Abstract
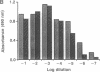
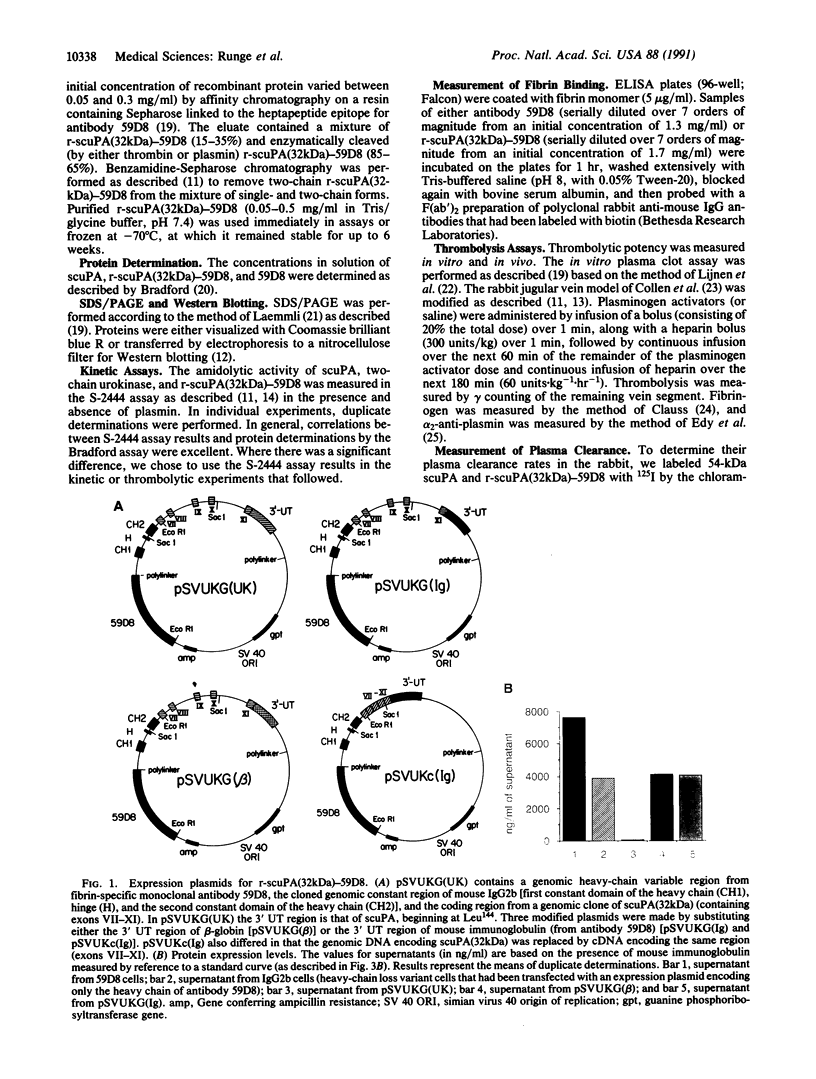
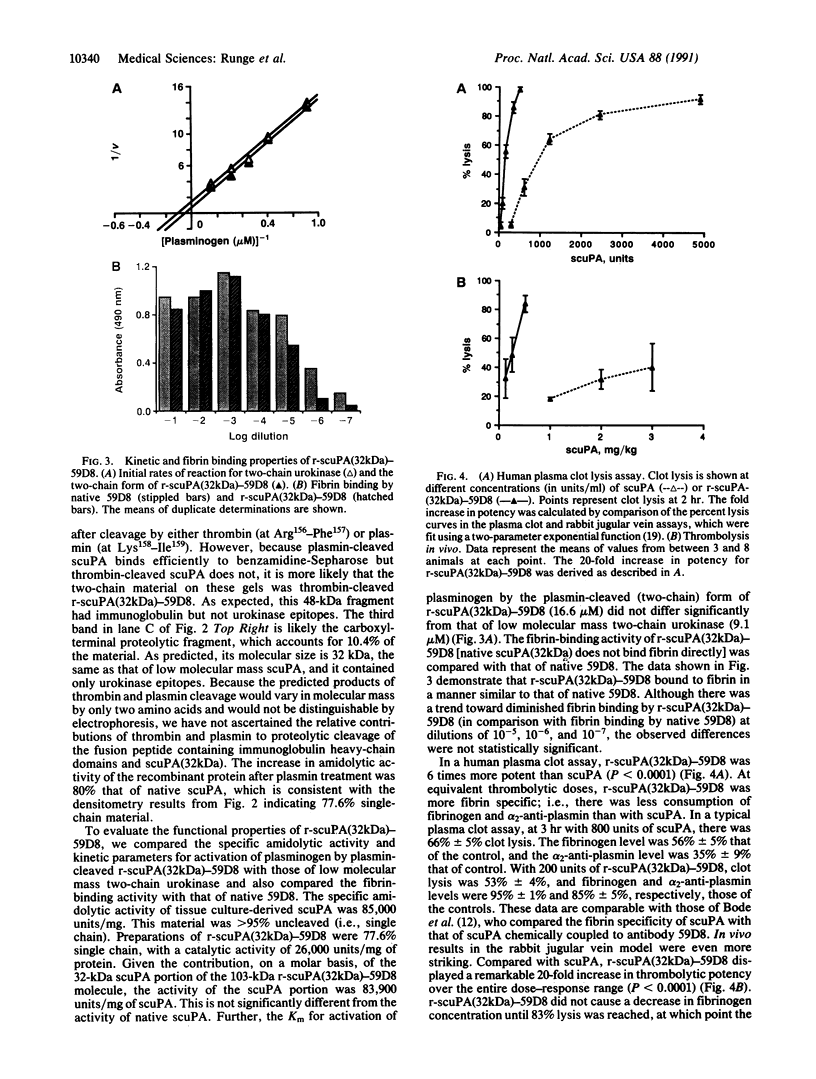
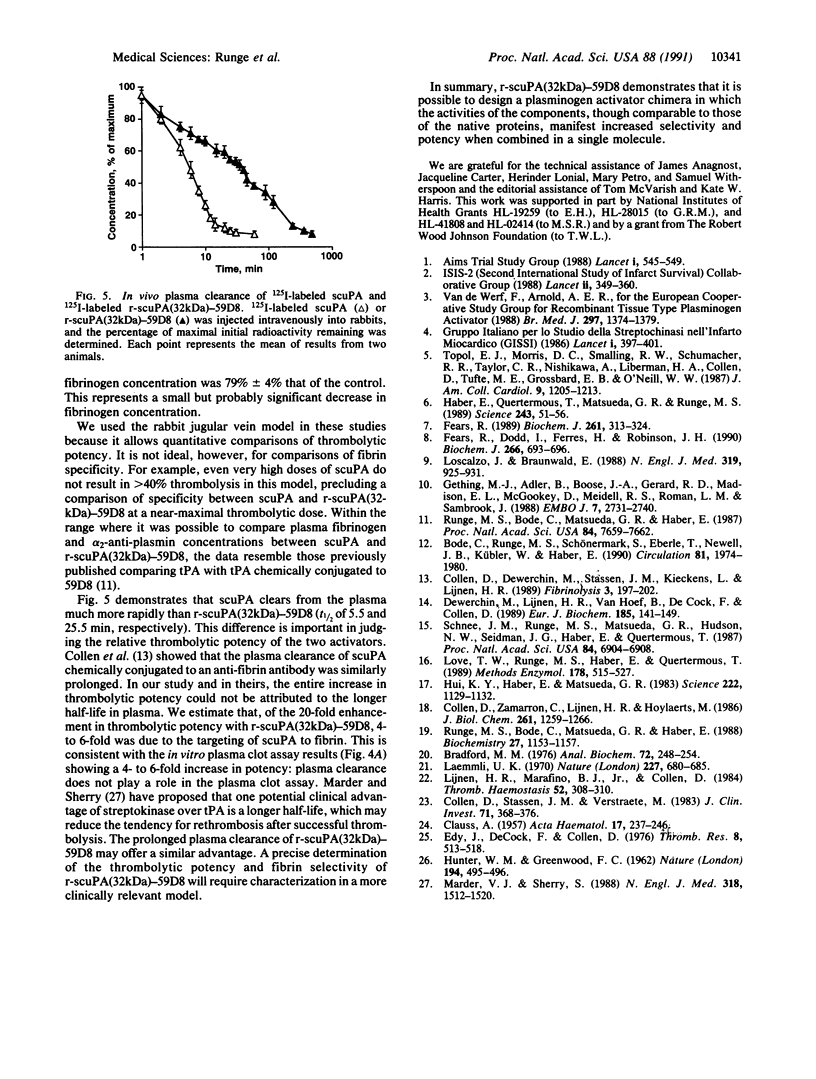
A recombinant plasminogen activator with high fibrin affinity and specificity was expressed by transfecting hybridoma cells with a plasmid that combines sequence coding for low molecular mass (32 kDa) single-chain urokinase-type plasminogen activator [scuPA(32kDa)] and anti-fibrin monoclonal antibody 59D8. The expression of the recombinant molecule [r-scuPA(32kDa)-59D8] was optimized by replacing the 3' untranslated region (initially that of high molecular mass scuPA) in the plasmid with the 3' untranslated region of either beta-globin or mouse immunoglobulin. This modification resulted in a greater than 100-fold improvement in the level of protein expression. The 103-kDa r-scuPA(32kDa)-59D8 protein displayed catalytic activity indistinguishable from that of high molecular mass scuPA and fibrin binding comparable to that of native antibody 59D8. r-scuPA(32kDa)-59D8 was 6 times more potent than high molecular mass scuPA in lysing a human plasma clot in vitro and was 20 times more potent than high molecular mass scuPA in the rabbit jugular vein model of thrombolysis. Molecules of this type may serve as prototypes for highly specific, antibody-targeted enzymes suitable for human use.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode C., Runge M. S., Schönermark S., Eberle T., Newell J. B., Kübler W., Haber E. Conjugation to antifibrin Fab' enhances fibrinolytic potency of single-chain urokinase plasminogen activator. Circulation. 1990 Jun;81(6):1974–1980. doi: 10.1161/01.cir.81.6.1974. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Collen D., Stassen J. M., Verstraete M. Thrombolysis with human extrinsic (tissue-type) plasminogen activator in rabbits with experimental jugular vein thrombosis. Effect of molecular form and dose of activator, age of the thrombus, and route of administration. J Clin Invest. 1983 Feb;71(2):368–376. doi: 10.1172/JCI110778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D., Zamarron C., Lijnen H. R., Hoylaerts M. Activation of plasminogen by pro-urokinase. II. Kinetics. J Biol Chem. 1986 Jan 25;261(3):1259–1266. [PubMed] [Google Scholar]
