Abstract
Objective
To investigate whether G protein-coupled estrogen receptor (GPER, also known as GPR30 and GPER1) stabilizes Hypoxia inducible factor 1α (HIF-1α) in eutopic endometrium (EuEM) of endometriosis?
Design
Immunohistochemical analysis and experimental in vitro study.
Setting
University hospital
Patient(s)
Patients with or without endometriosis
Intervention(s)
The EuEM and normal control endometrium (CoEM) were obtained by curettage. Primary cultured endometrial stromal cells (ESCs) were treated with 17β-estrogen (E2), G1 or G15.
Main Outcome Measure(s)
The EuEM and CoEM were collected for immunohistochemistry. Western blot, PCR, Elisa, and dual luciferase experiments were used to detect expression of GPER, HIF-1α, VEGF, and MMP9 in ESCs. E2 and G1 were used as agonists of GPER while G15 as an antagonist. Migration of ESCs and endothelial tube formation of HUVECs cultured in medium collected from ESCs were measured.
Results
Protein levels of GPER and HIF-1α were higher in EuEM than in CoEM. HIF-1α protein levels but not HIF-1α mRNA levels increased concurrently with GPER after E2 and G1 treatment. Furthermore, expression and activity of VEGF and MMP9 increased under E2 and G1 stimulation. However these effects disappeared when GPER was blocked.
Conclusion
GPER stabilizes HIF-1α thus promotes HIF-1α induced vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP9) in ESCs, which plays critical roles in endometriosis.
Keywords: Estrogen, G protein-coupled estrogen receptor (GPER), Hypoxia inducible factor 1 α (HIF-1α), endometriosis
Introduction
Endometriosis, defined as the presence of endometrial-like tissue outside the uterus, is a common benign gynaecological disease affecting 6–10% of general female population (1). Endometriosis causes pelvic pain and infertility, and has been associated with several types of cancer and other chronic diseases (2). Visual inspection of the pelvis at laparoscopy is the gold standard investigation for diagnosis, but it is invasive and results in long delays (3). Current therapeutic success is often unsatisfactory because of limited insight into disease mechanisms. The most widely accepted theory, retrograde menstruation, is insufficient to explain why most women have retrograde menstruation but only some of them develop endometriosis (4). Recent studies have focused on eutopic endometrium (EuEM) of endometriosis, which is possible to be collected simply and comfortably, as it appears to be biochemically, functionally, and genetically different compared with normal endometrium (CoEM) (5–7). It is possible that the EuEM may therefore play a key role in the pathogenesis of endometriosis.
Although a benign disease, endometriosis shares some similar features with malignancy, such as angiogenesis and metastasis (8). Recently studies suggested that hypoxia is vital for tumor formation and hypoxia inducible factor 1 α (HIF-1α) plays a key role in tumor progression by upregulating genes that control angiogenesis and metastasis (9, 10). Under normoxia conditions, HIF-1α is bound by the von Hippel-Lindau (VHL) protein for proteasomal degradation. While under hypoxia condition, the hydroxylation reaction is inhibited, allowing HIF-1α to escape degradation and increasing HIF-1α stability. Stabilized HIF-1α enters into nuclear and initiates the transcription of target genes (11). In fact, vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP9) are target genes of HIF-1α (12, 13). Even though the presence and function of hypoxia and HIF-1α in menstrual physiology remain controversial (14), increasing evidence validated that hypoxia played vital roles in endometriosis and HIF-1α was upregulated with the development of endometriosis (15–18). In our previous studies, we discovered that expression of HIF-1α in ectopic endometrium (EcEM) was higher than that in CoEM (19), which was consistent with the results of others. In fact EuEM shares changes with EcEM which were distinguish from CoEM and the view that primary defect in endometriosis is to be found in EuEM has advanced (20, 21). So we compared EuEM and CoEM and found that EuEM also showed higher HIF-1α than CoEM. What’s more, we found that expression levels of VEGF and MMP9 were increased in EuEM (22, 23). Therefore, we hypothesized that high level of HIF-1α in EuEM may increase VEGF and MMP9 expression, which was involved in the formation of endometriosis. But in the same microenvironment, what cause the different expression of HIF-1α in EuEM and CoEM? The underlying mechanism remains unknown.
As we all know, estrogen is one of the admitted factors of endometriosis (1). G protein-coupled estrogen receptor (GPER, also known as GPR30 and GPER1), a seven transmembrane-domain G protein coupled receptor, was identified as a novel estrogen receptor that mediates the balance between non-genomic and genomic activity in response to 17β-estrogen (E2) (24). Researches have proven the pathological roles of GPER in a diverse array of disorders and GPER is emerging as a novel therapeutic target and prognostic indicator (24). In endometriosis, GPER expression in EuEM has been demonstrated to be relatively higher than in CoEM (25–27). However, there is no report to explore its follow-up effects after activation by E2 or other ligands. The actual role elicited by GPER in endometriosis is still controversial. While in cancer research, GPER has been found to play important roles in activating signaling mediated by HIF-1α (28, 29). Following the background information above, we hypothesized that GPER may be involved in the pathogenesis of endometriosis through acting on HIF-1α.
