as a reservoir from which DG is produced. DG, which does not accumulate in the cell, is produced at a rate corresponding with its export to hemolymph (Canavoso and Wells, 2000). Studies in Drosophila have also shown a large accumulation of LDs in the midgut epithelium when the transport of lipid to the fat body is impaired (Palm et al., 2012; Panakova et al., 2005). TG storage in midgut may also serve the purpose of minimizing the amount of DG accumulating in midgut to prevent toxic effects and yet efficiently absorb FA from the lumen (Canavoso and Wells, 2000; Soulages and Wells, 1994). Since PLIN1 is not detected in M. sexta midgut (data not shown), PLIN2 is the only Perilipin regulating the processes of accumulation and/or mobilization of TG in midgut. To examine the relationship between PLIN2 and lipid storage in the midgut, we determined the expression of PLIN2 in 5th instar larvae (day 2–3) in starvation and after re-feeding on the standard diet. Samples of midgut total RNA and LDs were prepared from fed and starved (24h starvation) animals and also after 2h, 6h and 24h from the initiation of re-feeding. Starvation promoted a large decrease in the expression of PLIN2 mRNA and protein (Fig 10). PLIN2 protein was practically undetectable after 24h of starvation (Fig 10B). This condition was quickly reversed when feeding was resumed. PLIN2 levels (protein and mRNA) showed a significant increase 2h after refeeding and reached the fed state levels 6h after refeeding began. Following 24h of starvation the levels of DGAT mRNA were also significantly reduced (Fig 10A), as expected due to lack of FA for the synthesis of TG. Changes in DGAT mRNA followed a similar pattern suggesting that changes in PLIN2 levels are associated with the storage of newly synthesized TG in the lipid droplet of midgut cells. The clear effect of the level of nutrients in the expression of PLIN2 and the correlation between PLIN2 and DGAT levels in the midgut was very similar to that observed in fat body of the young larva after starvation and refeeding (Fig 9).
Figure 10. Effect of starvation and refeeding in the expression of PLIN2 in the midgut of larvae.
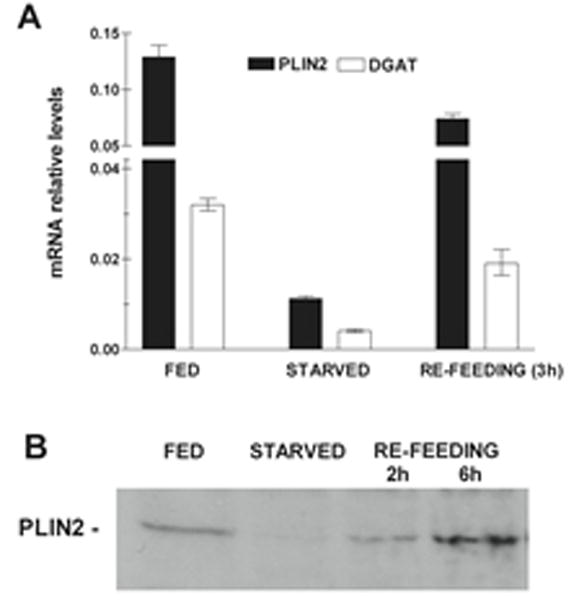
Total RNA and LDs were obtained from the midgut of at least three insects. FED: feeding 5th-instar larvae, day 2; STARVED: 5th-larvae, day 2, subjected to 24h starvation; REFEEDING: 5th-larvae, day 2, starved for 24h followed by refeeding for 2h, and 6h. A) mRNA Levels: The relative levels of PLIN2 and DGAT mRNA were determined by qPCR using mRNA RpS3 as reference. The assays were performed by triplicate and the relative values are plotted as the mean ± SD. B) PLIN2 western blot: Each gel lane was loaded with 20μg of LDs proteins.
