Abstract
The largest arthropod cuticular protein family, CPR, has the Rebers and Riddiford (R&R) Consensus that in an extended form confers chitin-binding properties. Two forms of the Consensus, RR-1 and RR-2, have been recognized and initial data suggested that the RR-1 and RR-2 proteins were present in different regions within the cuticle itself. Thus, RR-2 proteins would contribute to exocuticle that becomes sclerotized, while RR-1s would be found in endocuticle that remains soft. An alternative, and more common, suggestion is that RR-1 proteins are used for soft, flexible cuticles such as intersegmental membranes, while RR-2s are associated with hard cuticle such as sclerites and head capsules. We used TEM immunogold detection to localize the position of several RR-1 and RR-2 proteins in the cuticle of Anopheles gambiae. RR-1s were localized in the procuticle of the soft intersegmental membrane except for one protein found in the endocuticle of hard cuticle. RR-2s were consistently found in hard cuticle and not in flexible cuticle. All RR-2 antibodies localized to the exocuticle and four out of six were also found in the endocuticle. Hence the location of RR-1s and RR-2s depends more on properties of individual proteins than on either hypothesis.
Keywords: Rebers & Riddiford Consensus, EM immunolocalization, Endocuticle, Exocuticle, Sclerite, Intersegmental membrane
1. Introduction
1.1. Introducing RR-1 and RR-2
The majority of cuticular proteins (CPs) in every arthropod species examined to date belong to the CPR family named after the R&R Consensus first recognized by Rebers and Riddiford (1988). Subsequent to the first description, the Consensus region has been modified by adding amino acids upstream of a 24 amino acid core and deleting some downstream. The extended Consensus recognized as pfam00379 (Chitin_bind_4) now has about 53 amino acids and has been shown to be necessary and sufficient for chitin binding, first by Rebers and Willis (2001) and subsequently confirmed by several others (Togawa et al., 2004, 2007; Qin et al., 2009). Other methods have been used to confirm the chitinbinding properties of CPRs (Tang et al., 2010; Dong et al., 2016). Three distinct forms of the Consensus were recognized and named by Anderson (1998, 2000): RR-1, RR-2 and RR-3. RR-3 has been assigned to few proteins, is not clearly defined (Ioannidou et al., 2014), and will not be discussed further. Two Web sites allow one to classify CPRs (Cuticle DB: http://bioinformatics2.biol.uoa.gr/cuticleDB/index.jsp and CutProtFam-Pred: http://aias.biol.uoa.gr/CutProtFam-Pred/home.php). The second site uses the same Hidden Markov Model method for group prediction as the first but provides scores that enable one to assess the accuracy of the assignment as well as to identify additional families of CPs (Magkrioti et al., 2004; Ioannidou et al., 2014). The Consensus region of RR-2s is highly conserved. Fig.1 provides WebLogos (Crooks et al., 2004) for RR-1 and RR-2 CPs from Anopheles gambiae. It also compares the WebLogos of the RR-2 form of the Consensus from An. gambiae with that from 62 holometabolous insects. Previously published WebLogos (Willis, 2010) showed that the high similarity in the RR-2 Consensus extends to Crustacea and Chelicerata. In contrast, the Consensus region of RR-1s is variable in length and less conserved in sequence in An. gambiae and even more variable when other species are compared (Supplementary File 1A; Willis, 2010). In general, RR-1 proteins have more acidic isoelectric points and fewer histidines, but only the Consensus region is appropriate for group assignment. While it is tempting to speculate that the uniformity among the Consensus of RR-2s contributes to their participation in hard cuticle, it is important to note that the range of lengths of the mature proteins is greater in RR-2s than in RR-1s (Supplementary File 1A). Given the predominance of CPRs in the cuticulome and the consistent differences between proteins classified as either RR-1 or RR-2, we need to learn more about how they participate in cuticle.
Fig. 1.
WebLogos showing difference between RR-1 and RR-2 proteins from Anopheles gambiae. WebLogos were constructed at http://weblogo.berkeley.edu/logo.cgi. Additional information on the two groups is in Supplementary File 1A.
Eight sequence clusters, groups of genes, generally linked, that code for proteins that have a high degree of similarity account for 66 of the 101 RR-2 genes in An. gambiae (Cornman et al., 2008; Cornman and Willis, 2008). There are 42 CPRs that have been classified as RR-1.
1.2. Cuticle nomenclature
We will follow the terminology proposed by Locke (2001) for naming the regions of cuticle. From the apical surface: the envelope,10–30 nm; epicuticle, about 1 µm in thickness and chitin-free; the procuticle, the region that combines chitin and CPs. The procuticle has been further subdivided into exo- and endo-cuticle. Between the procuticle and epidermis, sometimes one sees an assembly zone where chitin and proteins are presumed to first interact. Definitions of these divisions differ. Some call the pigmented, sclerotized region exocuticle and the region closest to the epidermis endocuticle. Others, including the authors of this paper, prefer to use the term exocuticle for the region secreted prior to ecdysis with the endocuticle being synonymous with post-ecdysial cuticle.
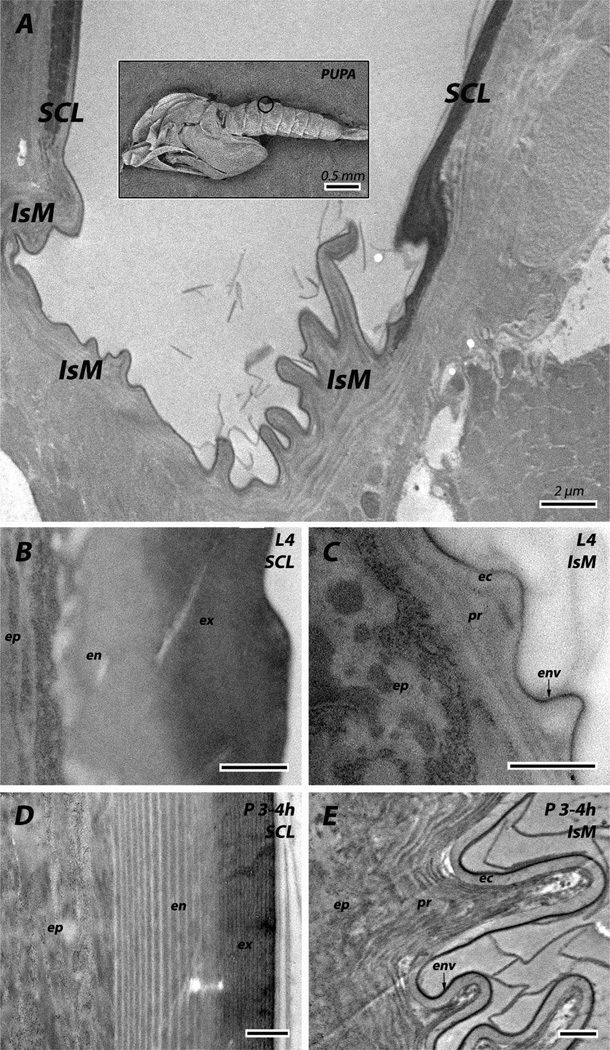
We did not see distinguishing morphological characteristics between the pre- and post-ecdysial cuticle of the intersegmental membranes or the soft cuticle of the adult head, so we just call this region procuticle. We also could not distinguish between envelope and epicuticle in sclerites (Fig. 2).
Fig. 2.
Overview of the abdominal cuticle. Cuticle of sclerites (SCL) and intersegmental membranes (IsM) are somewhat different in 4th instar larvae (L4) and pupae 3–4 h after pupation (P 3–4h). ec: epicuticle; en: endocuticle; env: envelope; ep: epidermis; ex: exocuticle; pr: procuticle. Scale bars for B–E are 500 nm.
1.3. Differentiation between RR-1 and RR-2 based on location within the cuticle
The first paper to define the RR-1 and RR-2 groups of CPRs (Andersen,1998) noted the difference between proteins used in the exo- (pre-ecdysial) and endo- (post-ecdysial) cuticles. Prior to their work, several studies had found a marked change in protein composition between pre- and post-ecdysial cuticle (e.g. Roberts and Willis, 1980; Andersen and Højrup, 1987; Lemoine et al., 1989, 1990; Nøhr and Andersen, 1993; Jensen et al., 1997). Andersen (1998) built on these findings by isolating spots on 2D gels that differed between samples harvested before and after ecdysis and sequencing them via MALDI-MS (Matrix-assisted laser ionization desorption mass spectrometry) and PDMS (plasma desorption mass spectrometry). Only RR-1 proteins were found in spots unique to animals harvested after ecdysis. Subsequent studies have tried to use the timing of mRNAs to predict whether a protein would be used in exo- or endo-cuticle. Togawa et al. (2008) looked at transcript levels (with RT-qPCR) of more than 150 CPRs from An. gambiae at 19 precisely timed points from hatching to adult eclosion. The majority of the genes had transcripts that appeared first in a pharate stage and persisted into the next stage, making it impossible even to speculate about the localization of the corresponding proteins. More recently, Shahin et al. (2016) carried out an extensive RT-qPCR analysis on wing discs and pupal wings of Bombyx mori, once again finding that most CPR genes had transcripts that appeared before pupation, although transcripts from RR-1s persisted longer. Thus, they concluded: “From the present results, RR-2 CP appears to be involved in the exocuticle and RR-1 in exo- and endocuticle layers.” A complication of using transcript appearance to predict protein localization is the evidence that newly secreted CPs can be interspersed in older regions of the cuticle, a phenomenon called intussusception (Carter and Locke, 1993). Obviously transcript data are only a very indirect way of assessing the location of CPR proteins.