The aim of this study was to determine whether expression levels of GPER and HIF-1α were different between EuEM and CoEM; and whether HIF-1α was activated by GPER. First, we investigated localization and protein levels of GPER and HIF-1α in CoEM and EuEM. Then, we examined the correlation between GPER and HIF-1α in the primary endometrial stromal cells (ESCs) under E2 and G1stimulation. To be more convincing, we next examined the expression of HIF-1α target genes-VEGF and MMP9 simultaneously. Finally, we examined the effect of blocking GPER on VEGF and MMP9 expression. Our studies suggested that GPER stabilized HIF-1α in EuEM and play a key role in endometriosis angiogenesis and metastasis.
Methods
Patients and tissues
Ethical approval was obtained from the local Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent was obtained from all participants. Human tissues were obtained in accordance with the guidelines of The Declaration of Helsinki. Twenty samples of EuEM (mean age: 26±5) were curetted from patients with endometriosis in stages III and IV diagnosed by both pathology and laparoscopic findings according to the revised classification of The American Fertility Society (30). Sufficient CoEM (mean age: 28±4) were available from 72 patients with tube infertility (no previous history of pelvic inflammatory disease, chronic pelvic pain, dysmenorrhea, or dyspareunia) and conformed without endometriosis by laparoscopy. None of them had received hormonal treatments or sex steroids, and none used intrauterine contraception for at least 6 months prior to surgery. Recruited patients had regular menstrual cycles (between 26 and 32 days) with confirmation of their menstrual history. At the time of tissue collection, all patients in the early proliferative phase of the menstrual cycle. All samples of EuEM and 20 samples of CoEM were fixed in 4% buffered formalin for immunohistochemistry evaluations. The remaining 52 CoEM biopsies were collected and transported to the laboratory for ESCs culture establishment.
Immunohistochemistry
Paraffin-embedded endometrial sections were subjected to immunohistochemistry as described previously using rabbit anti-human GPER (1:50 ab39742, Abcam) and HIF-1α (1:150 AF1009, Affinity) antibodies (22). The stained slides were evaluated by light microscope and digitally scanned images by two independent pathologists. All scoring was performed unaware of patient outcome. The immunohistochemical scores (IHS) were calculated by positive rate (PR) and staining intensity (SI) of cells reactive with antibodies. PR was categorized as 0 (no positive cells), 0 (<10% positive cells), 1 (10–25% positive cells), 2 (26–50% positive cells), or 3(50%–75% positive cells) 4 (76 – 100% positive cells) and SI was categorized as 0 (negative), 1 (weak), 2(moderate), or 3 (strong). The scoring pattern for staining was multiplied to give a total IHS and IHS ranged from 0 to 12. Scores of 0 – 2 points were considered as negative (0); 3 – 5 points as weak staining (+); 6 – 8 points as intermediate (++); and 9 – 12 points as strong staining (+++).
Cell culture
ESCs were isolated from CoEM as previously described (23). Briefly, fragments were minced, digested with collagenase II (0.1%; Sigma, USA), filtered through 150 and 37.4 μm sieves, centrifuged and suspended in Red Blood Cell Lysis Buffer (C3702, Beyotime, China). After a second centrifuge, ESCs were resuspended in full medium. Then cells were seeded on 25 cm2 culture flasks and maintained in a humidified 5% CO2 incubator at 37°C. When ESCs were nearly confluent, cells were regularly digested and plated in 6-well plates (1×106 cells/well) for western blot and in 24-well plates (2×105 cells/well) for Elisa. In each experiment, cells were divided into three groups. Cells in group one were stimulated with 10nM E2 (E-2758, Sigma, Aldrich) for different times (0, 5, 10, 15 30, 60, 120min); Cells in group two were stimulated with 100nM G1(CAS 881639-98-1, Cayman, USA) at the same time points. After identifying the most effective stimulation time, cells in group three were treated with 10nM E2 or 100nM G1 for the most effective stimulation time, with or without pretreatment with 100nM GPER inhibitor G15 (CAS 1161002-05-6, Cayman) for 30min. The supernatant was collected after stimulation and stored at −80°C until Elisa assay and in vitro HUVEC tube formation assay. The cells were washed twice with PBS and extracted for mRNA and protein assay. Each experiment was repeated at least three times with different cell preparations. The greater than 95% purity of ESCs was confirmed by positive staining for Vimentin (1:100; Cell Signaling Technology, USA) and negative staining for E-cadherin (1:150; Cell Signaling Technology, USA) in immunocytochemistry.
Human Umbilical Vein Endothelial Cells (HUVECs) were purchased from ATCC and cultured at 37°C in a humidified atmosphere of 5% CO2 in air.
Quantitative Real-Time Reverse Transcription PCR
RNA was isolated from the tissues using TRIzol reagent according to the manufacturer’s protocol. Reverse transcription and amplification for cDNA were carried out as described previously(22). The melting curve was analyzed following the reactions to check for primer dimer formation and nonspecific product amplification. The 2−ΔΔCT method was employed for the determination of relative transcript abundance.