3.6. Lipid Droplet Size
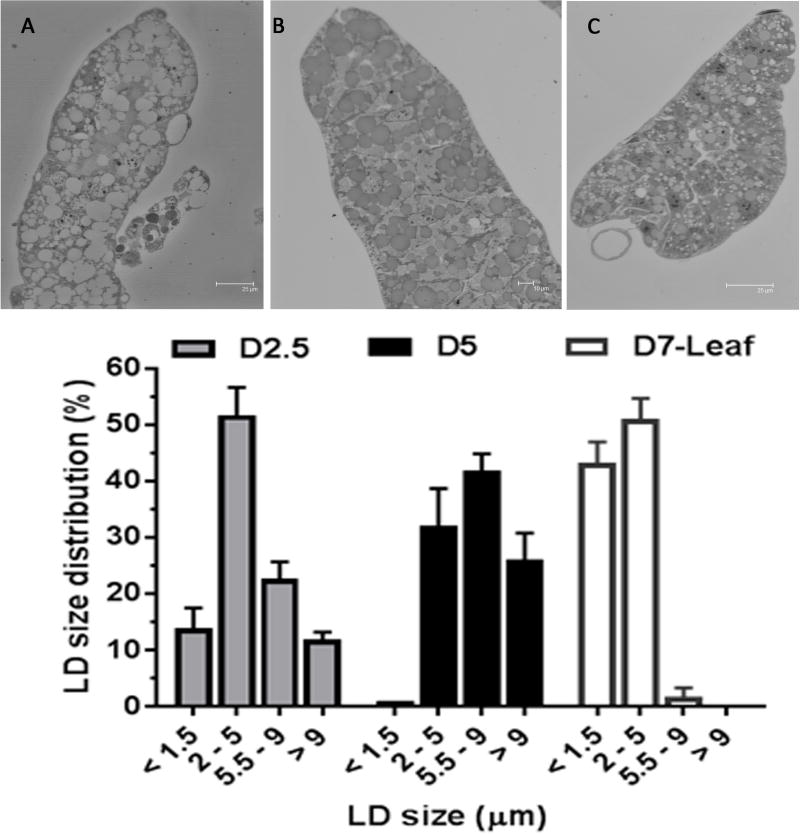
To better understand the relationship between MsPLIN2 and lipid accumulation in the fat body of the feeding larva, we examined the sizes of the fat body LDs in 5th instar larvae feeding regular diet at the middle (day 2.5, Fig 11A) and end of the feeding period (day 5, Fig 11B). The average lipid droplet size increases during the development. For example, 65.5 % of LDs have diameters lower than 5μm at day 2.5 whereas this size group represented 35% of the LD population at the end of the instar (Fig 11). Conversely, at the end of the period, when maximal lipid accumulation occurs, lipids are packed mainly in large LDs. The largest LD population (>9 μm) was significantly (p=0.0104) more abundant (26.1 ± 4.7 %) than at the midpoint (day 2.5) of the feeding stage (11.8 ± 1.3%). Thus, the decrease in PLIN2 protein that is observed in fat body of 5th-instar larva when comparing day 2.5 with day 5 (Fig 7) correlates with the decrease of the abundance of the smaller size present at day 5, or conversely, with the higher proportion of large LD found at day 5 (Fig 11). We also examined the LD distribution in 5th instar larvae feeding leaves at day 7 (Fig 11C). The lipid content in the fat body of these animals was lower than those of the day 2–3 of the larvae fed with the artificial diet (Fig 8). In this case 94% of LDs were smaller than 5μm adding support to the notion that at the beginning of the process of lipid accumulation lipids are packed in small droplets. The comparison of the distribution of LDs sizes and the occurrence of PLIN2 in M. sexta fat body of feeding larvae showed that PLIN2 abundance correlates with a higher proportion of smaller LDs.
Figure 11. Distribution of LDs sizes in the fat bodies of larva of M. sexta.
Representative images for each fat body type are shown above: (A)-5th-instar larva Day 2.5, (B) Day 5, and (C) 5th-instar larva fed on leaves, Day 7. Grey bars, black bars and white bars depict values (mean % ± SD) corresponding to samples shown in A, B, and C, respectively. The main proportion of very small LDs (<1.5μm) was found in 5th-instar larva fed on leaves, Day 7 (pD2.5 vs D5 < 0.05; pD2.5 vs D7< 0.0001; pD5 vs D7< 0.0001). The main proportion of medium size LDs (2 – 5 μm) were more abundant in Day 2.5 and larva fed on leaves-Day 7 (pD2.5 vs D5 < 0.05; pD2.5 vs D7 =ns; pD5 vs D7< 0.05). The main proportion of large LDs (5.5–9μm) was found in Day 5 (pD2.5 vs D5 < 0.001; pD2.5 vs D7< 0.005; pD5 vs D7< 0.0001). The main proportion of very large LDs (>9μm) were present in Day 5 (pD2.5 vs D5 < 0.05; pD2.5 vs D7< 0.05; pD5 vs D7< 0.0001).