EM immunolocalization of an RR-2 protein from Tribolium castaneum (TcCPR27) localized it in both laminae and vertical pore canals of the elytra’s hard cuticle (Noh et al., 2014). Since only cuticle from pharate adults was examined, no information is available about whether this protein might also be found in the post-ecdysial endocuticle.
1.4. Differentiation between RR-1 and RR-2 based on anatomical location
The second hypothesis about the deployment of RR-1s and RR-2s was that RR-1s and RR-2s would be used to build soft (flexible) and hard (rigid) cuticle, respectively (Andersen, 1998). This generalization came from earlier studies with protein extracts from carefully dissected samples, in situ hybridization, immunolocalization, and Northern analyses with RNA extracted from tissues taken from specific regions from six species (A summary of some of these data can be found in Table 1 of Willis et al., 2012). Perhaps the most impressive data came from in situ hybridization where abrupt borders were seen between intersegmental membranes and sclerites with mRNA for RR-1 proteins in the former, and RR-2 in the latter (Charles et al., 1992; Rebers et al., 1997; Mathelin et al., 1998). Yet, Gu and Willis (2003) presented quantitative data on transcript levels that indicated that message from a spatially “inappropriate” mRNA might be present, but from 25- to 3125-fold less abundant.
Table 1.
Peptides (protein) used to raise the primary antibodies.
| Primary antibody | R&R group | Peptide/Protein | Antigens matched |
|---|---|---|---|
| Anti-CPR12 = 13 | RR-1 | ETSNGIAAHEEGLG | CPR12, CPR13 |
| Anti-CPR22 | DQNTKVLRYENVQD | CPR22 | |
| Anti-CPR61 | DIRANPPNDPDFDA | CPR61 | |
| Anti-CPR75 | IRGQETGTLKRANS | CPR75 | |
| Anti-CPR125 | FQPPGKLQLNRTPD | CPR125 | |
| Anti-CPR133 = CPR153 | NDGRYYPEYYETKW | CPR133, CPR153 | |
| Anti-CPR151 | HHQQQSLPRYKEIP | CPR151 | |
| Anti-2RA | RR-2 | AAQPEEYDANPHYS | 6/6 proteins in 2RA |
| Anti-2RB | VVHREPLAVKAVAP | 9/10 proteins in 2RB |
|
| Anti-3RC | Entire mature protein of CPR97 |
10/10 proteins in 3RC |
|
| Anti-CPR59/70 | PDGSVRTVDYTADD | CPR59, CPR70 | |
| Anti-CPR60 | YGSEHQLDAGHHEY | CPR60 | |
| Anti-CPR140 | TGFHATVTNSRDQV | CPR140 |
See Supplementary File 1 for AGAP #s and sequences of antigens displayed on targeted proteins.
The issue of differential spatial expression of RR-1 vs. RR-2 has recently been addressed in T. castaneum (Dittmer et al., 2012). Microarray data revealed that elytra (rigid, hard cuticle) and hind wings (flexible, soft cuticle) of T. castaneum did not have an exclusive presence of one type of CPR; rather the distinguishing feature was transcript abundance with significantly higher levels of RR-1s and RR-2s in hindwing and elytra, respectively (Dittmer et al., 2012).
1.5. This study
Despite the many studies focused on this topic, resolution of whether RR-1 and RR-2 CPs can be distinguished by anatomical or intra-cuticular location has not yet had a clear answer. There is a technique, EM immunolocalization, which could provide direct information about the intra-cuticular localization of these two groups. For at least 30 years, this technique has been employed by others to study the location of CPs in the cuticle, first with frozen ultrathin sections (Wolfgang et al., 1986), and shortly thereafter with conventionally embedded tissue (Leung et al., 1989). Most often the antibodies used were raised against proteins from cuticle extracts or extracted from electrophoretic gels and, with few exceptions, their sequences were not known. The only CPR to be localized was an RR-2 protein from Tenebrio molitor, TmACP20, localized to the exocuticle of the adult sclerite (Bouhin et al., 1992, 1993). Our previous studies with this technique (Vannini et al., 2014, 2015), did not investigate CPRs.
In this study, we have taken advantage of the wealth of information about sequences, transcript levels, and anatomical distribution of RR-1 and RR-2 proteins in An. gambiae to learn directly about their distribution by examining their presence within the cuticle itself. We now report the precise localization of CPRs within the cuticle of An. gambiae by immunogold labeling of electron microscopic sections. At the same time, we obtained information about anatomical locations.
2. Materials and methods
2.1. Mosquito rearing
The colony of An. gambiae (G3 strain) is maintained in an insectary at the University of Georgia Entomology Department. Newly hatched first instar larvae were kept at 27 °C in a 12/12 h L/D photoperiod. Larvae were fed ground Koi Food Staple Diet (Foster and Smith Aquatics, Rhinelander, WI USA), and adults had access to an 8% fructose solution. To obtain developmentally synchronized animals, larvae were collected when they showed signs of a recent molt because of a pale head capsule; pupae were collected at hourly intervals and maintained in small groups until they reached the desired age. Adults were collected on the morning after emergence (d 0).
2.2. Antibody production
Twelve of the 13 primary antibodies used were raised against synthetic peptides. Anti-3RC was generously provided by Ellen Dotson and had been raised against the mature protein CPR97 (formerly AGCP2B) (Dotson et al., 1998). CPR97 belongs to the 3RC sequence cluster and should recognize 10 proteins (Table 1 and Supplementary File 1).
Antigenic peptides were identified at Genscript Inc. (Piscataway, NJ) where peptide synthesis, polyclonal antibody production and affinity purification were also carried out. All the peptides were checked for possible homology with other An. gambiae protein sequences using Blastp (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) to avoid nonspecific targets.
We used a single antigen for each of three sequence clusters (2RA, 2RB and 3RC). There were 2 RR-1 pairs of identical proteins (CPR12, CPR13 and CPR133, CPR153). Although CPR59 and CPR70 have limited identity, the peptide we used comes from both. See Table 1 and Supplementary File 1 for details. The secondary antibody was colloidal-gold (10 nm particle size) conjugated goatanti-rabbit (Sigma–Aldrich Co. St. Louis, MO). The presence of chitin was monitored with colloidal-gold (15 nm particle size) conjugated Wheat Germ Agglutinin (WGA) obtained from EY Laboratories Inc. (San Mateo, CA).
2.3. Western blots
For Western blots, protein extracts were obtained from different body parts at different stages (Table 2). Due to the differing solubility of the different CPRs, protein extraction was performed with 8 M urea, pH 8.0 with protease inhibitors (cOmplete, Mini, Roche) or by boiling samples in 1% SDS and 50 mM DTT.
Table 2.
Details about tissue, sample age, protein extraction method and antibody dilution ([ ]) used for Immunolocalization and Western blots. Immunolocalization samples: young (3–4 h-old) 4th instar larvae (L4), pharate adults (PA), pupae (0–6 h-old) (P), adults just emerged (0d-A), 1-day-old (1d-A). Western blot samples: 48 h-old 4th instar larvae (L4), pupae (0–2 h-old) (P) and 5-day-old adults (5d-A).
| Primary antibody | Immunolocalization |
Western blot |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| [ ] | Tissue Age |
[ ] | Tissue Age |
Protein extraction method | |||||
| RR-1 | |||||||||
| Anti-CPR12 = 13 | [1:100] | Abdomen L4 |
[1:1K] | Head 5d-A |
8M urea | ||||
| Anti-CPR22 | [1:100] | Abdomen L4 |
[1:5K] | Whole body L4 |
1% SDS | ||||
| Anti-CPR61 | [1:100] | Abdomen L4 |
[1:500] | Whole body L4 |
1% SDS | ||||
| Anti-CPR75 | [1:100] | Abdomen 0d-A |
[1:5K] | Head 5d-A |
8M urea | ||||
| Anti-CPR125 | [1:100] | Abdomen P |
Leg 1d-A |
[1:5K] | Leg 5d-A |
1% SDS | |||
| Anti-CPR133 = 153 | [1:100] | Abdomen L4 |
[1:5K] | Whole body L4 |
1% SDS | ||||
| Anti-CPR151 | [1:100] | Abdomen P |
Abdomen 1d-A |
Leg 1d-A |
Leg 5d-A |
1% SDS | |||
| RR-2 | |||||||||
| Anti-2RA | [1:100] | Abdomen L4 |
Abdomen P |
[1:5K] | Whole body L4 |
1% SDS | |||
| Anti-2RB | [1:100] | Abdomen P |
Leg PA |
Leg 1d-A |
[1:5K] | Leg 5d-A |
1% SDS | ||
| Anti-3RC | [1:10K] | Abdomen P |
[1:10K]a | Whole body P |
1% SDS | ||||
| Anti-CPR59/70 | [1:100] | Abdomen P |
Leg PA |
Leg 1d-A |
[1:5K] | Leg 5d-A |
1% SDS | ||
| Anti-CPR60 | [1:100]a | Head 0d-A |
[1:5K] | Antennae 5d-A |
8M urea | ||||
| Anti-CPR140 | [1:100] | Abdomen P |
Leg PA |
Leg 1d-A |
[1:5K] | Antennae 5d-A |
1% SDS | ||
Sections were treated for antigen retrieval before Immunolocalization.