Western blot analysis
Protein concentrations from cultured ESCs were quantified using the BCA protein assay kit (P0010S, Beyotime, China). Equal amount of protein (30μg) was subjected to 12% sodium dodecylsulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (0.45mm, Millipore, USA). After blocked for 1 hour at room temperature (RT) with blocking buffer (0.1% Tris-buffered saline with Tween (TBST) with 5% fat-free dried milk powder), the blots were incubated with primary antibodies against GPER (1: 500, ab39742, Abcam) or HIF-1α (1:1000, #AF1009, Affinity) at 4°C for overnight. The target proteins were visualized by the ECL western blotting detection system (Millipore) after incubation with a secondary antibody (1:5000 diluted with 5% fat-free dried milk powder in 0.1% 5 TBST).
Enzyme-linked immunosorbent assay
ESCs were cultured and divided into three groups as described previously. Expression levels of VEGF and MMP9 secreted into the conditioned media derived from treated and untreated cells was determined according to manufacturer guidelines using VEGF (DVE00) and MMP9 Elisa kit (DMP900) from R&D Systems Minnesota, USA. All samples were assayed in duplicate. The amount of protein secreted was determined as an optical-density value using a microplate reader at a wavelength of 450 nm, with the correction wavelength set at 570 nm. A standard-curve analysis was included on each plate, and protein secretion was compared against this curve.
Immunofluorescence
ESCs were seeded on glass coverslips sitting on the bottom of six-well plates. Fresh medium was provided to the cells 24 h before the experiment. The cells were pretreated or not with 100nM G15 for 30min, followed by treated with E2 or G1 for 15 min. The cells were then fixed (4% paraformaldehyde, 20min, RT), permeabilized (0.1% Triton X-100 in PBS, 20min, RT), blocked (5% BSA, 1h, RT), and incubated (overnight, 4°C) with primary antibody against GPER (1:100 ab39742, Abcam). A secondary antibody conjugated with Cy3 (1:100, Google biological technology, China) was used to visualize GPER. The cells were counterstained with DAPI stain (Sigma, Milan, Italy) to visualize nuclei. Sections were examined with an Olympus FV1000 laser scanning confocal microscope (Olympus).
Dual Luciferase Experiments
The 2050bp (−2000 to 50bp) sequence of wild-type VEGF promoter and the 2020bp (−1900 to 119) of wild-type MMP9 promoter were cloned from human genomic DNA and sub-cloned into pcDNA3.0 basic vectors. ESCs were seeded into 24-well plates the night before transfection. Cells were always co-transfected with the internal control plasmid pRL-SV40 (Promega, USA) containing the Renilla luciferase gene for 24h. Then cells were pretreated or not with 100nM G15 for 30min, followed by treated with E2 or G1 for 120min. After cells harvested, firefly and Renilla luciferase activities were measured using the dual luciferase assay system kit (Promega).
In vitro migration assays
In vitro migration assays were performed using transwell insert (Corning Costar, Tewksbury, MA, USA) with 8μm pore membrane filters. Briefly, matrigel (Sigma, Aldrich) was pre-coated and 104/ml of ESCs were plated in the upper chamber in a low serum medium (5%) and the units were transferred to a serum gradient (20%) in the lower chamber for 16 h. Then ESCs were treated with E2 or G1 for 120min, with or without pretreatment with G15 for 30min. The non-invasive cells and matrigel on the upper side were removed with a cotton swab. The membrane was then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of migrating cells was determined using light microscopy (Olympus, Japan) on each membrane in 5 random fields. The values reported were the averages of triplicate experiments. Duplicate wells were used per condition in each independent experiment.
In vitro HUVEC tube formation assay
A 96-well plate was evenly loaded with Matrigel (0.05 ml/well) (Sigma, Aldrich) and incubated at 37 °C for 30 min before seeding the HUVECs (5×104 cells/well). HUVECs were cultured using conditioned medium from ESCs previously treated with E2 or G1 for 120min, with or without pretreatment with G15 for 30min and incubated under normal condition. Tube formation was quantified 18 to 20 hours later and photographed using light microscopy (Olympus). Tube formations were measured blind on three randomly chosen microscopic fields per well by an independent observer, giving: (1) the total length of tube-like cells; and (2) the number of junctions or joint forming cell–cell networks. Experiments performed for the analysis of tubular formation were repeated at least three times.
Statistical analysis
Each experiment was performed in triplicate or quadruplicate. Statistical analysis was performed by GraphPad Prism 5 and results were expressed as mean ± SEM. Wilcoxon’s matched pairs test was used for the comparison of quantitative differences in the staining of GPER and HIF-1α between CoEM and EuEM. One way Anova followed by the Newman-Keuls test were used to mean comparisons between groups. Pearson correlation was used to investigate the correlation between GPER and HIF-1α protein levels in ESCs under different time points of E2 or G1. A p<0.05 was considered statistically significant.