LDs are subjected to fast and substantial variations in size and number in response to hormones and the availability of nutrients. A thirty-fold increase in LD-volume was observed within hours when Drosophila S2 cells were accumulating TG following incubation with oleic acid (Guo et al., 2008). Mammalian LDs can grow transferring lipids between droplets through specific contact sites (Grahn et al., 2013). On the other hand, TG hydrolysis induces LD fragmentation (Marcinkiewicz et al., 2006; Mizushima, 2007) and the formation of small and micro LDs (< 1 μm) (Ariotti et al., 2012; Hashimoto et al., 2012; Paar et al., 2012). Smaller LDs would form from the fission of large LDs, whereas micro LDs would be formed with TG produced by the re-esterification of FA (Hashimoto et al., 2012). Moreover, the distribution and/or abundance of PAT proteins among LDs of different sizes are not homogenous (Heid et al., 2014). ADRP has been found to be predominately associated with small LDs (Wolins et al., 2006). Studies in Drosophila have shown that PLIN2 is more abundant in small LDs (Bi et al., 2012; Diaconeasa et al., 2013). The analysis of the LD size distribution in the M. sexta fat body suggested an inverse relationship between LD size and content of PLIN2. Because the maximal content of PLIN2 occurred when smaller droplets were more abundant, it seems that PLIN2 is mainly associated with small LDs. This observation is in line with previous findings in Drosophila (Bi et al., 2012; Diaconeasa et al., 2013). On the other hand, the decrease of PLIN2 abundance when TG is stored in large cytosolic droplets in the fat body of late 5th instar larvae suggests alternative mechanism/s protecting TG from the action of lipases beyond the PLIN2 barrier on the lipid droplet surface. Moreover, the decrease of PLIN2 when TG content reached the maximum also suggests that the lipid level stored in the adipocyte of M. sexta could be a factor modulating PLIN2 abundance.
4. CONCLUSIONS
MsPLIN2 was exclusively associated with the LDs (Fig 3) and its abundance in fat body correlated with the abundance of smaller LDs (< 5μm) (Fig 11). In feeding larva, MsPLIN2 was mainly expressed when the fat body is synthesizing TG (Fig 7–9). These three results are in line with previous studies in Drosophila showing that DmPLIN2 was required for normal TG storage by acting as barrier to lipases (Gronke et al., 2003; Teixeira et al., 2003) and it was preferentially found in small droplets (Bi et al., 2012; Diaconeasa et al., 2013). In the midgut- a tissue in which PLIN2 is the only occurring PAT protein- the expression of PLIN2 also paralleled TG synthesis, suggesting for the first time a role for MsPLIN2 in TG storage in the midgut of an insect (Fig 10).
The study on MsPLIN2 highlighted two novel and interesting aspects. First, MsPLIN2 was most abundant in the adult fat body (Fig 4–5), which is characterized by a high rate TG hydrolysis, lipid mobilization and also a fast glyceride turnover. Whether MsPLIN2 is directly involved in lipolysis, the secretion of lipids or the insertion of re-synthesized glycerides in LDs is unknown. Nonetheless, since adult M. sexta is losing TG, this observation shows that MsPLIN2 expression by itself does not guaranteed an increase in fat accumulation. However, in the adult fat body MsPLIN2 could be associated with a pool of LDs not used for lipolysis. As discussed above, such pool could derive from re-esterified FA produced during lipolysis. The role of MsPLIN2 in fat body of adults would still be in connection to lipid storage, yet the fat body is not accumulating but rather losing lipids through FA oxidation and secretion to the hemolymph.
The second remarkably aspect of the MsPLIN2 study was the decline of PLIN2 when the larval fat body was storing TG at the maximal capacity (the end of the 5th instar of larvae feeding the artificial diet) (Fig 7). Some of the possible implications of this finding are: 1- The level of lipid storage could modulate PLIN2 expression and /or degradation: PLIN2 could be required for the storage of TG in small LDs to a certain lipid level, beyond which the size of LDs would increase and the abundance of PLIN2 decline. Likewise, the lipid level stored in the adipocyte of M. sexta could be a factor controlling PLIN2 abundance; 2- MsPLIN2 is not required to preserve TG in large droplets given the inverse relationship between PLIN2 abundance and LD size; 3- Alternative mechanism/s to shield TG from the action of lipases apart from PLIN2 must be present in the fat body of the larva of M. sexta. In the absence of PLIN2 lipids must still be protected from lipases suggesting alternative mechanism/s perhaps involving a different PAT protein. Current work dealing with some of these points will be reported in a separate study. Altogether, we found that MsPLIN2 is needed when the synthesis of glycerides, DG and TG, is active disregard of whether the insect is accumulating or consuming TG. The new findings even if they were specific to this model system, which is characterized by a large capacity to store fat in the larval stage and a very dynamic lipid mobilization in the adult, contribute to a better understanding on the role of PLIN2 in TG metabolism in insects.