- Dewerchin M., Lijnen H. R., Van Hoef B., De Cock F., Collen D. Biochemical properties of conjugates of urokinase-type plasminogen activator with a monoclonal antibody specific for cross-linked fibrin. Eur J Biochem. 1989 Oct 20;185(1):141–149. doi: 10.1111/j.1432-1033.1989.tb15095.x. [DOI] [PubMed] [Google Scholar]
- Edy J., De Cock F., Collen D. Inhibition of plasmin by normal and antiplasmin-depleted human plasma. Thromb Res. 1976 Apr;8(4):513–518. doi: 10.1016/0049-3848(76)90229-2. [DOI] [PubMed] [Google Scholar]
- Fears R. Binding of plasminogen activators to fibrin: characterization and pharmacological consequences. Biochem J. 1989 Jul 15;261(2):313–324. doi: 10.1042/bj2610313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears R., Dodd I., Ferres H., Robinson J. H. Kinetic studies on novel plasminogen activators. Demonstration of fibrin enhancement for hybrid enzymes comprising the A-chain of plasmin (Lys-78) and B-chain of tissue-type plasminogen activator (Ile-276) or urokinase (Ile-159). Biochem J. 1990 Mar 15;266(3):693–696. doi: 10.1042/bj2660693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Adler B., Boose J. A., Gerard R. D., Madison E. L., McGookey D., Meidell R. S., Roman L. M., Sambrook J. Variants of human tissue-type plasminogen activator that lack specific structural domains of the heavy chain. EMBO J. 1988 Sep;7(9):2731–2740. doi: 10.1002/j.1460-2075.1988.tb03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber E., Quertermous T., Matsueda G. R., Runge M. S. Innovative approaches to plasminogen activator therapy. Science. 1989 Jan 6;243(4887):51–56. doi: 10.1126/science.2492113. [DOI] [PubMed] [Google Scholar]
- Hui K. Y., Haber E., Matsueda G. R. Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science. 1983 Dec 9;222(4628):1129–1132. doi: 10.1126/science.6648524. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Marafino B. J., Jr, Collen D. In vitro fibrinolytic activity of recombinant tissue-type plasminogen activator in the plasma of various primate species. Thromb Haemost. 1984 Dec 29;52(3):308–310. [PubMed] [Google Scholar]