Proteins were separated on NuPAGE 4–12% Bis-Tris gel (Novex, Carlsbad, CA) with a MES SDS Running buffer (Novex, Carlsbad, CA) and transferred to polyvinylidene fluoride (PVDF) (Millipore) filters. Filters were blocked with 5% non-fat milk powder in PBST (PBS + 1% Tween-20) overnight at 4 °C and washed three times with PBST (15 min at room temperature). Primary antibody incubation was done in PBST–10% block solution for 1 h at room temperature. After four washes with PBST, filters were incubated with anti-rabbit secondary antibodies that were conjugated to peroxidase at a dilution of 1:30,000 in PBST for 1 h at room temperature. Filters were finally washed three times with PBST and three times with PBS. Chemiluminescence was developed with ECL Select Western Blotting Detection kit (GE Healthcare, Little Chalfont, UK) and then detected with a FluorChem 8900 (Alpha Innotech, Santa Clara, CA). As controls, blots were processed in the same way without the primary antibody incubation step.
2.4. Immunohistochemistry
Tissues were fixed in 4% formaldehyde, 0.3% glutaraldehyde + 4% sucrose in phosphate buffer 1× (PBS) (pH 7.4) overnight at 4 °C, and embedded in LR White resin (Electron Microscopy Sciences) with the last step carried out under nitrogen at 55 °C degrees for two days. A more detailed description is in Vannini et al. (2014).
Previous results from in situ hybridization, Western blots and RT-qPCR (Togawa et al., 2008) guided the tissues and ages to select for EM immunolocalization. Primary antibodies were diluted in 0.5 M NaCl, 0.1% BSA, 0.05% TWEEN 20 and 5% FBS. The dilutions used are given in Table 2 as well a list of the tissues for which localization results will be presented and discussed. The colloidal-gold conjugated anti-rabbit antiserum was diluted 1:50. All treatments were done as already described in Vannini et al. (2014). The grids with sections were incubated face down on drops of rinsing and blocking solutions and primary and then secondary antibodies. All steps were performed at room temperature except the incubation of the primary antibody that was performed at 4 °C. As a negative control, sections were treated in the same way without the primary antibody incubation step. Additional controls were pre-immune serum (when available) and flow-through from the affinity columns used to purify the antibodies.
For CPR60, post-embedding antigen retrieval (AR) procedure was necessary and performed following Saito et al. (2003). The ultrathin sections mounted on a nickel grid were placed on 100% EtOH for 3 min at room temperature, then submerged in 200 µl of 0.05 M TRIS pH 10 for 40 min at 99 °C. After a brief step (3 min) in the blocking solution, the reaction with the primary antibody and the protocol following were done as usual.
Chitin is not present in epicuticle, so in order to distinguish epicuticle from procuticle in the intersegmental membrane of larvae and pupae, labeling with WGA-gold that binds chitin, was performed following Lee et al. (1990). Section were treated with saturated aqueous sodium metaperiodate (1 h), water (5 min), 1% BSA in PBS pH 7.2 (10 min), WGA-gold 1:10 in PBS (45 min), PBS (5 min), water (5 min).
Sections were examined in a JEOL JEM-1011 transmission electron microscope (JEOL USA Inc. Peabody, MA) at 80 kV. The images were captured with a XR80M Wide-Angle Mid-Mount CCD Camera from AMT (Advanced Microscopy Techniques, Woburn, MA).
2.5. Display of RT-qPCR data
To facilitate interpretation of figures, we have added data to most figures showing the RT-qPCR results using the appropriate panels from Togawa et al. (2008), modified to show precisely when samples were harvested. The panels for the sequence clusters come from Fig. 3 and the others from Supplementary Fig. 3 of Togawa et al. (2008). That paper did not normalize its data, so they are shown on our figures as R0 divided by 1000. (R0 = fluorescence proportional to the amount of transcript.) At the stages used, R0 for the ribosomal protein S7 would be about 2 (2000), so that most of the genes had high levels of mRNA at peak expression. For sequence clusters, the Y-axis shows the mean of relative expression level, which is the ratio of R0 to the summation of R0 through all stages examined. Blue and red bars represent males and females, respectively.
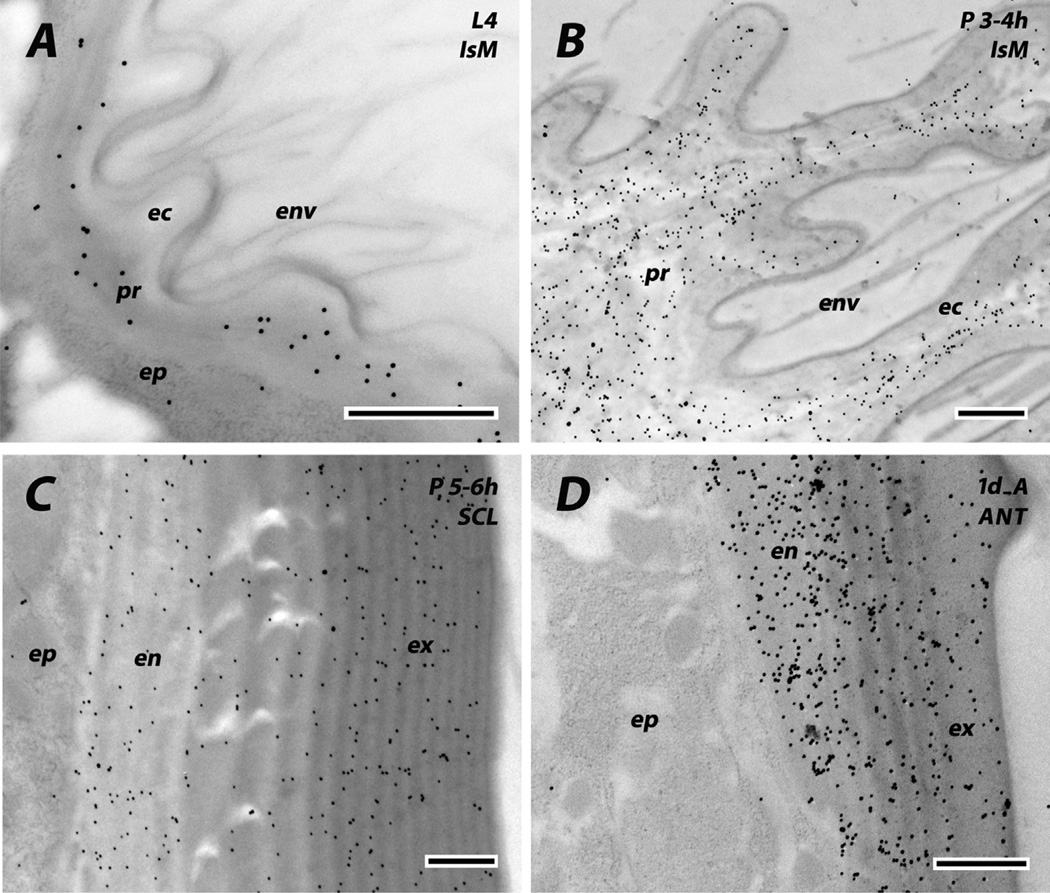
Fig. 3.
Identification of chitin in cuticular regions using colloidal gold labeled wheat germ agglutinin (WGA). Chitin is absent in envelope (env) and epicuticle (ec), and present in procuticle (pr) of intersegmental membranes of both last instar larvae (A) and young pupae fixed 3–4 h after pupation (B). It is also present in both exo- and endo-cuticle of pupal sclerites (C) and the adult antenna (D). Scale bars are 500 nm.
3. Results and discussion
3.1. Validation of technique
EM immunolocalization of CPs is an established technique, but this is the first time it has been used to address the problem of deployment of RR-1 and RR-2 CPRs within the cuticle. We selected stages and tissues to examine based on earlier work from our laboratory, especially an extensive RT-qPCR analysis of transcript levels throughout development (Togawa et al., 2008).
Several controls were used: preimmune serum, flow through from the affinity columns, and no primary antibody. Some of the sections treated with preimmune serum had sparse deposits of gold particles (Supplementary File 2). Except for some samples with CPR125, their density was conspicuously lower than comparable sections treated with the purified antibody. Since our antibodies had been affinity purified, we thought a better control would be the flow-through from the affinity columns used to purify the antibodies. These controls had almost no gold particles (Supplementary File 2). Perhaps the background with preimmune serum might come from the rabbits having had environmental exposure to insects and the processing before affinity purification reduces its concentration. No gold particles were seen when the primary antibody was replaced with dilution buffer (Supplementary File 3). The density of gold particles was so high in many sections and the locations of antibody binding so different with different antibodies and tissue sources that we are confident that we are seeing specific reactions.
3.2. Western blots
The antibodies were tested against extracts of proteins from different developmental stages and anatomical regions (Table 2 and Supplementary File 4). For each antibody, except that for the 2RB sequence cluster, there was one dominant band recognized. The proteins in that sequence cluster recognized by the antibody fall into 2 classes: 3 have an average MW of 14,847; for the other 6 it is 17,842. Hence the two bands are further confirmation for the specificity of the antibodies. It is a common feature for MWs of CPs displayed on SDS gels not to match calculated MW for they are highly sensitive to gel conditions, ionic strength and pH (Cox and Willis, 1987), but some of the difference found here seem rather extreme.