Results
GPER and HIF-1α expression in CoEM and EuEM
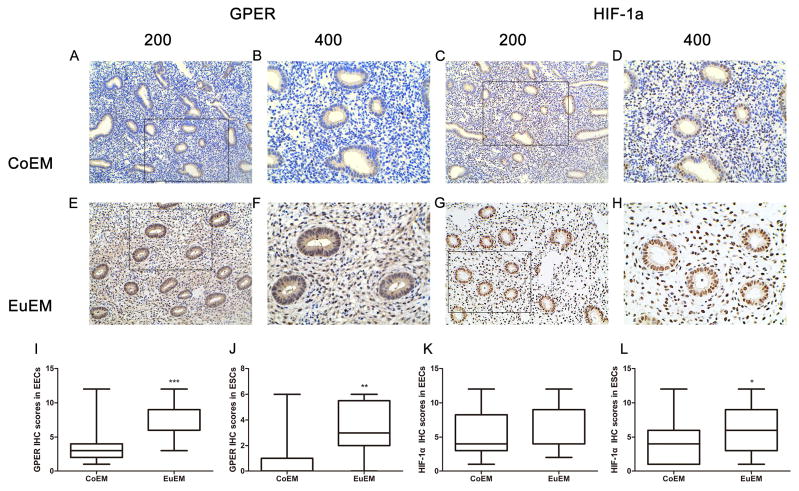
Expression and localization of GPER and HIF-1α were studied by immunohistochemistry staining in 20 CoEM and 20 EuEM. Both endometrium epithelial cells (EECs) and ESCs were analyzed. Representative staining examples are shown in Figure 1 and staining intensities are depicted in Supplemental Table 1. Generally, GPER staining was more intense in EECs than in ESCs. GPER was detected predominantly cytoplasmic in most EECs (Fig1. A, B, E, F) and the intensity was significantly higher in EuEM when compared to CoEM (p=0.009, Fig1. I). Staining was rarely seen in most of ESCs from CoEM (Fig1. A, B), while predominantly detected in the cytoplasm of ESCs from EuEM (Fig1. E, F), (P=0.0047, Fig1. J). HIF-1α was predominantly localized in the nuclear and was observed in both EECs and ESCs (Fig1.C, D, G, H). There was no significant difference between CoEM and EuEM in EECs (p=0.3746, Fig.1 K). While in ESCs, HIF-1α expression level was significant higher in EuEM than in CoEM (p=0.02179, Fig.1 L).
Figure 1.
GPER and HIF-1α expression and localization in eutopic endometrium (EuEM) of endometriosis and normal endometrium (CoEM). (A-D) Immunohistochemical analysis of GPER and HIF-1α protein expression and localization in CoEM. (E-F) Immunohistochemical analysis of GPER and HIF-1α protein in EuEM. Photographs were taken at magnifications of ×200 (left panels) and ×400 (right panels) respectively. (I-L) Quantitative comparison of the fold difference in the expression of GPER and HIF-1α protein. The data are presented as means ± SEM (*P<0.05; **P<0.01; ***P<0.001 by Wilcoxon’s matched pairs test). Data presented were from 20 independent experiments.
E2 and G1 induce GPER, HIF-1α, and HIF-1α target genes VEGF, and MMP9 expression in ESCs
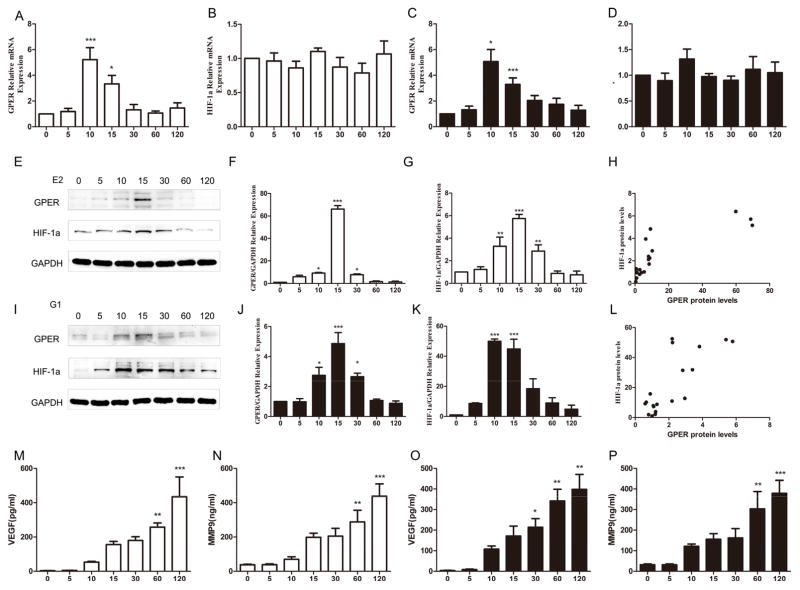
As E2 is a major factor in the pathogenesis of endometriosis, we hypothesized that it was E2 that induced higher level of GPER and HIF-1α in ESCs from EuEM. To determine the effects of E2 on GPER and HIF-1α level, we analyzed ESCs incubated with 10nM E2 for increasing time points (0, 5, 10, 15, 30, 60, 120min). In PCR assay, GPER mRNA was increased significantly after treated with E2 for 10 and 15min (Fig. 2A) while HIF-1α mRNA was similar at all the time points tested (Fig. 2B). In western blot analysis, there was a time-dependent increase in GPER and HIF-1α protein levels, and both reached peak effect at 15min (Fig. 2E-G). Furthermore, HIF-1α increased coincidently with GPER (Fig.2H). Results above suggested that the elevated level of GPER protein was in transcription dependent manner while HIF-1α protein was independent of HIF-1a transcription. We further examined expression of HIF-1α target genes VEGF and MMP9 in ESCs under E2 stimulation. Elisa analysis demonstrated that E2 significantly increased VEGF and MMP9 secretion and reached peak effect at 120min (Fig.2M and N). As E2 caused GPER-specific stimulation is difficult for the cross-reactivity of other estrogen receptors (ERs). In order to exclude the interference of other ERs, we repeated the above-mentioned experiments with G1, the first specific agonist of GPER (31). The results were consistent with the ones stimulated with E2 (Figure 2C, D, I-L, O, P). These findings above suggest that E2 and G1simultaneously promote protein levels of GPER, HIF-1α, and HIF-1α target genes VEGF and MMP9 in cultured ESCs.
