Abstract
Intramuscular pressure (IMP), defined as skeletal muscle interstitial fluid pressure, reflects changes in individual muscle tension and may provide crucial insight into musculoskeletal biomechanics and pathologies. IMP may be measured using fiber-optic fluid pressure sensors, provided the sensor is adequately anchored to and shielded from surrounding muscle tissue. Ineffective anchoring enables sensor motion and inadequate shielding facilitates direct sensor-tissue interaction, which result in measurement artifacts and force-IMP dissociation. The purpose of this study was to compare the effectiveness of polyimide and nitinol protective housing designs to anchor pressure sensors to muscle tissue, prevent IMP measurement artifacts, and optimize the force-IMP correlation. Anchoring capacity was quantified as force required to dislodge sensors from muscle tissue. Force-IMP correlations and non-physiological measurement artifacts were quantified during isometric muscle activations of the rabbit tibialis anterior. Housing structural integrity was assessed after both anchoring and activation testing. Although there was no statistically significant difference in anchoring capacity, nitinol housings demonstrated greater structural integrity and superior force-IMP correlations. Further design improvements are needed to prevent tissue accumulation in the housing recess associated with artificially high IMP measurements. These findings emphasize fundamental protective housing design elements crucial for achieving reliable IMP measurements.
Keywords: Skeletal muscle, Isometric activation, Microsensor, Tibialis anterior, Force
INTRODUCTION
The ability to measure individual muscle forces would improve clinical assessments of musculoskeletal function and health, but remains a challenge in the field. Intramuscular pressure (IMP), the hydrostatic fluid pressure generated in muscle tissue, correlates linearly with passive and active tension and may be used to estimate muscle forces.1,16,17 Historically, tools such as the needle manometer, wick catheter, and transducer-tipped catheter have been employed to measure IMP. Even the smallest of these tools has a diameter of 1.33 mm, which is excessively invasive for routine clinical use. Additionally, catheters are sensitive to hydrostatic artifacts,14,18 limiting their application in dynamic settings.
More recently, fiber optic-based pressure microsensors have gained interest for IMP applications due to their small size (< 300 µm diameter, Fig. 1a) and insensitivity to hydrostatic artifacts.5,10 Fiber-optic pressure sensors use optical interferometry to detect fluid pressure changes through the deflection of a deformable diaphragm at the sensor tip. However, this diaphragm is also sensitive to mechanical perturbations from solid materials, therefore pressure measurement applications in muscle tissue require special consideration to prevent measurement artifact.
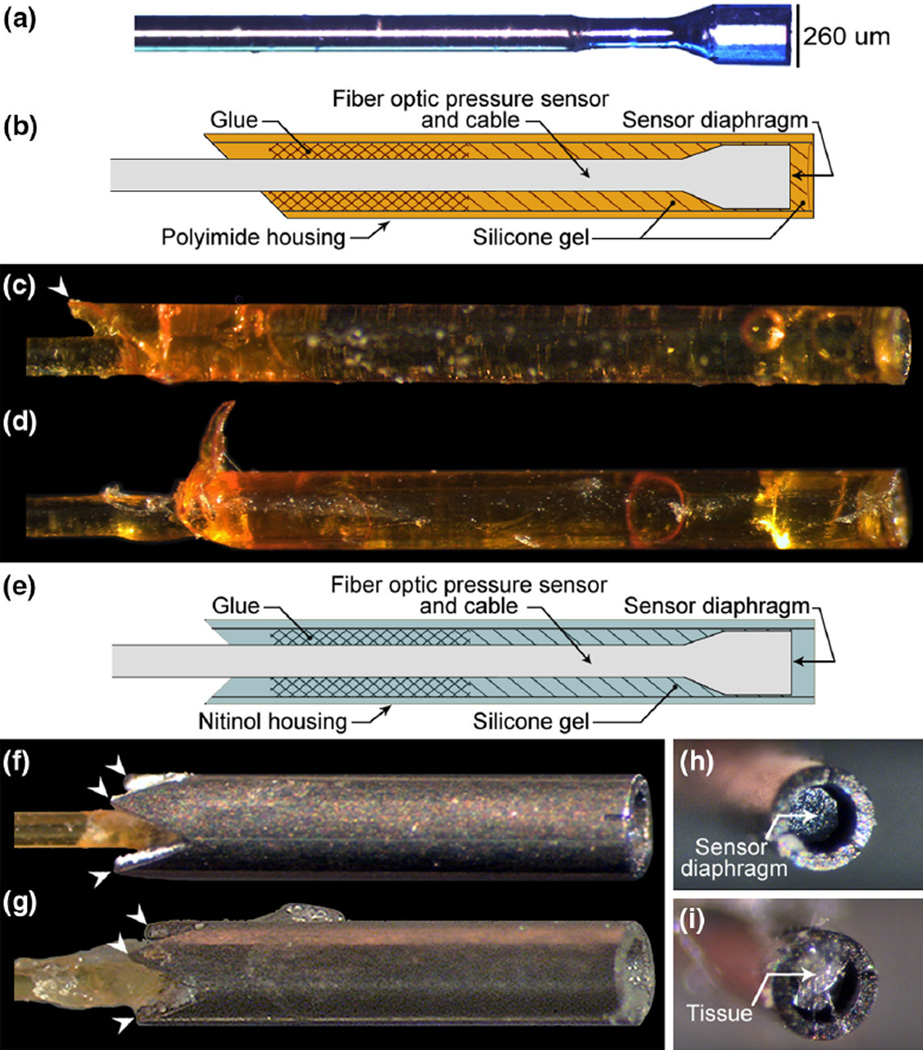
FIGURE 1.