- Loscalzo J., Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988 Oct 6;319(14):925–931. doi: 10.1056/NEJM198810063191407. [DOI] [PubMed] [Google Scholar]
- Love T. W., Runge M. S., Haber E., Quertermous T. Recombinant antibodies possessing novel effector functions. Methods Enzymol. 1989;178:515–527. doi: 10.1016/0076-6879(89)78037-x. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Sherry S. Thrombolytic therapy: current status (1). N Engl J Med. 1988 Jun 9;318(23):1512–1520. doi: 10.1056/NEJM198806093182306. [DOI] [PubMed] [Google Scholar]
- Runge M. S., Bode C., Matsueda G. R., Haber E. Antibody-enhanced thrombolysis: targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7659–7662. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge M. S., Bode C., Matsueda G. R., Haber E. Conjugation to an antifibrin monoclonal antibody enhances the fibrinolytic potency of tissue plasminogen activator in vitro. Biochemistry. 1988 Feb 23;27(4):1153–1157. doi: 10.1021/bi00404a012. [DOI] [PubMed] [Google Scholar]
- Schnee J. M., Runge M. S., Matsueda G. R., Hudson N. W., Seidman J. G., Haber E., Quertermous T. Construction and expression of a recombinant antibody-targeted plasminogen activator. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6904–6908. doi: 10.1073/pnas.84.19.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol E. J., Morris D. C., Smalling R. W., Schumacher R. R., Taylor C. R., Nishikawa A., Liberman H. A., Collen D., Tufte M. E., Grossbard E. B. A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction. J Am Coll Cardiol. 1987 Jun;9(6):1205–1213. doi: 10.1016/s0735-1097(87)80457-6. [DOI] [PubMed] [Google Scholar]
- Van de Werf F., Arnold A. E. Intravenous tissue plasminogen activator and size of infarct, left ventricular function, and survival in acute myocardial infarction. BMJ. 1988 Nov 26;297(6660):1374–1379. doi: 10.1136/bmj.297.6660.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]