3.3. Cuticle morphology
An overview of the regions we examined is in Fig. 2. In the pupa, sclerite (hard cuticle) and intersegmental membrane (soft, flexible cuticle) have clearly distinct morphologies. The exocuticle has closely spaced laminae, while those in the endocuticle are farther apart. The intersegmental membrane has a clear area about 200 nm thick that we have interpreted as epicuticle because it lacks chitin (Fig. 3A and B). In larvae, sclerites do not have a conspicuously laminar endocuticle and the intersegmental membrane has no laminae at all. Pharate adult and adult legs have a well-defined exocuticle, and an endocuticle without conspicuous laminae. Leg apodemes are interesting structures with endocuticle on the inside and exocuticle on the outside, neither with laminae (Vannini et al., 2014).
3.4. Intra-cuticular location of RR-1 proteins
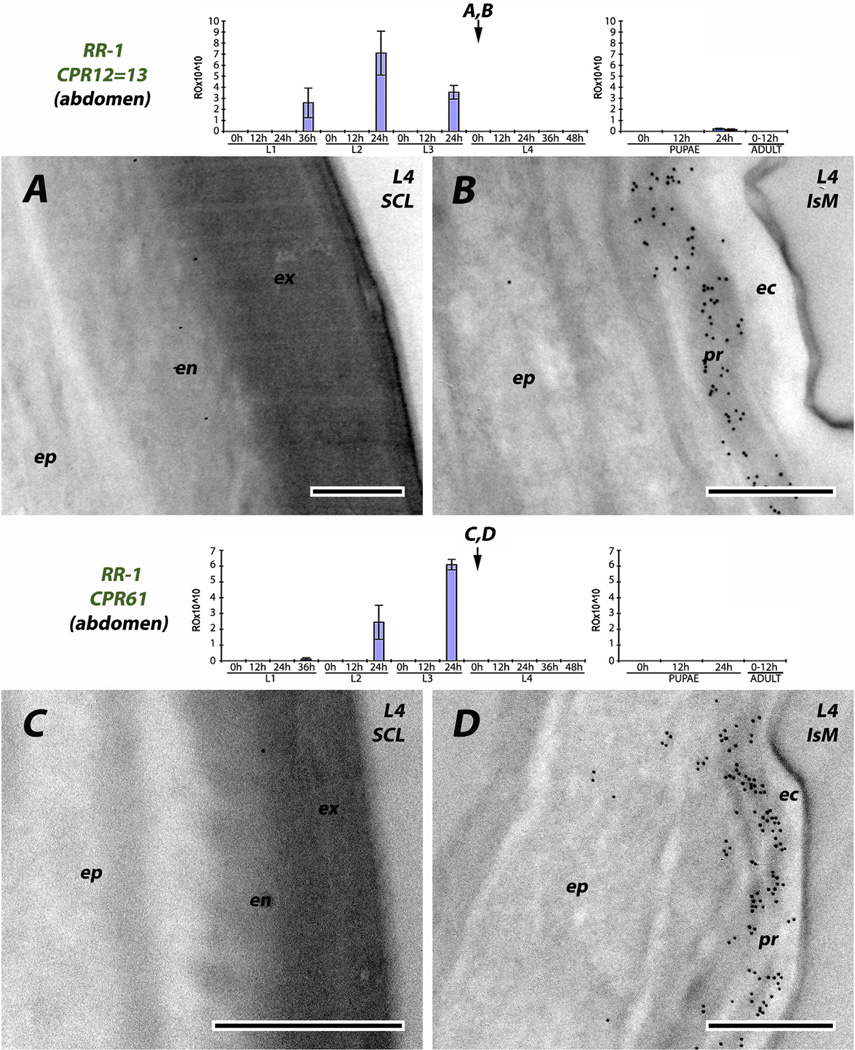
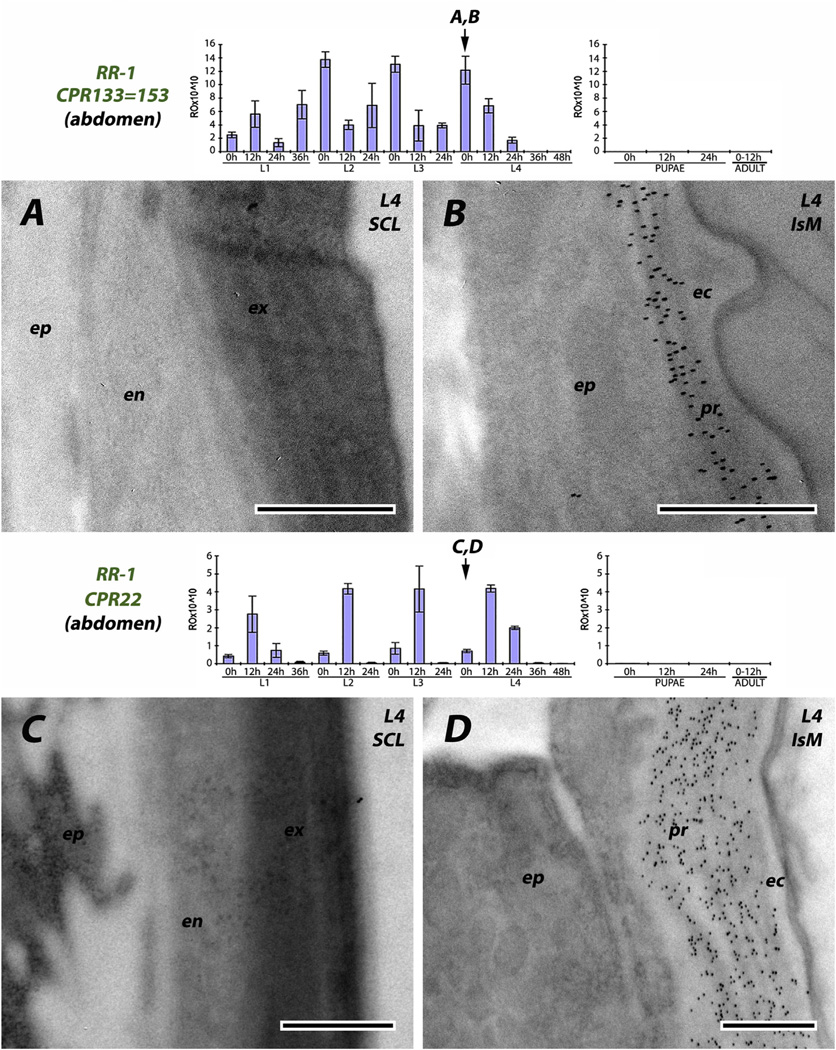
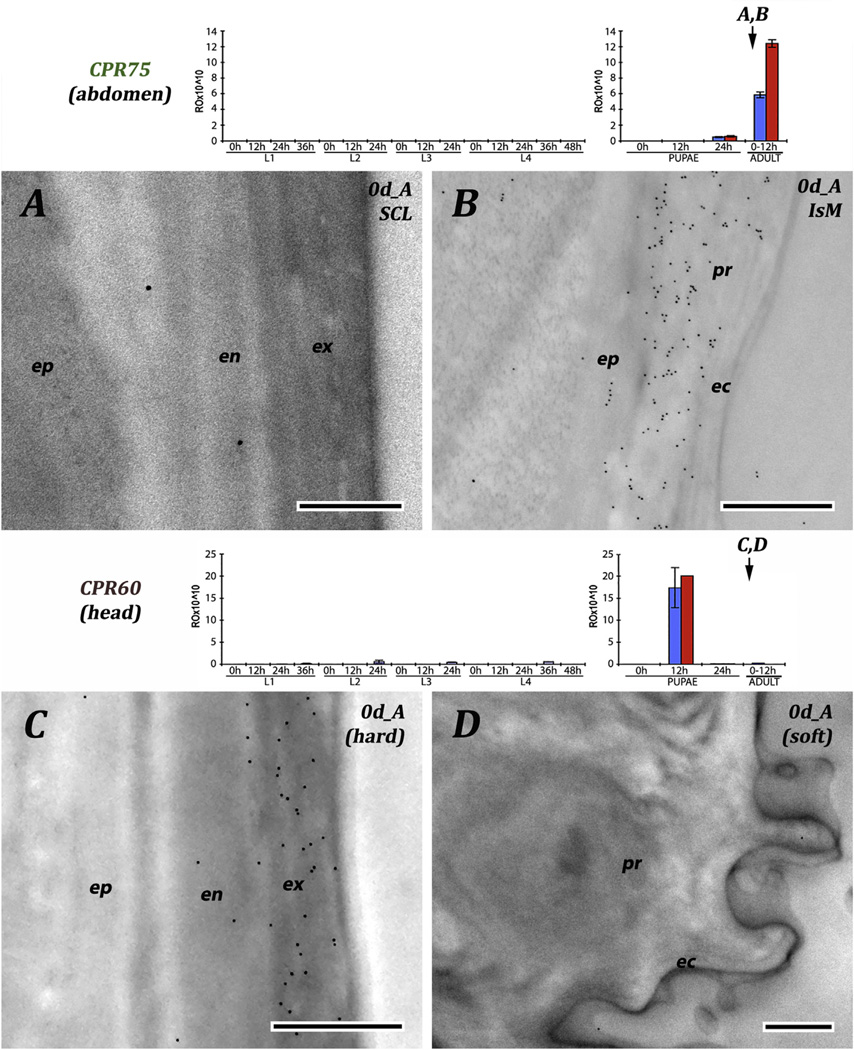
We used seven antibodies directed against RR-1 proteins. Four were reacted with sections of larval cuticle, one to pupal, one to adult, and one to pupal and adult cuticle (Table 2). The results are consistent across stages. The region directly below the envelope that we have interpreted as a thick epicuticle, was devoid of RR-1 proteins. Three of the antibodies (CPR12/13, CPR61 and CPR133/ 153) corresponded to genes that had transcript levels at the end of L3, so mRNA was present that could have coded for proteins present in the exocuticle of sclerites. While we could not distinguish exo-and endo-cuticle in the intersegmental membranes, label was found extending from the epidermis to the epicuticle and no label was found in the sclerites (Fig. 4 and Fig. 5A and B). Even CPR22, which had no transcript until 0 h L4, had label throughout the procuticle of the intersegmental membrane (Fig. 5D). Virtually no transcript for CPR151 was found in larvae, and none until the second time point examined after pupation (P12). Here, too, antibody binding was restricted to the intersegmental membrane (Fig. 6B and C). CPR75 was found exclusively in the procuticle of the intersegmental membrane of a recently eclosed adult, once again extending up to the epicuticle (Fig. 7B). It is possible, but highly unlikely given the results with the RR-2 proteins, that sclerotization prevented us from identifying RR-1 proteins in cuticle of sclerites with these 6 antibodies.