Supplementary Material
Figure S1. Nucleotide and deduced amino acid sequence from M. sexta PLIN2. cDNA nucleotide (1-1350) sequence, JF809664.1, is shown above the deduced amino acid sequence (1-304), AEJ33049. Amino acid residues are aligned with the second nucleotide of each codon. The amino acid sequences underlined represents the matched peptides obtained from the MS/MS analysis of M. sexta ovary lipid droplets. These peptides are 100% identical to Bombyx mori Lsd2 (NP_001138804). The stop codon TAA is marked by short dash line.
Figure S2. Protein sequence alignment of vertebrate PLIN2 and MsexPLIN2. Alignments were produced with Clustal Omega. The sequences identifiers and abbreviations used are: HsPLIN2: Homo sapiens perilipin-2 (NP_001113.2); MmPLIN2: Mus musculus perilipin-2 (NP_031434.3); XlPLIN2: Xenopus laevis perilipin 2 (NP_001081960.1); MsexPLIN2: Manduca sexta lipid storage droplet protein 2 or Perilipin 2 (AEJ33049.1).
Figure S3. A. Protein sequence alignment of PLIN2 from 13 insect species. Alignments were produced with Clustal Omega. The sequences identifiers and abbreviations used are: Hsal: Harpegnathos saltator (EFN86125.1); Mr: Megachile rotundata (XP_003703225.1); Bi: Bombus impatiens (XP_003485735.1); Bt: Bombus terrestris (XP_003397531.1); Am: Apis mellifera (XP_003249235.1); Af: Apis florea (XP_003697663.1); Bm: Bombix mori (NP_001138804.1); Msex: Manduca sexta (AEJ33049.1); Cc: Ceratitis capitata (XP_004524967.1); Dm: Drosophila melanogaster (NP_001036276.1); Md: Musca domestica (XP_005188653.1); Aa: Aedes aegypti (XP_001658058.1); Ag: Anopheles gambiae (XP_310971.5).
Figure S4. Identity matrix of PLIN2 proteins. The sequences identifiers and abbreviations used are: Hsal: Harpegnathos saltator (EFN86125.1); Mr: Megachile rotundata (XP_003703225.1); Bi: Bombus impatiens (XP_003485735.1); Bt: Bombus terrestris (XP_003397531.1); Am: Apis mellifera (XP_003249235.1); Af: Apis florea (XP_003697663.1); Bm: Bombix mori (NP_001138804.1); Msex: Manduca sexta (AEJ33049.1); Cc: Ceratitis capitata (XP_004524967.1); Dm: Drosophila melanogaster (NP_001036276.1); Md: Musca domestica (XP_005188653.1); Aa: Aedes aegypti (XP_001658058.1); Ag: Anopheles gambiae (XP_310971.5).
Figure S5. Supplement to Figure 7, Protein levels of PLIN2 in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) is shown in the figure. Labels: M, molecular weight marker; 1, 2, 3, 4, and 5 correspond to LDs samples from the fat body of 5th instar larvae on day 1, 2, 3,4 and 5, respectively. Alternatively, when the age of the larvae is expressed as a fraction of the total length of the feeding period (5 days), Day 1,2,3,4, and 5 of the 5th instar corresponds to fraction 0.2, 0.4, 0.6, 0.8 and 1, respectively.
Figure S6. Supplement to Figure 8, Effect of low caloric diet on the lipid accumulation and PLIN2 protein levels in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) is shown in the figure. Labels: M, molecular weight marker; 1, 2, 3, 4, 5, 6 and 7 correspond to LDs samples from the fat body of 5th instar larvae on day 3, 4, 5, 6, 7 and 8, respectively. Alternatively, when the age of the larvae is expressed as a fraction of the total length of the feeding period (9 days), Day 3, 4, 5, 6, 7, 8 and 9 of the 5th instar corresponds to fraction 0.33, 0.44, 0.56, 0.76, 0.89 and 1, respectively.
Figure S7. Supplement Figure 9, Effect of starvation and refeeding in the expression of PLIN2 in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) of the LDs isolated from the fat body of 5th instar larvae under the following conditions: Fed, day 2 feeding (lane 1); Starved, day 2 subjected to 24h starvation (lane 2); Refeeding, day 2, starved for 24h followed by refeeding for 2h (lane 3); 3h (lane 4); and 6h (lane 5) is shown in the figure.
Figure S8. Supplement Figure 10, Effect of starvation and refeeding in the expression of PLIN2 in the midgut of larvae. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) of the LDs isolated from the fat body of 5th instar larvae under the following conditions: Fed, feeding day 2 (lane 1); Starved, day 2, subjected to 24h starvation (lane 2); Refeeding, day 2, starved for 24h followed by refeeding for 2h (lane 3), 6h (lane 4) and 24h (lane 5).