Figure 2.
17β-Estradiol (E2) and G1 regulate GPER and HIF-1α expression in ESCs. (A, B, C, D) Time course of GPER and HIF-1α mRNA levels in ESCs treated with 10nM E2 or 100nM G1for 0, 5, 10, 15, 30, 60 and 120 min. (E, I) Time course of GPER and HIF-1α mRNA levels in ESCs treated with 10nM E2 or 100nM G1for 0, 5, 10, 15, 30, 60 and 120 min. (F, G, J, K) Quantitative comparison of the fold difference in the expression of GPER and HIF-1α proteins (*P<0.05, **P<0.01, ***P<0.001 by ANOVA). (H, L) Correlation between GPER and HIF-1α protein levels under different time cause of E2 (p<0.001, R=0.7014) or G1(p<0.001, R=0.6386). (M, N, O, P) Time course of VEGF and MMP9 secretion after treated with 10nM E2 or 100nM G1for 0, 5, 10, 15, 30, 60 and 120 min.
E2 and G1 induce HIF-1α mediated VEGF and MMP9 expression through GPER
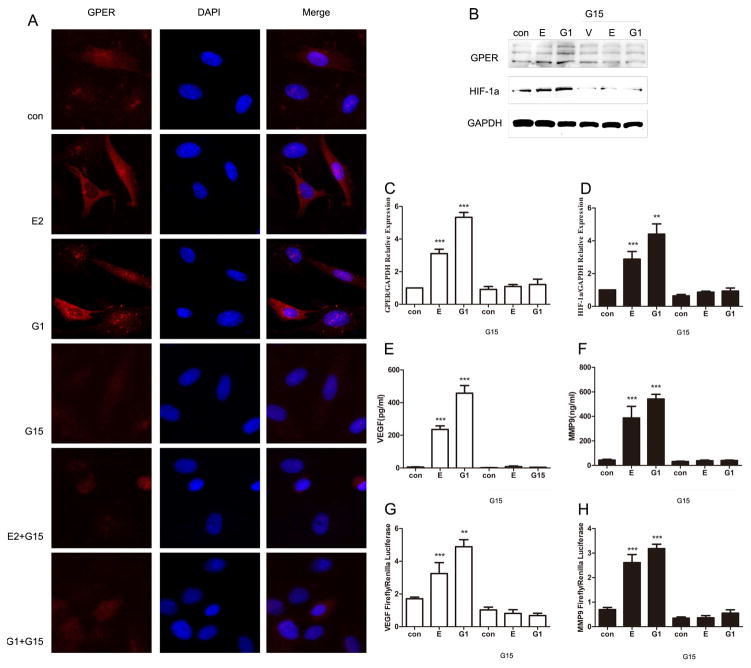
To further determine the role of GPER in E2 and G1mediated HIF-1α expression, we used G15, a antagonist of GPER activity. Immunofluorescence staining with GPER antibody revealed an intracellular pattern for GPER in ESCs. Its expression was significantly increased under the stimulation of E2 or G1 for 15 min; while significantly decreased when fore-stimulated with G15 for 30min (Fig.3A). Furthermore, to rule out the possibility that the protein level of HIF-1α was affected GPER, we performed western blotting analysis to examine the HIF-1α protein levels in different treatment of ESCs. Notably, Figure 3 B to D shows E2 and G1 upregulated HIF-1α expression level while the effect disappeared when blocking GPER. What’s more, Elisa assay revealed that stimulation of HIF-1α target gene VEGF and MMP9 in the medium also dependent on GPER expression (Fig. E, F). Accordingly, G1 and E2 transactivated VEGF and MMP9 promoter constructs (Fig. 3G and H) through GPER, as the luciferase activity was repressed when fore-stimulated with G15. All of the above suggested that E2 mediates HIF-1α activity in ESCs in a GPER-dependent manner.