Fig. 4.
EM immunolocalization of RR-1 proteins CPR12 = CPR13 and CPR61 in procuticle of soft, flexible intersegmental membranes of young 4th instar larvae. Also shown are figures of transcript levels from Togawa et al. (2008) modified with arrows to indicate ages when tissues were harvested. Abbreviations: ec: epicuticle; en: endocuticle; ep: epidermis; ex: exocuticle; IsM: (intersegmental membrane); pr: procuticle; SCL: (sclerite). Scale bars are 500 nm.
Fig. 5.
EM immunolocalization of RR-1 proteins CPR133 = CPR153 and CPR22 in young 4th instar larvae. CPs were detected only in procuticle of the soft, flexible intersegmental membranes. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Fig. 6.
EM immunolocalization of RR-1 protein CPR151. Sclerite and intersegmental membrane from mature pupa 5–6 h after pupation (A, B) and (C) intersegmental membrane from young adult. CPs were detected only in procuticle of the soft, flexible intersegmental membranes. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Fig. 7.
EM immunolocalization of RR-1 protein, CPR75 and RR-2 protein CPR60. Cuticles are from 0 day adults. Sclerite and intersegmental membrane (A, B) and hard and soft from head (C, D). CPR75 was detected only in procuticle of the soft, flexible intersegmental membranes. CPR60 is present in exocuticle, but probably not in endocuticle and not anywhere in the procuticle of a region of soft cuticle. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
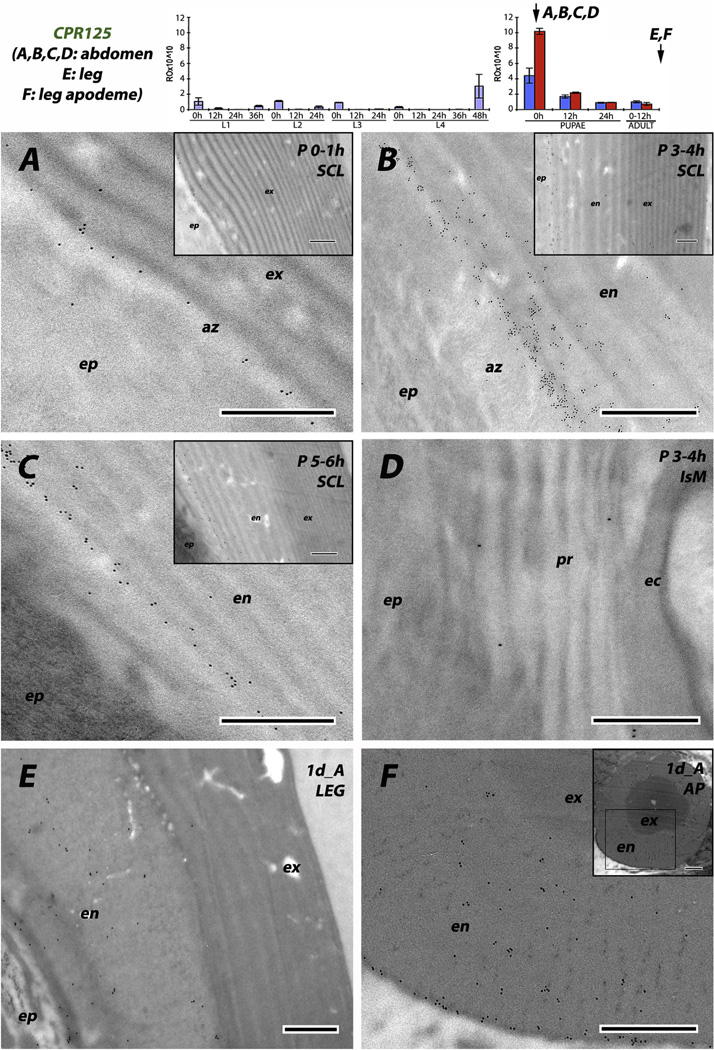
The data for CPR125 are different because this RR-1 protein was detected in hard cuticle. Abdominal tissue fixed immediately after pupation had a distinct row of gold particles at the junction between epidermis and endocuticle in sclerites (Fig. 8A). Animals fixed 3–4 or 5–6 h after pupation had even higher densities of gold particles, but still restricted to the innermost laminae of the endocuticle (Fig. 8B and C). We saw this pattern in tissues from several different animals. The situation in the hard cuticle of legs from young adults was somewhat different. Here CPR125 antigen was present throughout the endocuticle (Fig. 8E), and this was especially clear in the apodeme where endocuticle is at the outside of the structure (Fig. 8F). In contrast to the other RR-1 proteins, we found only scattered gold particles in intersegmental membranes, not enough to be sure they were reporting protein presence (Fig. 8D), especially given the background with preimmune serum (Supplementary File 2B). Thus the prediction of Andersen (1998) that RR-1 proteins would be found in the endocuticle of hard cuticles was only supported by the data from CPR125. The other RR-1 proteins were restricted to flexible, intersegmental membranes, supporting the hypothesis that the characteristics of the mature cuticle (hard or flexible) were the prime determinants of CP utilization.
Fig. 8.
EM immunolocalization of RR-1 protein CPR125. (A) young pupa no more than 1 h after pupation. (B, D) pupa 3–4 h after pupation. (C) Mature pupa 5–6 h after pupation. (E, F) leg and its apodeme from 1 d old adult. Note the presence of gold particles in the endocuticle of all hard structures, especially in the assembly zone (az) at the border with the epidermis. Transcript panels and other abbreviations as in Fig. 4. Scale bars are 500 nm.
A recent paper (Noh et al., 2015) reports that a CPR protein from T. castaneum (TcCPR4) is found exclusively in hard cuticle; RNAi knockdown, resulted in abnormal pore canals. They report this as an RR-1 protein, but on CutProtFam-Pred it has a score of 16.1, well below the stringent cutoff value for RR-1s (35). Nonetheless, it fails to score as an RR-2, and given the wide range of amino acids in the Consensus of RR-1 sequences, it clearly is another example of an RR-1-like protein in hard cuticle.