(a) A representative image of a pressure microsensor with no housing. (b) Detailed assembly drawing of the polyimide housing sensor. A barbed polyimide housing sensor (c) before and (d) after isometric activations of a rabbit tibialis anterior. (e) Detailed assembly drawing of the nitinol housing sensor. A nitinol housing sensor (f) before and (g) after isometric activations. White arrowheads indicate barbs. Inspection of nitinol housings after testing revealing (h) an unobstructed recess and visible sensor diaphragm and (i) tissue accumulation in the recess. Polyimide housing barbs were damaged after muscle activations. In contrast, nitinol housing barbs remained intact.
Previous efforts to adapt fiber optic-based sensors for IMP measurement have produced mixed results. Sensors were housed within a barbed polyimide tube to protect the diaphragm6,19 and anchor the transducer to the surrounding tissue.20 Polyimide is regularly used in the fiber optic sensor industry due to its biocompatibility, high ultimate strength (231 MPa),7 and light weight. These investigations showed a linear relationship between IMP and force in the rabbit tibialis anterior muscle with an exposed6 or intact20 anterior compartment. However, IMP experimental variability was much greater than force variability, with coefficients of variation generally exceeding 86%.6 Significant sensor motion was detected using high-speed videography, which may have led to the observed dissociation between force and IMP during muscle activation.19 It is possible that damage to the polyimide barbs and subsequent loss of anchoring capacity resulted in the observed motion. As an alternative, we designed protective housings from nitinol, a biocompatible nickel titanium alloy with higher ultimate strength than polyimide (1100–1390 MPa)13 that is commonly used in biomedical implants.15 Nitinol’s strength and manufacturability allowed for a more complex four-barbed design compared to the polyimide protective housing.
Sensor anchoring and motion may also be affected by the orientation of the sensor relative to the shortening muscle fibers. Previous literature suggests that inserting sensors parallel to the muscle fiber shortening direction minimizes trauma by allowing fibers and connective tissue to guide sensors along the path of least resistance during a contraction.18 Consequently, parallel insertion may allow sensors to slide easily relative to the shortening muscle, which would generate motion-induced pressure artifacts that do not reflect muscle force. In contrast, inserting sensors perpendicular to fibers may increase resistance to sensor motion relative to the shortening fibers.
The purpose of this study was two-fold: first, to compare the ability of two housing designs to anchor sensor to tissue, minimize IMP measurement artifact, and maximize the within-trial force-IMP correlation; and second, to determine whether sensor orientation would improve anchoring and influence IMP measurements. We hypothesized that the nitinol housing would have superior barb integrity and improved anchoring compared to a single-barbed polyimide housing, resulting in higher force-IMP correlations. We further hypothesized that orienting sensors perpendicular to muscle fibers would result in improved anchoring and higher force-IMP correlations compared to the correlations obtained when orienting them parallel.