Research Highlights.
PLIN2 is known for playing a role in the accumulation of fat presumably by acting as a barrier to lipases.
MsPLIN2 protein and mRNA levels change very rapidly when the availability of FA is increased or reduced.
MsPLIN2 declined when lipid levels reached a maximum in larval fat body implying the occurrence of alternative mechanism/s to shield TG.
Acknowledgments
This project was supported by Oklahoma Agricultural Experiment Station (OKL02398), Oklahoma State University and by Grant Number R01GM064677 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official use of the National Institute of General Medical Sciences or the National Institutes of Health. The authors are grateful to Janet Rogers and Lisa Whitworth for their excellent technical assistance in the experiments of mass spectrometry and microscopy, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariotti N, Murphy S, Hamilton NA, Wu L, Green K, Schieber NL, Li P, Martin S, Parton RG. Postlipolytic insulin-dependent remodeling of micro lipid droplets in adipocytes. Molecular biology of the cell. 2012;23:1826–1837. doi: 10.1091/mbc.E11-10-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Howard AD, Patel RT, Rimoldi OJ, Soulages JL. Mobilization of lipid stores in Manduca sexta: cDNA cloning and developmental expression of fat body triglyceride lipase, TGL. Insect Biochem Mol Biol. 2010;40:91–99. doi: 10.1016/j.ibmb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Rivera L, Hamada M, Mirza S, Hartson SD, Weintraub S, Soulages JL. Function and structure of lipid storage droplet protein 1 studied in lipoprotein complexes. Archives of biochemistry and biophysics. 2008a;473:42–47. doi: 10.1016/j.abb.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Rivera L, Hamada M, Soulages JL. Purification and characterization of recombinant lipid storage protein-2 from Drosophila melanogaster. Protein Pept Lett. 2008b;15:1027–1032. doi: 10.2174/092986608785849191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Rojas-Rivas BI, Wells MA. The use of decapitated insects to study lipid mobilization in adult Manduca sexta: effects of adipokinetic hormone and trehalose on fat body lipase activity. Insect Biochem Mol Biol. 1996;26:775–782. doi: 10.1016/s0965-1748(96)00024-0. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Saudale FZ, Soulages JL. Lipid Droplets as Signaling Platforms Linking Metabolic and Cellular Functions. Lipid insights. 2014;7:7–16. doi: 10.4137/LPI.S11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annual review of entomology. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Wells MA. Adipokinetic hormone-induced lipolysis in the fat body of an insect, Manduca sexta: synthesis of sn-1,2-diacylglycerols. J Lipid Res. 1997;38:68–76. [PubMed] [Google Scholar]
- Attrill H, Falls K, Goodman JL, Millburn GH, Antonazzo G, Rey AJ, Marygold SJ, FlyBase C. FlyBase: establishing a Gene Group resource for Drosophila melanogaster. Nucleic acids research. 2016;44:D786–792. doi: 10.1093/nar/gkv1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilstein F, Bouchoux J, Rousset M, Demignot S. Proteomic analysis of lipid droplets from Caco-2/TC7 enterocytes identifies novel modulators of lipid secretion. PLos One. 2013;8:e53017–e53017. doi: 10.1371/journal.pone.0053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Annals of the Entomological Society of America. 1976;69:365–373. [Google Scholar]
- Beller M, Bulankina AV, Hsiao HH, Urlaub H, Jackle H, Kuhnlein RP. PERILIPIN-Dependent Control of Lipid Droplet Structure and Fat Storage in Drosophila. Cell Metab. 2010;12:521–532. doi: 10.1016/j.cmet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Characterization of the Drosophila lipid droplet subproteome. Molecular & cellular proteomics : MCP. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- Bi J, Xiang Y, Chen H, Liu Z, Gronke S, Kuhnlein RP, Huang X. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. Journal of cell science. 2012;125:3568–3577. doi: 10.1242/jcs.101329. [DOI] [PubMed] [Google Scholar]
- Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- Canavoso LE, Wells MA. Metabolic pathways for diacylglycerol biosynthesis and release in the midgut of larval Manduca sexta. Insect Biochemistry and Molecular Biology. 2000;30:1173–1180. doi: 10.1016/s0965-1748(00)00094-1. [DOI] [PubMed] [Google Scholar]
- Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol. 2006;16:1783–1795. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- Diaconeasa B, Mazock GH, Mahowald AP, Dubreuil RR. Genetic Studies of Spectrin in the Larval Fat Body of Drosophila melanogaster: Evidence for a Novel Lipid Uptake Apparatus. Genetics. 2013;195:871–881. doi: 10.1534/genetics.113.155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer RGH, Matthews JR. Patterns of lipid distribution and utilization in insects. American Zoologist. 1976;16:733–745. [Google Scholar]
- Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauny JD, Silber J, Zider A. Drosophila Lipid Storage Droplet 2 gene (Lsd-2) is expressed and controls lipid storage in wing imaginal discs. Dev Dyn. 2005;232:725–732. doi: 10.1002/dvdy.20277. [DOI] [PubMed] [Google Scholar]
- Fernando-Warnakulasuriya GJP, Tsuchida K, Wells MA. Effect of dietary lipid content on lipid transport and storage during larval development of Manduca sexta. Insect Biochemistry. 1988;18:211–214. [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gies A, Fromm T, Ziegler R. Energy-Metabolism in Starving Larvae of Manduca-Sexta. Comp Biochem Phys A. 1988;91:549–555. [Google Scholar]
- Grahn THM, Zhang Y, Lee MJ, Sommer AG, Mostoslavsky G, Fried SK, Greenberg AS, Puri V. FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochemical and Biophysical Research Communications. 2013;432:296–301. doi: 10.1016/j.bbrc.2013.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, Kuhnlein RP. Control of fat storage by a Drosophila PAT domain protein. Curr Biol. 2003;13:603–606. doi: 10.1016/s0960-9822(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Segawa H, Okuno M, Kano H, Hamaguchi HO, Haraguchi T, Hiraoka Y, Hasui S, Yamaguchi T, Hirose F, Osumi T. Active involvement of micro-lipid droplets and lipid-droplet-associated proteins in hormone-stimulated lipolysis in adipocytes. Journal of cell science. 2012;125:6127–6136. doi: 10.1242/jcs.113084. [DOI] [PubMed] [Google Scholar]
- Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y, Kuhn C, Franke WW. On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS One. 2014;9:e90386. doi: 10.1371/journal.pone.0090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:7856–7860. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Arrese EL, Cao X, Chen YR, Chellapilla S, Goldsmith MR, Grosse-Wilde E, Heckel DG, Herndon N, Jiang H, Papanicolaou A, Qu J, Soulages JL, Vogel H, Walters J, Waterhouse RM, Ahn SJ, Almeida FC, An C, Aqrawi P, Bretschneider A, Bryant WB, Bucks S, Chao H, Chevignon G, Christen JM, Clarke DF, Dittmer NT, Ferguson LC, Garavelou S, Gordon KH, Gunaratna RT, Han Y, Hauser F, He Y, Heidel-Fischer H, Hirsh A, Hu Y, Jiang H, Kalra D, Klinner C, Konig C, Kovar C, Kroll AR, Kuwar SS, Lee SL, Lehman R, Li K, Li Z, Liang H, Lovelace S, Lu Z, Mansfield JH, McCulloch KJ, Mathew T, Morton B, Muzny DM, Neunemann D, Ongeri F, Pauchet Y, Pu LL, Pyrousis I, Rao XJ, Redding A, Roesel C, Sanchez-Gracia A, Schaack S, Shukla A, Tetreau G, Wang Y, Xiong GH, Traut W, Walsh TK, Worley KC, Wu D, Wu W, Wu YQ, Zhang X, Zou Z, Zucker H, Briscoe AD, Burmester T, Clem RJ, Feyereisen R, Grimmelikhuijzen CJ, Hamodrakas SJ, Hansson BS, Huguet E, Jermiin LS, Lan Q, Lehman HK, Lorenzen M, Merzendorfer H, Michalopoulos I, Morton DB, Muthukrishnan S, Oakeshott JG, Palmer W, Park Y, Passarelli AL, Rozas J, Schwartz LM, Smith W, Southgate A, Vilcinskas A, Vogt R, Wang P, Werren J, Yu XQ, Zhou JJ, Brown SJ, Scherer SE, Richards S, Blissard GW. Multifaceted biological insights from a draft genome sequence of the tobacco hornworm moth, Manduca sexta. Insect Biochem Mol Biol. 2016;76:118–147. doi: 10.1016/j.ibmb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin A directs lipid droplet fragmentation and dispersion. Journal of Biological Chemistry. 2006;281:11901–11909. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Zabaronick W, Schaack J, Orlicky DJ. Lipid droplet targeting domains of adipophilin. J Lipid Res. 2003;44:668–673. doi: 10.1194/jlr.C200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes & Development. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Nijhout H, Davidowitz G, Roff D. A quantitative analysis of the mechanism that controls body size in Manduca sexta. Journal of Biology. 2006;5:1–15. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF, Riddiford LM, Mirth C, Shingleton AW, Suzuki Y, Callier V. The developmental control of size in insects. Wires Dev Biol. 2014;3:113–134. doi: 10.1002/wdev.