3.5. Intra-cuticular location of RR-2 proteins
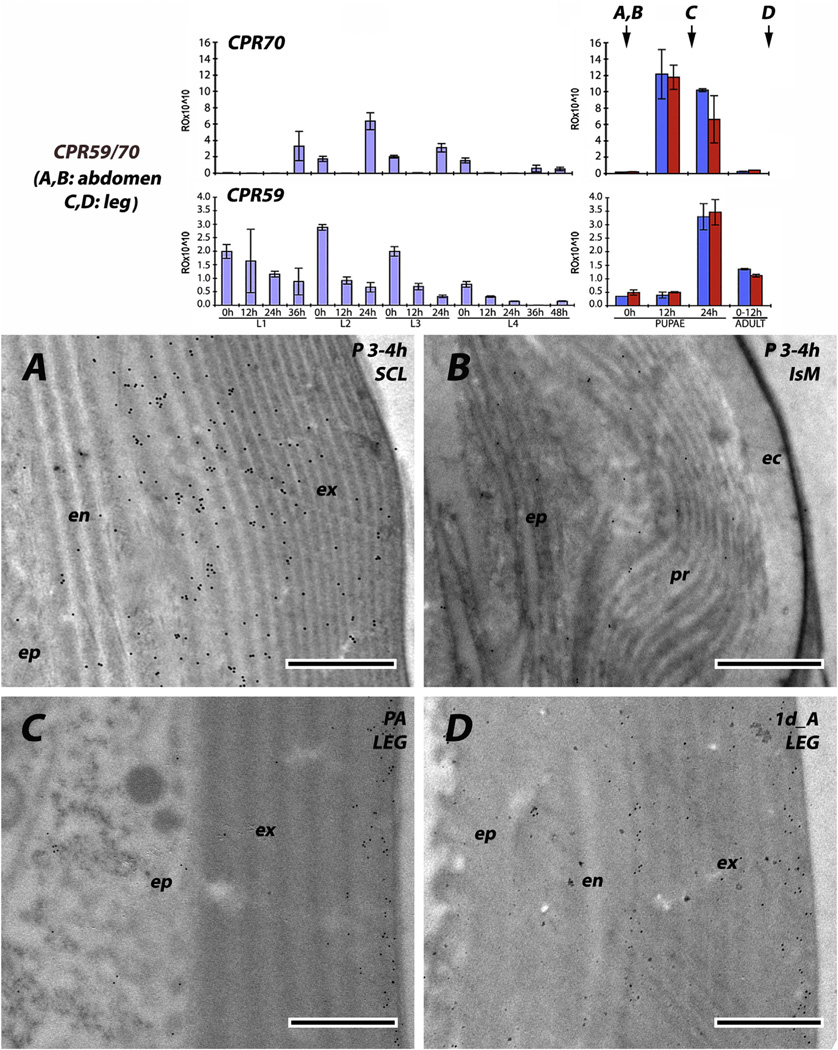
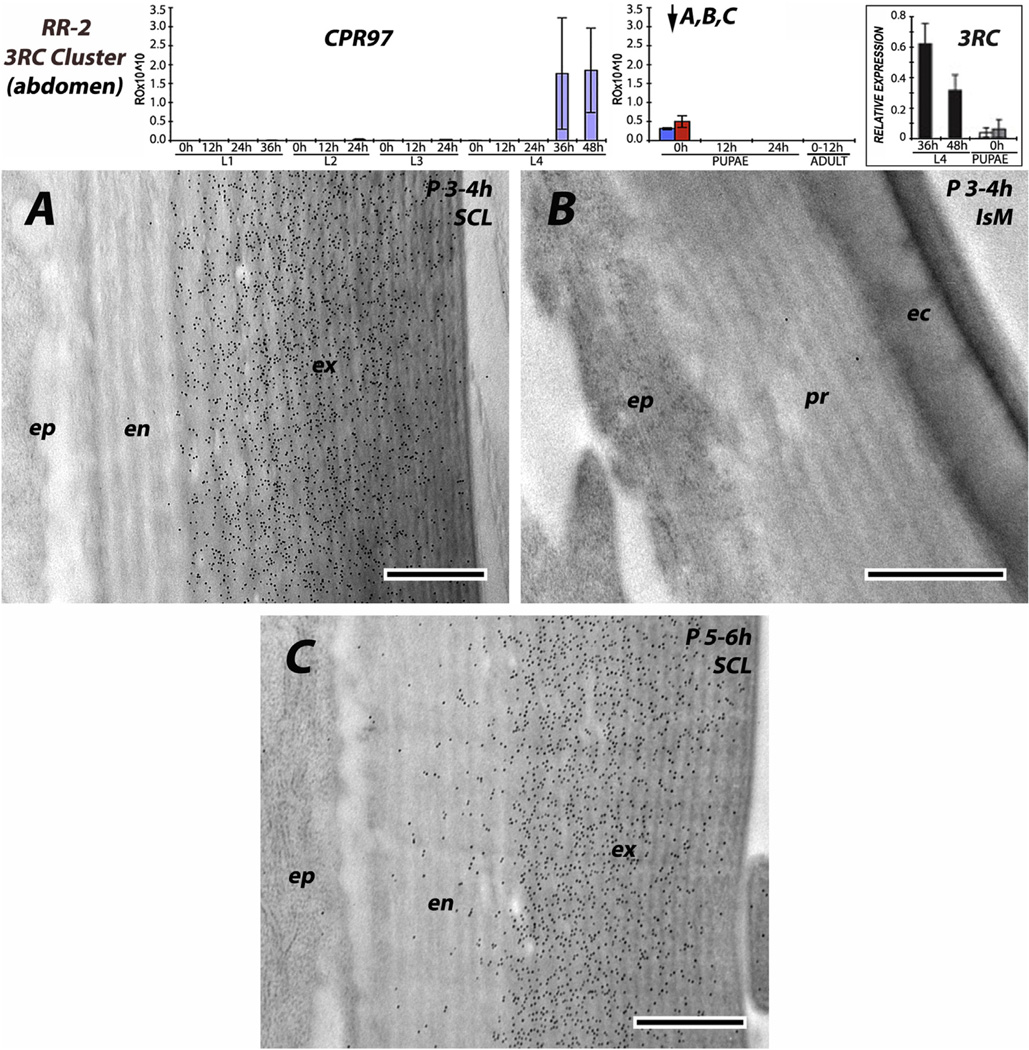
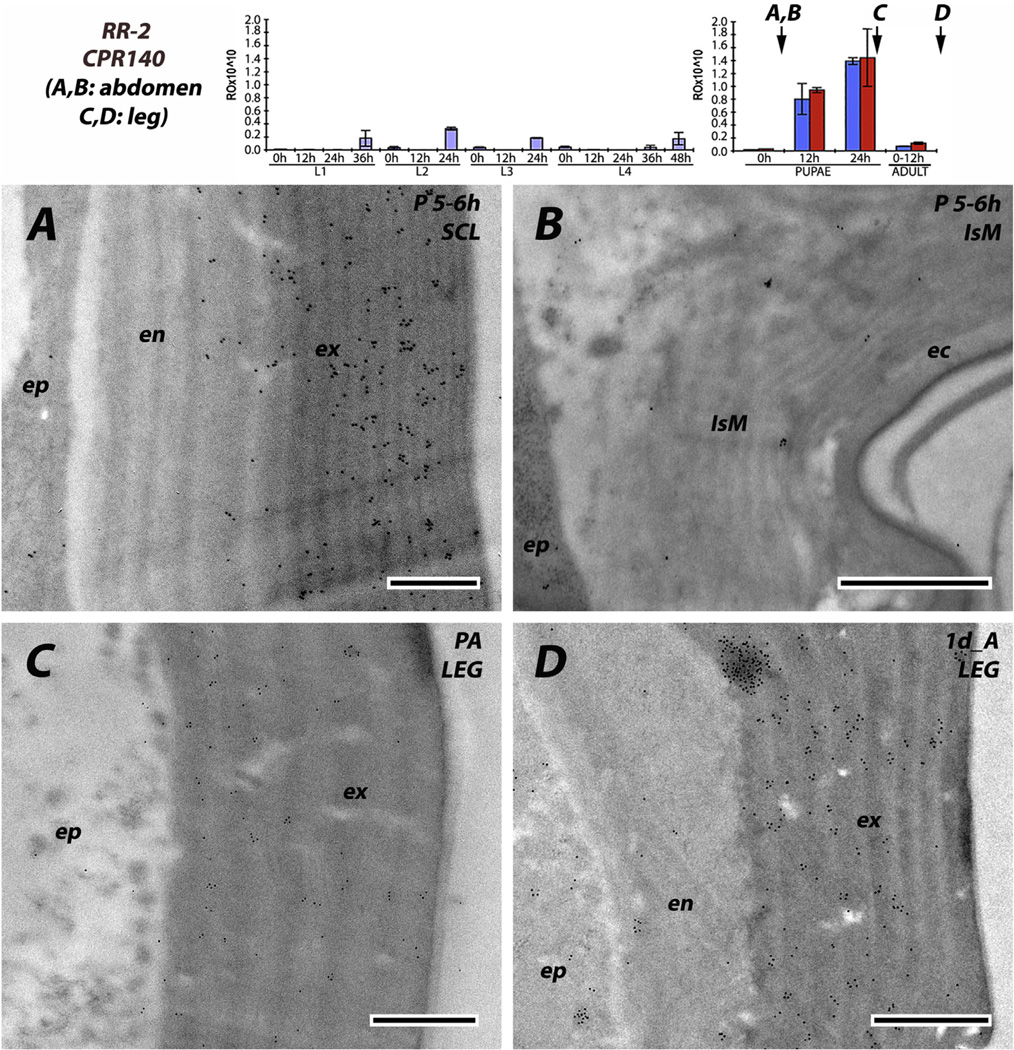
The six antibodies raised against RR-2 proteins were consistent; irrespective of stage examined from 0 h pupae to P24 (pharate adults) to adults, and we never saw any evidence for RR-2 proteins being present in intersegmental membranes. Beyond that, there were two patterns. Three of the antibodies (anti-2RA, anti-CPR59/CPR70 and to a lesser extent anti-3RC) had reacted with antigens in both exo- and endo-cuticle. For anti-2RA, this dual labeling was seen in both the young sclerites of L4 and the mature pupal cuticle (Fig. 9A and C). Interestingly, the density of gold particles was far lower in the outer regions of the exocuticle (Fig. 9A and C), something we did not see with any other antibodies. Perhaps as sclerotization proceeds, some proteins become unavailable as antigens. Anti-CPR59/CPR70 also showed labeling in both exo- and endo-cuticles of slightly younger pupae (Fig. 10A), as well as the leg of a young adult (Fig. 10D). The antibody that recognizes the 3RC sequence cluster reacted predominantly with the exocuticle in the sclerites of young and mature pupae (Fig. 11A and C). CPR140 had a hint of label in the endocuticle of a mature pupa (Fig. 12A), but almost none in the leg of a 1 d adult (Fig. 12D). Interestingly, anti-CPR140, raised against the largest CPR, was the only antibody that was visualized in small clusters of spots, suggesting aggregates of this protein within the cuticle (Fig. 12D).