MATERIALS AND METHODS
Protective Housing Design
A polyimide and a nitinol housing design were compared in this study (Fig. 1). Based on material strength limitations, a single barb was formed in the polyimide housing with an acute angle cut, while four barbs were laser cut into the nitinol housing. The polyimide housing outer diameter (OD) was 307 µm and the nitinol housing OD was 457 µm. These housing sizes were selected based on the materials’ minimum available wall thickness and an inner diameter constraint of 260 µm, which is the sensor OD. The housings were cut to a 2–3 mm length, which created sufficient interior surface area for effective adhesion between the housing and the sensor.
Housings were attached to fiber optic pressure sensors (model FOP-M260, FISO Technologies, Inc.) with barbs pointing toward the cable (Fig. 1). Sensors were recessed relative to the flat end of the housings to protect the pressure sensing diaphragm from the tissue. Polyimide housing sensors were manufactured with a silicone gel meniscus in the recess, which is commonly used in biomedical pressure sensing applications9 to prevent tissue accumulation and enable sensor reuse. Nitinol housing sensors were manufactured without silicone gel for comparison. Sensors were cleaned between uses in a stirred Tergazyme solution (Alconox, White Plains, NY).
Animals
The experimental model was the tibialis anterior (TA) muscle of female New Zealand white rabbits (n = 18; mass: 3.14 ± 0.46 kg), chosen for its accessibility and parallel fiber arrangement.11 The protocol was approved by the University of California Institutional Animal Care and Use Committee. All experimental procedures adhered to guidelines set forth by the National Institutes of Health. All animals were euthanized with pentobarbital upon testing completion.
Anchoring Efficacy Assessments
Anchoring efficacy of the housings was assessed by qualitatively inspecting housing barbs at the conclusion of activation testing and, in separate experiments, by quantifying the force to remove sensors from muscle tissue. These assessments are described in greater detail in the following sections.
Barb Integrity
To test the hypothesis that nitinol would exhibit improved barb integrity over polyimide, housings were visually inspected for the presence or absence of damage, such as bending or fracture, incurred during muscle activation or removal from the muscle. Nitinol housing sensor recesses were also inspected with a dissecting microscope at the conclusion of activation testing to evaluate tissue accumulation.
Pullout Force
To test the hypotheses that (1) the nitinol housing would improve anchoring over polyimide housings and (2) perpendicular insertion would improve anchoring over parallel insertion the pullout force, defined as the peak force required to dislodge sensors from the tissue, was quantified as sensors were removed from non-activated rabbit TA muscles (n = 11). Sensors were inserted approximately 2 mm into the muscle mid-belly using a 22-gauge catheter oriented parallel or perpendicular to the fiber shortening direction. Each sensor cable was attached to a hand-held dynamometer and a ramped force was applied. Ten nitinol housing sensors were tested under parallel (n = 10 trials) and perpendicular (n = 12) insertion direction conditions. Three barbed polyimide housing sensors were tested under the parallel insertion condition (n = 6). Additionally, two barbless polyimide housing sensors were tested under the parallel insertion condition (n = 3) as a control for the barbed polyimide housing sensors. Sensor housings were inspected between pullout force trials and were not re-used if damaged.
Force and Intramuscular Pressure Measurements
To test the effect of housing design on IMP artifacts and the force-IMP correlation, force and IMP were simultaneously measured in rabbit TA muscles during isometric activations. The animal testing protocol was previously described by Davis et al.6 and Ward et al.19 In brief, animals were anesthetized (n = 7), a midline incision was made from the ankle to the mid-thigh, and the fascia was removed to expose the TA. The distal tendon was transected, attached to a force transducer-instrumented servo-motor (Model 310B, Aurora Scientific, Ontario, Canada), and aligned parallel to the motor’s force-generating axis. The leg was immobilized and secured to a custom jig at the mid-tibia and distal femur. A cuff electrode was placed around the peroneal nerve for direct muscle activation and the stimulation threshold was determined by increasing the current delivered until the maximum contraction force was elicited. The optimum muscle length was determined by applying a supramaximal stimulation at different muscle lengths until the length corresponding with the maximum force was identified as previously described.6
Polyimide (n = 4) or nitinol (n = 7) housing sensors were inserted in the same manner described in the previous section. At most three sensors were inserted per trial so as to ensure adequate sensor insertion depth and spacing, and to minimize cumulative muscle damage. Force and pressure were recorded simultaneously during 100 Hz tetanic isometric activations (pulse width 0.3 ms at 5–10 V, over a 450 ms period) at optimal muscle length. Up to 20 optimal length isometric activations were performed per animal. This protocol was performed in conjunction with other activations that included concentric and eccentric length changes, which were not included in the analysis. All activations were separated by 3-min rest intervals to prevent fatigue.