Figure 3.
GPER mediates upregulation of HIF-1α and HIF-1α target gene expression induced by E2 and G1. ESCs were treated with E2 or G1, with or without pretreatment with G15 for 30min. (A) Evaluation of GPER protein expression by immunofluorescent microscopy in treated or untreated ESCs. (B) Immunoblots showing GPER and HIF-1α protein expression in ESCs under different treatment. (C, D) Quantitative comparison of the fold difference in GPER and HIF-1α protein expression. (E, F) Elisa assay showing VEGF and MMP9 secretion from ESCs of different conditions. (G, H) The transactivation of the VEGF and MMP9 promoters in ESCs by different treatment. (*P<0.05, **P<0.01, ***P<0.001 by ANOVA).
GPER is involved in VEGF-mediated tube formation
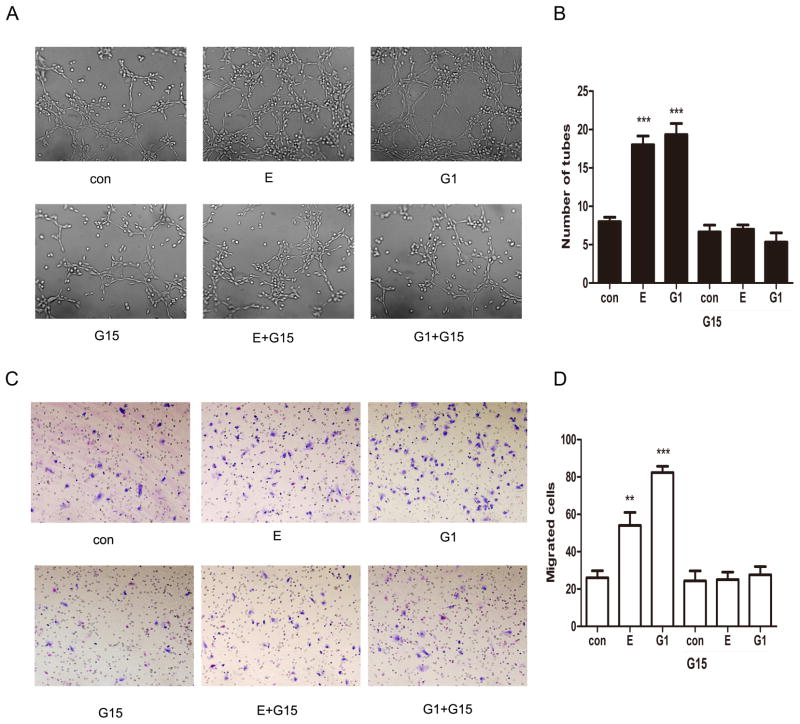
Previous results suggest that GPER mediates HIF-1α induced upregulation of VEGF secretion of ESCs. The influence of GPER can be also observed in an assay much closer to the in vivo situation of angiogenesis-in vitro tube formation of HUVECs. HUVECs were cultured using conditioned medium from ESCs previously treated with E2 or G1 for 120 min, with or without pretreatment with G15 for 30min. Interestingly, a ramified network of tubules was generated in HUVECs grown in medium from ESCs treated with E2 and G1; however, there were no effects when knocking down the expression of GPER by G15. These results, recapitulated in Figure 4A and B, indicate that VEGF may be considered as a target of the estrogenic GPER mediated signaling toward new blood vessels formation.
Figure 4.
E2 and G1enhance endothelial tube formation and ESCs migration through GPER. For endothelial tube formation, ESCs were treated with E2 or G1 for 120min, with or without pretreatment with GPER inhibitor G15 for 30min. Tube formation was evaluated in HUVECs cultured for 16–18 hours in medium collected from ESCs. (A) Representative photomicrographs of tube formation under different medium. (B) Quantified results of tube formation assay. For migration assay, ESCs were cultured in the upper chamber for 16h and then treated with E2 or G-1 for 120min, with or without pretreatment with GPER inhibitor G15 for 30min. (C) Representative photomicrographs of ESCs migration under different conditions. (D) Quantified results of migration assay. (*P<0.05, **P<0.01, ***P<0.001 by ANOVA).. (*P<0.05, **P<0.01, ***P<0.001 by ANOVA).
GPER is involved in MMP9-mediated cell migration
To determine whether the GPER participates in HIF-1α mediated ESCs migration, ESCs were stimulated under different conditions. As shown in Figure 4C and D, E2 and G1 significantly increased ESCs migration (P< 0.05, respectively). However, pre-incubated ESCs with G15 abolished the effect, leading to a significant decrease in the number of migrated cells (P< 0.05, respectively). Taken together, these data indicate that HIF-1α may function as a downstream factor in GPER mediated promotion of ESCs migration.