Fig. 9.
EM immunolocalization of all 6 RR-2 proteins in the 2RA sequence cluster. (A, B) from recently molted 4th instar larva; (C, D) from mature pupa 5–6 h after pupation. Here antibody has reacted with proteins in both exo- and endo-cuticles of the sclerites but not of the intersegmental membrane. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Fig. 10.
EM immunolocalization of RR-2 proteins with antibody that recognizes both CPR59 and CPR70. (A, B) Tissues from young pupae 3–4 h after pupation. Antibody has reacted with proteins in both exo- and endo-cuticle, but not with the intersegmental membrane. Protein is present in legs of both pharate adult (C) and 1d old adult (D). Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Fig. 11.
EM immunolocalization of all 10 RR-2 proteins in the 3RC sequence cluster recognized by an antibody raised against the mature form of CPR97. Abdominal tissues (A, B) from pupa 3–4 h after pupation; (C) from sclerite of mature pupa 5–6 h after pupation. Note presence of scattered gold particles in endocuticle of the older pupal sclerite. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Fig. 12.
EM immunolocalization of RR-2 protein CPR140. Tissues (A, B) from abdomen of mature pupae, 5–6 h after pupation, (C) leg from pharate adult 24 h after pupation, a few hours before adult eclosion and (D) leg from adult 1 d after eclosion. Traces of protein were recognized in the endocuticle of the sclerite, while more gold particles were seen in the exocuticle. Note that gold particles frequently appear in clusters. Almost no antibody was detected in the intersegmental membrane or endocuticle of the leg. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
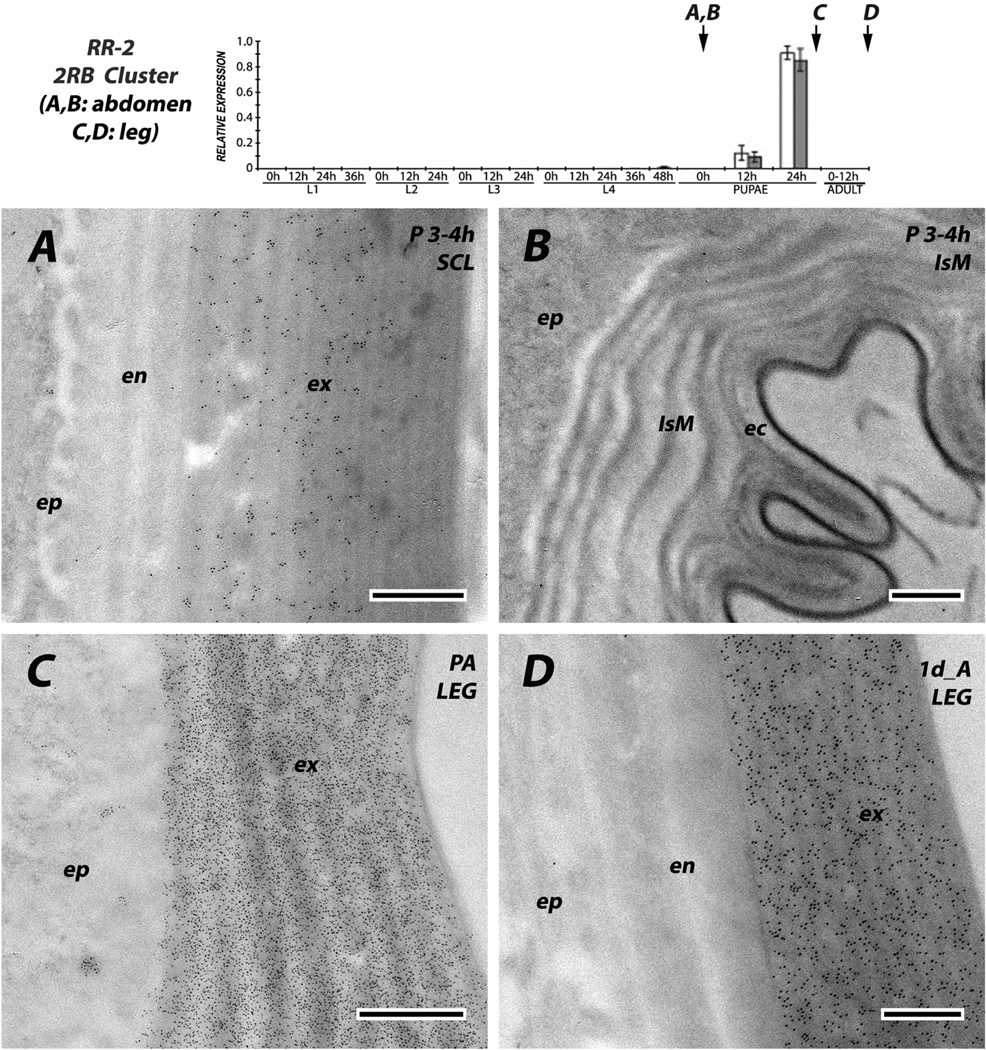
The other three anti-RR-2 antibodies reacted solely with proteins in the exocuticle of hard cuticles. The 2RB sequence cluster proteins were in exocuticle of sclerites from young pupae (Fig.13A). Anti-2RB was also tested against the legs of pharate adults, when no endocuticle is present (Fig. 13B) and in the legs of 1 d adults, with a well formed endocuticle that lacked label although the exocuticle had remained heavily labeled (Fig.13D). CPR60, although with few gold particles, still showed the difference between exo-and endo-cuticle in cuticle from the head (Fig. 7C).
Fig. 13.
EM immunolocalization of 9/10 RR-2 proteins in the 2RB sequence cluster. (A, B) Tissues from abdomen of pupae 3–4 h after pupation and (C) leg from pharate adult 24 h after pupation, a few hours before adult eclosion and (D) leg from adult 1 day after eclosion. Protein was only recognized in the exocuticle of hard cuticle and not in the endocuticle or intersegmental membrane. Transcript panels and abbreviations as in Fig. 4. Scale bars are 500 nm.
Thus, it is clear that even the outermost regions of flexible cuticle of the intersegmental membranes are not built with RR-2 proteins. But the suggestions that RR-2 proteins would be restricted to exocuticle did not receive universal support. Obviously, there must be subtle differences in these proteins to affect whether their secretion persists after ecdysis so that they can appear in the endocuticle. Thus it is intriguing that all four RR-2 antibodies that reacted with endocuticle come from proteins with several AAP(A/V) repeats, whereas no more than an occasional presence of such a repeat was found in any of the other proteins we tested (Supplementary File 1). Andersen et al. (1995) were the first to comment on this motif and pointed out that it should have “a strong tendency to form turns, several conformations can be present in equilibrium … When the sequence occurs regularly in a protein, as it does in many of the cuticular proteins …, it can be suggested that the result will be proteins folded into a more or less regular helix, which is easily and reversibly deformed by external forces, thereby resembling elastin.”
4. Conclusions
At last we have direct evidence for the location of RR-1 and RR-2 proteins in the cuticle. While generalization awaits comparable analyses in other species, in An. gambiae we found support for both anatomical and intra-cuticular specialization for these two groups of CPRs. For both RR-1 and RR-2, a subset of the proteins deviated from the others in its properties and negated the universality of one of the hypotheses. RR-1s, with one exception, were not found in hard cuticle such as sclerites and legs; rather they were restricted to soft cuticle such as intersegmental membranes. The one exception, CPR125, was found in the endocuticle of hard cuticle. Conversely, RR-2s were restricted to hard cuticles, and never appeared in intersegmental membranes. But within the hard cuticle, a subset of the RR-2s we examined was present in both endo- and exo-cuticle. Thus, the physical properties of the cuticle (hard, soft) more than intra-cuticular location appear to be the main, but not the sole factor determining CPR group localization.
Supplementary Material
Acknowledgments
We thank Mark R. Brown and Anne Elliot for maintaining the mosquito facility from which the animals were obtained and M.R. Brown for advice on immunolabeling. We thank Yihong Zhou for advice on protein isolation, John Hunter Bowen for help with the sequence analyses and both for comments on the MS. We also thank Mary B. Ard and John P. Shield of the Center for Advanced Ultrastructural Research at the University of Georgia for technical support. Two anonymous reviewers provided helpful suggestions. This research was funded by a grant from the U.S. National Institutes of Health R01AI055624.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.asd.2016.10.002.