Data Analysis
Distribution normality was determined using the Anderson–Darling test using a p value cutoff of 0.05 and statistical tests were selected accordingly. Student’s t-tests were performed to test the hypotheses that a greater pullout force would be required for: (1) barbed versus barbless polyimide housing sensors; (2) nitinol housing sensors versus barbed polyimide housing sensors, both inserted parallel to the fiber shortening direction; and (3) nitinol housing sensors inserted perpendicular versus parallel to the fiber shortening direction. Cohen’s d effect size was quantified for significant differences.3
All IMP measurements (46 polyimide, 47 nitinol) were treated as independent trials regardless of the number of sensors in a muscle for a given activation. Baseline (median) resting force and IMP during the 100 ms period preceding each stimulation were subtracted from total force and IMP, respectively. All data were filtered using a second order lowpass Butterworth filter (200 Hz cutoff). To characterize the force-pressure agreement during the stimulation period, the coefficient of determination (COD) was calculated for each trial using MATLAB (The MathWorks, Natick, MA). Interpretation of the COD is limited since it is sensitive to measurement artifacts (i.e. instances where pressure does not reflect changes in force) but does not describe the nature of these artifacts. Two forms of artifact were observed in our data: high peak pressures and negative pressure measurements. High peak pressures may indicate mechanical interaction between the sensor diaphragm and muscle tissue, while negative pressures may be attributed to measurement error since it is generally agreed that muscle pressures are positive.12,14 To quantify these artifacts, the maximum (IMPmax) and minimum (IMPmin) pressure of each trial were identified for further analysis.
COD, IMPmax, and IMPmin were not normally distributed; therefore, significant differences in median COD, IMPmax, and IMPmin due to housing design were assessed using the Mann–Whitney–Wilcoxon test. Two sub-analyses were also performed on the nitinol housing sensor data using the Mann–Whitney–Wilcoxon test. First, the effect of sensor insertion direction on median COD, IMPmax, and IMPmin was assessed. Second, because muscle tissue remnants were frequently noted in the housing recess during post-testing sensor assessment, the median COD, IMPmax, and IMPmin distribution were also compared between trials associated with tissue accumulation versus those associated with an unobstructed recess. Cohens’ r effect size was quantified for groups with significant differences.4 Effect size values of 0.8, 0.5, and 0.2 represent large, moderate, and small effects, respectively.3 Statistical analyses were performed using JMP (The SAS Institute Inc. Cary, NC) with α = 0.05. Distribution statistics are reported as either means ± standard deviations, or medians.
RESULTS
Anchoring Efficacy Assessments
Barb Integrity
Nitinol housings were more resistant to damage compared to polyimide housings, but were susceptible to tissue accumulation in the housing recess. One out of four polyimide housings sustained structural damage during force and IMP testing; two out of three polyimide housings sustained structural damage during pullout force testing (Fig. 1c, 1d). No nitinol housing barbs were damaged (Fig. 1f, 1g). Inspection of the nitinol housing recess after testing confirmed an unobstructed recess for 24 trials (Fig. 1h) and tissue accumulation for 23 trials (Fig. 1i).