Discussion
The current study demonstrated that GPER and HIF-1α expressed in ESCs were higher in EuEM than in CoEM. What’s more, we found that E2 and G1 promoted HIF-1α expression in a GPER dependent manner. As a biological counterpart, we have evidence showing that GPER promoted HIF-1α mediates migration of ESCs and endothelial tube formation of HUVECs cultured in medium from ESCs. The present findings provide novel insights into the potential role of GPER in endometriosis angiogenesis and migration mediate through HIF-1α.
Multiple factors contribute to angiogenesis and migration of endometriosis. Changes in the expression of HIF-1α could be involved in these processes. Recently, more researchers studied the role of HIF-1α in endometriosis and several groups reported up-regulation of HIF-1α in EcEM (15, 17). In our study, we focused on EuEM and compared HIF-1α expression between EuEM and CoEM to exclude the interference of peritoneal fluid environment. Most previous studies were designed to clarify how HIF-1α induced expression of downstream genes that regulate proliferation, angiogenesis and, metastasis of endometriosis (15–17, 32). While mechanisms responsible for aberrant expression of HIF-1α remain enigmatic. In fact, cytokines, growth factors, and hormones beyond hypoxia were shown to upregulate HIF-1α expression (33). As endometriosis is an estrogen dependent disease, we hypothesized that high E2 stimulation resulted in increased expression of HIF-1α in EuEM.
The endogenous HIF-1a protein level is mainly depended on the rate of protein translation and degradation (34). Under normoxia, HIF-1α is posttranslationally modified by a mechanism that involves ubiquitylation by the Hippel-Lindau tumor suppressor E3 ligase complex and rapidly degradation. Conversely, under hypoxia, this process is inhibited by hypoxia, allowing stabilized HIF-1α accumulation and transcriptional activation (35). Recently, regulation of HIF-1α protein levels by E2 has been reported. E2 triggers multiple biological responses mainly through the specific receptors-ER alpha and ER beta (36) . In this study, we focused on GPER, a newly found receptor that is sensitive to estrogen. Beth J. Plante at el demonstrated cycle-regulated expression of GPER in normal human endometrium, with maximal expression in the proliferative phase and in EuEM and EcEM GPER were overexpressed (25). Our immunohistochemical results showed that EuEM expressed higher GPER and HIF-1α. Previous studies conducted clarify the relationship between GPER and HIF-1α. Anna Grazia Recchia and JUAN REN found that GPER was up-regulated by HIF-1α (37, 38), while Damiano Cosimo Rigiracciolo and Ernestina Marianna De Francesco certified that HIF-1α was up-regulated by GPER (28, 39). EuEM and CoEM exit under the same environment and the striking difference is that EuEM produce higher E2. So we hypothesize that GPER promotes HIF-1α expression in EuEM. In order to prove this, we isolated and cultured ESCs, and stimulated with E2, G1, and G15 respectively, and then examined HIF-1α and HIF-1α target genes VEGF and MMP9 expression. The results showed that E2 and G1 could increase HIF-1α protein expression in a transcriptional independent manner and enhance migration and angiogenesis of the cells while G15 could block these effects.
Even as a common disease, the pathogenesis of endometriosis is still ambiguous. High incidence and recurrence rate, lack of convenient and effective diagnosis and treatment methods, make endometriosis a major problem in gynecology. Early noninvasive diagnosis and efficient treatment are needed. In this study, we found that both GPER and HIF-1α were upregulated in EuEM. What’s more, we demonstrated that E2 stabilizes HIF-1α by GPER to promote ESCs invasion and angiogenesis. In summary, these findings provide new etiological insight into the development of endometriosis as well as shed light on the design of new diagnostic and therapeutic strategies. However, we did not clarify the regulation mechanism of estrogen on GPER, and the mechanism how GPER stabilizes HIF-1α protein. These need to be further studied in the future.
Supplementary Material
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant No.81471439 Y.L.) and the NIH Award (NIH HD 076257).