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi A, Hull JJ, Kaji M, Hashimoto K, Lee JM, Tsuneizumi K, Suzuki T, Dohmae N, Matsumoto S. Hormone signaling linked to silkmoth sex pheromone biosynthesis involves Ca2+/calmodulin-dependent protein kinase II-mediated phosphorylation of the insect PAT family protein Bombyx mori lipid storage droplet protein-1 (BmLsd1) J Biol Chem. 2011;286:24101–24112. doi: 10.1074/jbc.M111.250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paar M, Jungst C, Steiner NA, Magnes C, Sinner F, Kolb D, Lass A, Zimmermann R, Zumbusch A, Kohlwein SD, Wolinski H. Remodeling of Lipid Droplets during Lipolysis and Growth in Adipocytes. Journal of Biological Chemistry. 2012;287:11164–11173. doi: 10.1074/jbc.M111.316794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S. Lipoproteins in Drosophila melanogaster--assembly, function, and influence on tissue lipid composition. PLoS genetics. 2012;8:e1002828. doi: 10.1371/journal.pgen.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Patel RT, Soulages JL, Hariharasundaram B, Arrese EL. Activation of the lipid droplet controls the rate of lipolysis of triglycerides in the insect fat body. J Biol Chem. 2005;280:22624–22631. doi: 10.1074/jbc.M413128200. [DOI] [PubMed] [Google Scholar]
- Penno A, Hackenbroich G, Thiele C. Phospholipids and lipid droplets. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2013;1831:589–594. doi: 10.1016/j.bbalip.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Richardson KC, Jarett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain technology. 1960;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Analytical biochemistry. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Siegert K, Ziegler R. A hormone from the corpora cardiaca controls fat body glycogen phosphorylase during starvation in tobacco hornworm larvae. Nature. 1983;301:526–527. [Google Scholar]
- Soulages JL, Firdaus SJ, Hartson S, Chen X, Howard AD, Arrese EL. Developmental changes in the protein composition of Manduca sexta lipid droplets. Insect Biochemistry and Molecular Biology. 2012;42:305–320. doi: 10.1016/j.ibmb.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulages JL, Wu Z, Firdaus SJ, Mahalingam R, Arrese EL. Monoacylglycerol and diacylglycerol acyltransferases and the synthesis of neutral glycerides in Manduca sexta. Insect Biochem Mol Biol. 2015;62:194–210. doi: 10.1016/j.ibmb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang XZ, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. Journal of Biological Chemistry. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- Storey SM, McIntosh AL, Senthivinayagam S, Moon KC, Atshaves BP. The phospholipid monolayer associated with perilipin-enriched lipid droplets is a highly organized rigid membrane structure. American journal of physiology Endocrinology and metabolism. 2011;301:E991–E1003. doi: 10.1152/ajpendo.00109.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. Iubmb Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Rabouille C, Rorth P, Ephrussi A, Vanzo NF. Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech Dev. 2003;120:1071–1081. doi: 10.1016/s0925-4773(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Wells MA. Digestion, Absorption, Transport and Storage of Fat during the Last Larval Stadium of Manduca-Sexta - Changes in the Role of Lipophorin in the Delivery of Dietary-Lipid to the Fat-Body. Insect Biochemistry. 1988;18:263–268. [Google Scholar]
- Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, Gindhart JG, Gross SP. Regulation of lipid-droplet transport by the perilipin homolog LSD2. Curr Biol. 2005;15:1266–1275. doi: 10.1016/j.cub.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Ziegler R. Changes in Lipid and Carbohydrate Metabolism during Starvation in Adult Manduca sexta. J Comp Physiol B. 1991;161:125–131. doi: 10.1007/BF00262874. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Eckart K, Law JH. Adipokinetic hormone controls lipid metabolism in adults and carbohydrate metabolism in larvae of Manduca sexta. Peptides. 1990;11:1037–1040. doi: 10.1016/0196-9781(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Willingham LA, Sanders SJ, Tamensmith L, Tsuchida K. APOLIPOPHORIN-III AND ADIPOKINETIC HORMONE IN LIPID-METABOLISM OF LARVAL MANDUCA SEXTA. Insect Biochemistry and Molecular Biology. 1995;25:101–108. doi: 10.1016/0965-1748(94)00039-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Nucleotide and deduced amino acid sequence from M. sexta PLIN2. cDNA nucleotide (1-1350) sequence, JF809664.1, is shown above the deduced amino acid sequence (1-304), AEJ33049. Amino acid residues are aligned with the second nucleotide of each codon. The amino acid sequences underlined represents the matched peptides obtained from the MS/MS analysis of M. sexta ovary lipid droplets. These peptides are 100% identical to Bombyx mori Lsd2 (NP_001138804). The stop codon TAA is marked by short dash line.