Pullout Force
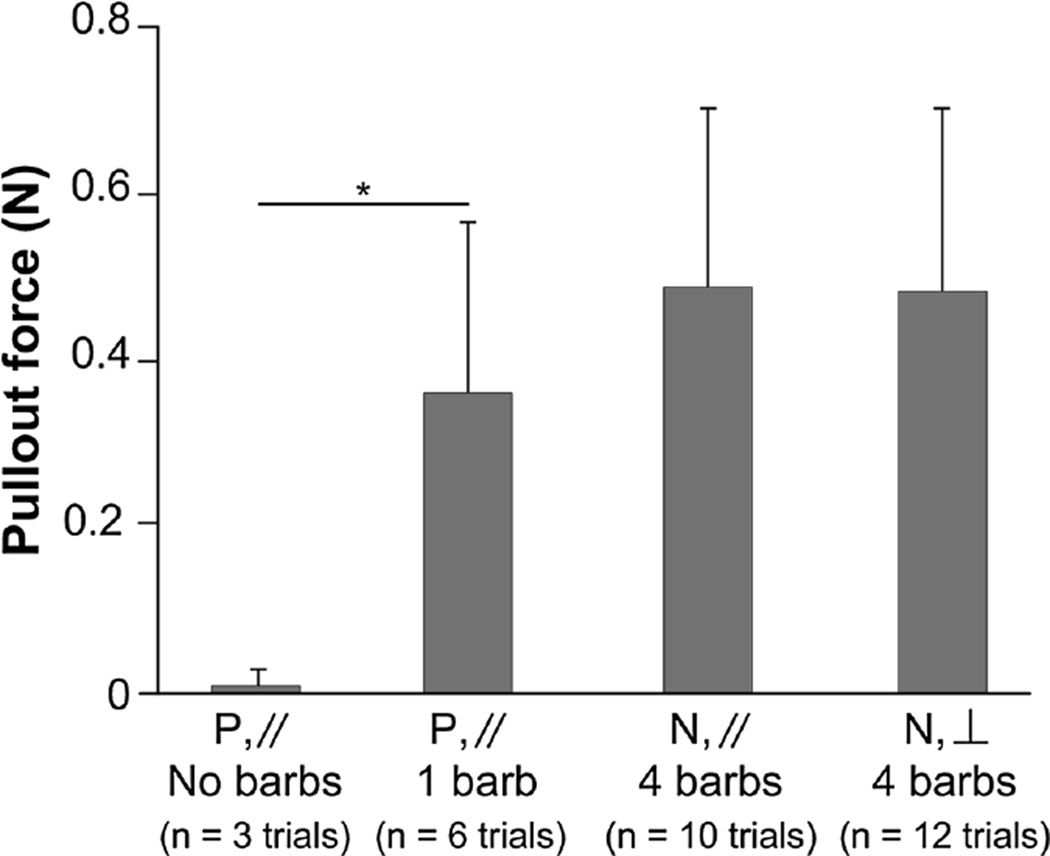
Significantly higher forces (p < 0.01) were required to extract barbed polyimide housings (0.36 ± 0.21 N) compared to barbless polyimide housings (0.01 ± 0.17 N). Cohen’s d effect size (1.37) suggests a high practical significance. No significant difference (p > 0.2) was found in the pullout force between barbed polyimide and nitinol housings (0.49 ± 0.21 N) or between parallel and perpendicular insertion directions (0.48 ± 0.22 N) for the nitinol housing (Fig. 2).
FIGURE 2.
Comparison of pullout forces of sensors with a single barbed polyimide (P) and four-barbed nitinol (N) housings inserted into the rabbit tibialis anterior either parallel (//) or perpendicular (⊥) to the direction of fiber shortening. Barbed polyimide housings required a significantly greater pullout force compared to barbless polyimide housings (p < 0.001). No significant difference was found between nitinol housings and barbed polyimide housings.