Footnotes
Disclosure statement: the authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giudice LC, Kao LC. Endometriosis. The Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–16. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 4.D’Hooghe TM, Kyama C, Debrock S, Meuleman C, Mwenda JM. Future directions in endometriosis research. Ann N Y Acad Sci. 2004;1034:316–25. doi: 10.1196/annals.1335.034. [DOI] [PubMed] [Google Scholar]
- 5.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Human Reproduction Update. 2011;17:637–53. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 6.Brosens I, Brosens JJ, Benagiano G. The eutopic endometrium in endometriosis: are the changes of clinical significance? Reprod Biomed Online. 2012;24:496–502. doi: 10.1016/j.rbmo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Lang JH. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med Sci Monit. 2011;17:RA92–9. doi: 10.12659/MSM.881707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swiersz LM. Role of endometriosis in cancer and tumor development. Ann N Y Acad Sci. 2002;955:281–92. doi: 10.1111/j.1749-6632.2002.tb02788.x. discussion 93–5, 396–406. [DOI] [PubMed] [Google Scholar]
- 9.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 10.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tafani M, De Santis E, Coppola L, Perrone GA, Carnevale I, Russo A, et al. Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression. Biomed Pharmacother. 2014;68:1–5. doi: 10.1016/j.biopha.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor--HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–7. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 13.Choi JY, Jang YS, Min SY, Song JY. Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions. J Breast Cancer. 2011;14:88–95. doi: 10.4048/jbc.2011.14.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maybin JA, Critchley HOD. Menstrual physiology: implications for endometrial pathology and beyond. Human Reproduction Update. 2015;21:748–61. doi: 10.1093/humupd/dmv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol. 2007;170:590–8. doi: 10.2353/ajpath.2007.060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril. 2014;101:568–76. doi: 10.1016/j.fertnstert.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Filippi I, Carrarelli P, Luisi S, Batteux F, Chapron C, Naldini A, et al. Different Expression of Hypoxic and Angiogenic Factors in Human Endometriotic Lesions. Reprod Sci. 2016;23:492–7. doi: 10.1177/1933719115607978. [DOI] [PubMed] [Google Scholar]
- 18.Zhan L, Wang W, Zhang Y, Song E, Fan Y, Wei B. Hypoxia-inducible factor-1alpha: A promising therapeutic target in endometriosis. Biochimie. 2016;123:130–7. doi: 10.1016/j.biochi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Xiong W, Zhang L, Xiong Y, Liu H, Liu Y. Hypoxia Promotes Invasion of Endometrial Stromal Cells via Hypoxia-Inducible Factor 1alpha Upregulation-Mediated beta-Catenin Activation in Endometriosis. Reprod Sci. 2016;23:531–41. doi: 10.1177/1933719115607999. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–47. doi: 10.1111/j.1749-6632.2001.tb03797.x. [DOI] [PubMed] [Google Scholar]
- 21.Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, et al. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10:29–38. doi: 10.1093/humupd/dmh007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Xiong W, Xiong Y, Liu H, Li N, Du Y, et al. Intracellular Wnt/Beta-Catenin Signaling Underlying 17beta-Estradiol-Induced Matrix Metalloproteinase 9 Expression in Human Endometriosis. Biol Reprod. 2016;94:70. doi: 10.1095/biolreprod.115.135574. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Xiong W, Xiong Y, Liu H, Liu Y. 17 beta-Estradiol promotes vascular endothelial growth factor expression via the Wnt/beta-catenin pathway during the pathogenesis of endometriosis. Mol Hum Reprod. 2016 doi: 10.1093/molehr/gaw025. [DOI] [PubMed]
- 24.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–26. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19:684–93. doi: 10.1177/1933719111431000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samartzis N, Samartzis EP, Noske A, Fedier A, Dedes KJ, Caduff R, et al. Expression of the G protein-coupled estrogen receptor (GPER) in endometriosis: a tissue microarray study. Reprod Biol Endocrinol. 2012;10:30. doi: 10.1186/1477-7827-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Yuan X, Zhang Y. The co-expression of GPER and Gankyrin in ovarian endometriosis and its correlation with the rASRM stages. Arch Gynecol Obstet. 2016;293:133–41. doi: 10.1007/s00404-015-3807-x. [DOI] [PubMed] [Google Scholar]
- 28.De Francesco EM, Pellegrino M, Santolla MF, Lappano R, Ricchio E, Abonante S, et al. GPER mediates activation of HIF1alpha/VEGF signaling by estrogens. Cancer Res. 2014;74:4053–64. doi: 10.1158/0008-5472.CAN-13-3590. [DOI] [PubMed] [Google Scholar]
- 29.Rigiracciolo DC, Scarpelli A, Lappano R, Pisano A, Santolla MF, De Marco P, et al. Copper activates HIF-1alpha/GPER/VEGF signalling in cancer cells. Oncotarget. 2015;6:34158–77. doi: 10.18632/oncotarget.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 31.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 32.Lin SC, Wang CC, Wu MH, Yang SH, Li YH, Tsai SJ. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab. 2012;97:E1515–23. doi: 10.1210/jc.2012-1450. [DOI] [PubMed] [Google Scholar]
- 33.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–40. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Sudhagar S, Sathya S, Lakshmi BS. Rapid non-genomic signalling by 17beta-oestradiol through c-Src involves mTOR-dependent expression of HIF-1alpha in breast cancer cells. Br J Cancer. 2011;105:953–60. doi: 10.1038/bjc.2011.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 36.Leitman DC, Paruthiyil S, Vivar OI, Saunier EF, Herber CB, Cohen I, et al. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–36. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recchia AG, De Francesco EM, Vivacqua A, Sisci D, Panno ML, Ando S, et al. The G protein-coupled receptor 30 is up-regulated by hypoxia-inducible factor-1alpha (HIF-1alpha) in breast cancer cells and cardiomyocytes. J Biol Chem. 2011;286:10773–82. doi: 10.1074/jbc.M110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren J, Guo H, Wu H, Tian T, Dong D, Zhang Y, et al. GPER in CAFs regulates hypoxia-driven breast cancer invasion in a CTGF-dependent manner. Oncol Rep. 2015;33:1929–37. doi: 10.3892/or.2015.3779. [DOI] [PubMed] [Google Scholar]
- 39.De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1alpha/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.