Figure S2. Protein sequence alignment of vertebrate PLIN2 and MsexPLIN2. Alignments were produced with Clustal Omega. The sequences identifiers and abbreviations used are: HsPLIN2: Homo sapiens perilipin-2 (NP_001113.2); MmPLIN2: Mus musculus perilipin-2 (NP_031434.3); XlPLIN2: Xenopus laevis perilipin 2 (NP_001081960.1); MsexPLIN2: Manduca sexta lipid storage droplet protein 2 or Perilipin 2 (AEJ33049.1).
Figure S3. A. Protein sequence alignment of PLIN2 from 13 insect species. Alignments were produced with Clustal Omega. The sequences identifiers and abbreviations used are: Hsal: Harpegnathos saltator (EFN86125.1); Mr: Megachile rotundata (XP_003703225.1); Bi: Bombus impatiens (XP_003485735.1); Bt: Bombus terrestris (XP_003397531.1); Am: Apis mellifera (XP_003249235.1); Af: Apis florea (XP_003697663.1); Bm: Bombix mori (NP_001138804.1); Msex: Manduca sexta (AEJ33049.1); Cc: Ceratitis capitata (XP_004524967.1); Dm: Drosophila melanogaster (NP_001036276.1); Md: Musca domestica (XP_005188653.1); Aa: Aedes aegypti (XP_001658058.1); Ag: Anopheles gambiae (XP_310971.5).
Figure S4. Identity matrix of PLIN2 proteins. The sequences identifiers and abbreviations used are: Hsal: Harpegnathos saltator (EFN86125.1); Mr: Megachile rotundata (XP_003703225.1); Bi: Bombus impatiens (XP_003485735.1); Bt: Bombus terrestris (XP_003397531.1); Am: Apis mellifera (XP_003249235.1); Af: Apis florea (XP_003697663.1); Bm: Bombix mori (NP_001138804.1); Msex: Manduca sexta (AEJ33049.1); Cc: Ceratitis capitata (XP_004524967.1); Dm: Drosophila melanogaster (NP_001036276.1); Md: Musca domestica (XP_005188653.1); Aa: Aedes aegypti (XP_001658058.1); Ag: Anopheles gambiae (XP_310971.5).
Figure S5. Supplement to Figure 7, Protein levels of PLIN2 in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) is shown in the figure. Labels: M, molecular weight marker; 1, 2, 3, 4, and 5 correspond to LDs samples from the fat body of 5th instar larvae on day 1, 2, 3,4 and 5, respectively. Alternatively, when the age of the larvae is expressed as a fraction of the total length of the feeding period (5 days), Day 1,2,3,4, and 5 of the 5th instar corresponds to fraction 0.2, 0.4, 0.6, 0.8 and 1, respectively.
Figure S6. Supplement to Figure 8, Effect of low caloric diet on the lipid accumulation and PLIN2 protein levels in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) is shown in the figure. Labels: M, molecular weight marker; 1, 2, 3, 4, 5, 6 and 7 correspond to LDs samples from the fat body of 5th instar larvae on day 3, 4, 5, 6, 7 and 8, respectively. Alternatively, when the age of the larvae is expressed as a fraction of the total length of the feeding period (9 days), Day 3, 4, 5, 6, 7, 8 and 9 of the 5th instar corresponds to fraction 0.33, 0.44, 0.56, 0.76, 0.89 and 1, respectively.
Figure S7. Supplement Figure 9, Effect of starvation and refeeding in the expression of PLIN2 in 5th-larval fat body. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) of the LDs isolated from the fat body of 5th instar larvae under the following conditions: Fed, day 2 feeding (lane 1); Starved, day 2 subjected to 24h starvation (lane 2); Refeeding, day 2, starved for 24h followed by refeeding for 2h (lane 3); 3h (lane 4); and 6h (lane 5) is shown in the figure.
Figure S8. Supplement Figure 10, Effect of starvation and refeeding in the expression of PLIN2 in the midgut of larvae. A representative PLIN2 western blot (right panel) image with the corresponding Ponceau S staining (left panel) of the LDs isolated from the fat body of 5th instar larvae under the following conditions: Fed, feeding day 2 (lane 1); Starved, day 2, subjected to 24h starvation (lane 2); Refeeding, day 2, starved for 24h followed by refeeding for 2h (lane 3), 6h (lane 4) and 24h (lane 5).