Force and Intramuscular Pressure Measurements
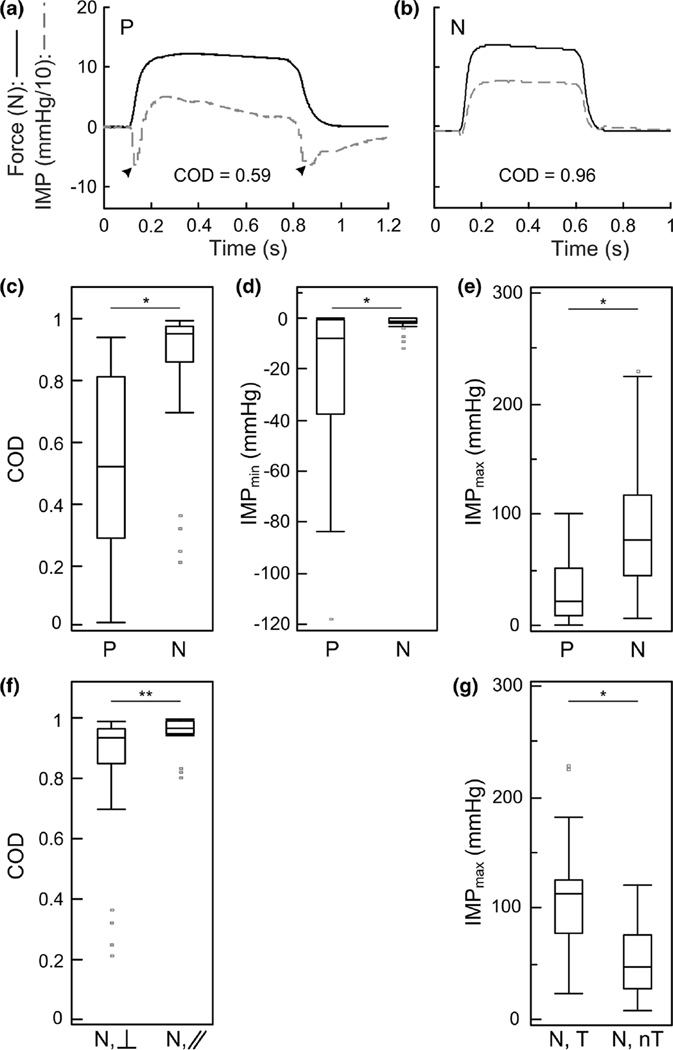
Nitinol housing sensors achieved a higher median and narrower force-pressure COD distribution compared to polyimide housing sensors (Fig. 3c; Table 1). The median nitinol COD was 0.96, and the median polyimide COD was 0.52. This difference was both statistically significant (p < 0.001) and considered to be of moderate practical significance (Cohen’s r = 0.67).
FIGURE 3.
Representative force (solid black) and intramuscular pressure (IMP; dashed grey) measured using a sensor with a (a) polyimide (P) housing and a (b) nitinol (N) housing during isometric activation of a rabbit tibialis anterior at optimal length. Black arrowheads indicate negative pressure artifacts. Box plots showing the (c) coefficient of determination (COD) between force and IMP, (d) minimum IMP (IMPmin) and (e) maximum IMP (IMPmax) measured using four polyimide housing sensors (n = 46 trials) and seven nitinol housing sensors (n = 47 trials). Sub-analyses of nitinol housing sensor on the effect of (f) insertion direction relative to fiber shortening direction (perpendicular, ⊥, n = 31 trials; parallel, //, n = 16 trials) on the COD and (g) the presence (tissue, T; n = 23 trials) or absence (no tissue, nT, n = 24 trials) of tissue accumulation in the recess on the IMPmax. Sensors with nitinol housings had a superior force-IMP correlation compared to polyimide housings and eliminated negative pressure artifacts. Parallel insertion resulted in a significantly greater COD compared to perpendicular insertion. The presence of tissue resulted in a significantly greater IMPmax compared to when tissue was not present in the recess. *p < 0.001, **p < 0.05.
TABLE 1.
Summary of isometric force-pressure coefficients of determination
| Insertion direction | Median | Interquartile range |
|---|---|---|
| Nitinol | ||
| Parallel | 0.96 | 0.95–0.99 |
| Perpendicular | 0.93 | 0.85–0.98 |
| Parallel + perpendicular | 0.96 | 0.86–0.99 |
| Polyimide | ||
| Parallel | 0.52 | 0.28–0.81 |
Representative force and IMP tracings demonstrate the frequently observed development of rapid and transient negative pressures in the polyimide housing sensor measurements (Fig. 3a), which were not observed in the nitinol housing sensor measurements (Fig. 3b). The median IMPmin for nitinol sensors (−1.2 mm Hg) was significantly higher than for polyimide sensors (−7.62 mm Hg, p < 0.001, Fig. 3d), although Cohen’s r effect size (0.36) suggests that this practical significance is small. Negative pressures as low as −118 mm Hg were measured with polyimide housing sensors, whereas nitinol housing sensors detected pressures no lower than −12 mm Hg (Fig. 3d). The median IMPmax for nitinol housing sensors (77.21 mm Hg) was significantly higher than for polyimide housing sensors (21.84 mm Hg, p < 0.001, Fig. 3e) and Cohen’s r effect size (0.54) suggests that this difference is of moderate significance.