References
- Andersen SO. Amino acid sequence studies on endocuticular proteins from the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 1998;28:421–434. doi: 10.1016/s0965-1748(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Andersen SO. Studies on proteins in post-ecdysial nymphal cuticle of locust, Locusta migratoria, and cockroach, Blaberus craniifer. Insect Biochem. Mol. Biol. 2000;30:569–577. doi: 10.1016/s0965-1748(00)00029-1. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Højrup P. Extractable proteins from abdominal cuticle of sexually mature locusts, Locusta migratoria. Insect Biochem. 1987;17:45–51. [Google Scholar]
- Andersen SO, Hojrup P, Roepstorff P. Insect cuticular proteins. Insect Biochem. Mol. Biol. 1995;25:153–176. doi: 10.1016/0965-1748(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Bouhin H, Charles JP, Quennedey B, Delachambre J. Developmental profiles of epidermal mRNAs during the pupal-adult molt of Tenebrio molitor and isolation of a cDNA clone encoding an adult cuticular protein: effects of a juvenile hormone analogue. Dev. Biol. 1992;149:112–122. doi: 10.1016/0012-1606(92)90268-l. [DOI] [PubMed] [Google Scholar]
- Bouhin H, Braquart C, Charles JP, Quennedey B, Delachambre J. Nucle-otide sequence of an adult-specific cuticular protein gene from the beetle Tenebrio molitor: effects of 20-hydroxyecdysone on mRNA accumulation. Insect Mol. Biol. 1993;2:81–88. doi: 10.1111/j.1365-2583.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Carter D, Locke M. Why caterpillar do not grow short and fat. Intl J. Insect Morphol. Embryol. 1993;22:81–102. [Google Scholar]
- Charles JP, Bouhin H, Quennedey B, Courrent A, Delachambre J. cDNA cloning and deduced amino acid sequence of a major, glycine-rich cuticular protein from the coleopteran Tenebrio molitor. Temporal and spatial distribution of the transcript during metamorphosis. Eur. J. Biochem. 1992;206:813–819. doi: 10.1111/j.1432-1033.1992.tb16989.x. [DOI] [PubMed] [Google Scholar]
- Cornman RS, Togawa T, Dunn WA, He N, Emmons AC, Willis JH. Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genomics. 2008;9:22. doi: 10.1186/1471-2164-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman RS, Willis JH. Extensive gene amplification and concerted evolution within the CPR family of cuticular proteins in mosquitoes. Insect Biochem. Mol. Biol. 2008;38:661–676. doi: 10.1016/j.ibmb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Willis JH. Analysis of the cuticular proteins of Hyalophora cecropia with two dimensional electrophoresis. Insect Biochem. 1987;17:457–468. [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer NT, Hiromasa Y, Tomich JM, Lu N, Beeman RW, Kramer KJ, Kanost MR. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J. Proteome Res. 2012;11:269–278. doi: 10.1021/pr2009803. [DOI] [PubMed] [Google Scholar]
- Dong Z, Zhang W, Zhang Y, Zhang X, Zhao P, Xia Q. Identification and characterization of novel chitin-binding proteins from the larval cuticle of silkworm, Bombyx mori. J. Proteome Res. 2016;15:1435–1445. doi: 10.1021/acs.jproteome.5b00943. [DOI] [PubMed] [Google Scholar]
- Dotson EM, Cornel AJ, Willis JH, Collins FH. A family of pupal-specific cuticular protein genes in the mosquito Anopheles gambiae. Insect Biochem. Mol. Biol. 1998;28:459–472. doi: 10.1016/s0965-1748(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Gu S, Willis JH. Distribution of cuticular protein mRNAs in silk moth integument and imaginal discs. Insect Biochem. Mol. Biol. 2003;33:1177–1188. doi: 10.1016/j.ibmb.2003.06.010. [DOI] [PubMed] [Google Scholar]
- Ioannidou ZS, Theodoropoulou MC, Papandreou NC, Willis JH, Hamodrakas SJ. CutProtFam-Pred: detection and classification of putative structural cuticular proteins from sequence alone, based on profile hidden Markov models. Insect Biochem. Mol. Biol. 2014;52:51–59. doi: 10.1016/j.ibmb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen UG, Rothmann A, Skou L, Andersen SO, Roepstorff P, Hojrup P. Cuticular proteins from the giant cockroach, Blaberus craniifer. Insect Biochem. Mol. Biol. 1997;27:109–120. doi: 10.1016/s0965-1748(96)00074-4. [DOI] [PubMed] [Google Scholar]
- Lee SA, Clarke BS, Jenner DW, Williamson FA. Cytochemical demonstration of the effects of the acylureas flufenoxuron and diflubenzuron on the incorporation of chitin into insect cuticle. Pestic. Sci. 1990;28:367–375. [Google Scholar]
- Lemoine A, Millot C, Curie G, Delachambre J. A monoclonal antibody against an adult-specific cuticular protein of Tenebrio molitor (Insecta, Coleoptera) Dev. Biol. 1989;136:546–554. doi: 10.1016/0012-1606(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Lemoine A, Millot C, Curie G, Delachambre J. Spatial and temporal variations in cuticle proteins as revealed by monoclonal antibodies. Immunoblotting analysis and ultrastructural immunolocalization in a beetle, Tenebrio molitor. Tissue Cell. 1990;22:177–189. doi: 10.1016/0040-8166(90)90020-a. [DOI] [PubMed] [Google Scholar]
- Leung H, Palli SR, Locke M. The localization of arylphorin in an insect, Calpodes ethlius. J. Insect Physiol. 1989;35:223–231. [Google Scholar]
- Locke M. The Wigglesworth Lecture: insects for studying fundamental problems in biology. J. Insect Physiol. 2001;47:495–507. doi: 10.1016/s0022-1910(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Magkrioti CK, Spyropoulos IC, Iconomidou VA, Willis JH, Hamodrakas SJ. cuticleDB: a relational database of arthropod cuticular proteins. BMC Bioinforma. 2004;5:138. doi: 10.1186/1471-2105-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelin J, Quennedey B, Bouhin H, Delachambre J. Characterization of two new cuticular genes specifically expressed during the post-ecdysial molting period in Tenebrio molitor. Gene. 1998;211:351–359. doi: 10.1016/s0378-1119(98)00125-5. [DOI] [PubMed] [Google Scholar]
- Noh MY, Kramer KJ, Muthukrishnan S, Kanost MR, Beeman RW, Arakane Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem. Molec Biol. 2014;53:22–29. doi: 10.1016/j.ibmb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet. 2015;11:e1004963. doi: 10.1371/journal.pgen.1004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr C, Andersen SO. Cuticular proteins from fifth instar nymphs of the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 1993;23:521–531. [Google Scholar]
- Qin G, Lapidot S, Numata K, Hu X, Meirovitch S, Dekel M, Podoler I, Shoseyov O, Kaplan DL. Expression, cross-linking, and characterization of recombinant chitin binding resilin. Biomacromolecules. 2009;10:3227–3234. doi: 10.1021/bm900735g. [DOI] [PubMed] [Google Scholar]
- Rebers JE, Niu J, Riddiford LM. Structure and spatial expression of the Manduca sexta MSCP14.6 cuticle gene. Insect Biochem. Mol. Biol. 1997;27:229–240. doi: 10.1016/s0965-1748(96)00090-2. [DOI] [PubMed] [Google Scholar]
- Rebers JE, Riddiford LM. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J. Mol. Biol. 1988;203:411–423. doi: 10.1016/0022-2836(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Rebers JE, Willis JH. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem. Mol. Biol. 2001;31:1083–1093. doi: 10.1016/s0965-1748(01)00056-x. [DOI] [PubMed] [Google Scholar]
- Roberts PE, Willis JH. The cuticular proteins of Tenebrio molitor. I. Electrophoretic banding patterns during postembryonic development. Dev. Biol. 1980;75:59–69. doi: 10.1016/0012-1606(80)90143-8. [DOI] [PubMed] [Google Scholar]
- Saito N, Konishi K, Takeda H, Kato M, Sugiyama T, Asaka M. Antigen retrieval trial for post-embedding immunoelectron microscopy by heating with several unmasking solutions. J. Histochem Cytochem. 2003;51:989–994. doi: 10.1177/002215540305100802. [DOI] [PubMed] [Google Scholar]
- Shahin R, Iwanaga M, Kawasaki H. Cuticular protein and transcription factor genes expressed during prepupal-pupal transition and by ecdysone pulse treatment in wing discs of Bombyx mori. Insect Mol. Biol. 2016:138–152. doi: 10.1111/imb.12207. [DOI] [PubMed] [Google Scholar]
- Tang L, Liang J, Zhan Z, Xiang Z, He N. Identification of the chitin-binding proteins from the larval proteins of silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2010;40:228–234. doi: 10.1016/j.ibmb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Togawa T, Dunn WA, Emmons AC, Willis JH. CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem. Mol. Biol. 2007;37:675e688. doi: 10.1016/j.ibmb.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Togawa T, Dunn WA, Emmons AC, Nagao J, Willis JH. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 2008;38:508–519. doi: 10.1016/j.ibmb.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa T, Nakato H, Izumi S. Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2004;34:1059–1067. doi: 10.1016/j.ibmb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Vannini L, Reed TW, Willis JH. Temporal and spatial expression of cuticular proteins of Anopheles gambiae implicated in insecticide resistance or differentiation of M/S incipient species. Parasit. Vectors. 2014;7:24. doi: 10.1186/1756-3305-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini L, Bowen JH, Reed TW, Willis JH. The CPCFC cuticular protein family: anatomical and cuticular locations in Anopheles gambiae and distribution throughout Pancrustacea. Insect Biochem. Mol. Biol. 2015;65:57–67. doi: 10.1016/j.ibmb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JH. Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010;40:189–204. doi: 10.1016/j.ibmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JH, Papandreou NC, Iconomidou VA, Hamodrakas SJ. Cuticular proteins. In: Gilbert LI, editor. Insect Molecular Biology and Biochemistry. London, Waltham & San Diego: Academic Press; 2012. pp. 134–166. [Google Scholar]
- Wolfgang WJ, Fristrom D, Fristrom JW. The pupal cuticle of Drosophila: differential ultrastructural immunolocalization of cuticle proteins. J. Cell Biol. 1986;102:306–311. doi: 10.1083/jcb.102.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.