Sub-analyses were performed on the nitinol housing pressure data to determine whether insertion direction (parallel, n = 16; perpendicular, n = 31, Fig. 3f) or tissue accumulation in the housing recess (tissue present, n = 23; unobstructed recess, n = 24, Fig. 3g) affected the COD, IMPmax, or IMPmin measurements. The median COD was significantly higher (p = 0.032) for the parallel insertions (0.96) compared to perpendicular insertions (0.93 Table 1, Fig. 3f). However, the practical significance of this difference is likely small (Cohen’s r = 0.31). Neither IMPmax nor IMPmin differed significantly between insertion directions (data not shown). The median IMPmax was significantly higher (p < 0.001) when tissue occluded the recess (113.24 mm Hg, Fig. 3g), than when the recess was unobstructed (47.1 mm Hg), and Cohen’s r value (0.56) suggests moderate practical significance. Neither IMPmin nor COD differed significantly between tissue accumulation conditions.
DISCUSSION
This study demonstrated improved IMP measurement during isometric activation using a new nitinol housing design over a polyimide housing design with a silicone gel meniscus. Compared to polyimide housings, nitinol housings were more resistant to muscle-induced damage. Although relatively high IMP was measured using a nitinol housing and was associated with tissue accumulation in the housing recess, the occurrence of negative pressure artifacts observed in polyimide housing pressure measurements was eliminated. Most importantly, sensors with nitinol housings yielded consistently higher correlations between force and IMP.
One possible reason why the nitinol housing design produced IMP measurements in better agreement with muscle tension is its five-fold greater material strength, which had implications on both the number of barbs manufactured and the structural integrity of these barbs. A greater number of intact barbs improves the likelihood that at least one barb latches on to the muscle tissue, which suggests a greater effectiveness at anchoring and resisting pullout. Nitinol’s greater material strength enabled a four barb design due to its ability to sustain the manufacturing forces without fracturing. In contrast, our attempts to manufacture multi-barbed polyimide housings consistently fractured the material and, thus, limited the design to a single barb. Furthermore, the effect of material strength on barb integrity was apparent during both pullout and activation testing, where two in three and one in four polyimide housings, respectively, exhibited damaged barbs, whereas none of the nitinol housings were damaged. The expected improvement in anchoring was subjectively observed during sensor insertion, where nitinol housing sensors anchored to the tissue more readily than polyimide housing sensors. Additionally, the mean nitinol housing pullout forces was greater than the polyimide housing pullout forces; however, this difference was not statistically significant. Our limited number of barbed polyimide housing sensors and their susceptibility to damage restricted the number of pullout trials that could be performed, resulting in inadequate statistical power (< 0.80).3 Additional testing is required to confirm these findings.
A second reason the nitinol housing design may have produced improved force-IMP agreement is the absence of the silicone gel meniscus filling the housing recess. Large negative gage pressure artifacts were only observed when using polyimide housing sensors. We were able to replicate these negative pressures using only the polyimide housing sensors by gently pressing the sensor tip against a piece of compliant rubber and releasing. This suggests that these artifacts may be caused by mechanical interaction between the silicone gel and muscle tissue. Silicone gel is highly effective as a cell culture substrate to measure traction forces.2 Therefore, it is possible that the gel could transiently adhere to muscle tissue, cause the diaphragm to deflect outward during a contraction, and generate a decreased pressure reading.
A consequence of the nitinol housing design was an increased outer diameter with respect to the polyimide housing design, resulting in 35% more surface area. While this may have also played a minor role in increasing resistance to sensor motion, the significantly higher pullout force measured using the single barbed polyimide housing compared to the barbless polyimide housing (with identical OD) provides strong evidence that the presence of barbs is the primary factor leading to enhanced anchoring overall.
We also hypothesized that insertion of sensors perpendicular to the muscle fiber shortening direction would improve the force-IMP correlation by enhancing the nitinol housing anchoring effectiveness and minimizing motion-related pressure artifacts. Contrary to this hypothesis, a statistically significant decrease in the COD between force and IMP was observed when the nitinol housing sensors were inserted perpendicular to fibers instead of parallel. However, the effect was marginal8 and we conclude that there is no meaningful difference between perpendicular and parallel insertion methods. It is possible that this difference may be more meaningful during isokinetic activations since large fiber length changes parallel to the sensing mechanism may induce motion artifacts in the pressure measurement.
The nitinol-based housing design has some limitations. We found visual evidence of tissue accumulation in the nitinol housing recess over the course of testing. The frequency of this occurrence was nearly fifty percent. Although this did not affect the within-trial force-IMP relationship (COD), presence of tissue was associated with significantly increased IMPmax (moderate effect size). It is possible that the tissue facilitated a direct mechanical force transmission mode between the muscle and sensor diaphragm, negating the benefits of recessing the sensor in the housing. There was no way to retrospectively identify the point when tissue accumulation occurred, but we observed pairs of activation trials where the same incremental force increase resulted in a greater increase in pressure over the previous trial. This further supports the proposed mechanism of mechanical interference. Additional development of this nitinol housing will require a mechanism to prevent tissue accumulation in the recess.
CONCLUSIONS
Our findings highlight protective housing design factors critical for reliable IMP measurements and suggest that the presented nitinol housing sensor is better suited for in vivo IMP measurement than a polyimide-based design. Insertion direction was not found to play a meaningful role in the IMP metrics under the prescribed experimental conditions. For IMP to be a useful in vivo tool, a mechanism must be designed to prevent tissue accumulation in the housing recess and the force-IMP relationship must be thoroughly investigated under isokinetic conditions.
Acknowledgments
This work was supported by the National Institutes of Health through the National Institute of Child Health and Human Development (R01HD31476), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32AR56950), the National Institute on Aging (F30AG050390), as well as the National Science Foundation Graduate Research Fellowship (1255833) and Mayo Graduate School. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation. Shanette Go thanks the Mayo Clinic Medical Scientist Training Program for fostering an outstanding environment for physician–scientist training.
ABBREVIATIONS
- COD
Coefficient of determination
- IMP
Intramuscular pressure
- IMPmax
Maximum intramuscular pressure
- IMPmin
Minimum intramuscular pressure
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Aratow M, Ballard RE, Crenshaw AG, Styf J, Watenpaugh DE, Kahan NJ, Hargens AR. Intramuscular pressure and electromyography as indexes of force during isokinetic exercise. J Appl. Physiol. 1993;74:2634–2640. doi: 10.1152/jappl.1993.74.6.2634. [DOI] [PubMed] [Google Scholar]
- 2.Burton K, Taylor DL. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature. 1997;385:450–454. doi: 10.1038/385450a0. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. p. 21. [Google Scholar]
- 4.Cooper HM, Hedges LV. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. p. 16. [Google Scholar]
- 5.Cottler PS, Karpen WR, Morrow DA, Kaufman KR. Performance characteristics of a new generation pressure microsensor for physiologic applications. Ann. Biomed. Eng. 2009;37:1638–1645. doi: 10.1007/s10439-009-9718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J, Kaufman KR, Lieber RL. Correlation between active and passive isometric force and intramuscular pressure in the isolated rabbit tibialis anterior muscle. J Biomech. 2003;36:505–512. doi: 10.1016/s0021-9290(02)00430-x. [DOI] [PubMed] [Google Scholar]
- 7.DuPont. Summary of properties for Kapton® polyimide films. http://www.dupont.com/content/dam/assets/products-and-services/membranes-films/assets/DEC-Kapton-summary-of-properties.pdf. [Google Scholar]
- 8.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp. Psychol. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 9.Goodyer PD, Fothergill JC, Jones NB, Hanning CD. The design of an optical fiber pressure transducer for use in the upper airways. Biomed. Eng. IEEE Trans. 1996;43:600–606. doi: 10.1109/10.495279. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman KR, Wavering T, Morrow D, Davis J, Lieber RL. Performance characteristics of a pressure microsensor. J Biomech. 2003;36:283–287. doi: 10.1016/s0021-9290(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 11.Lieber RL, Blevins FT. Skeletal muscle architecture of the rabbit hindlimb: functional implications of muscle design. J Morphol. 1989;199:93–101. doi: 10.1002/jmor.1051990108. [DOI] [PubMed] [Google Scholar]
- 12.Matsen FA, Mayo KA, Sheridan GW, Krugmire RB., Jr Monitoring of intramuscular pressure. Surgery. 1976;79:702–709. [PubMed] [Google Scholar]
- 13.Memry Corporation. Nitinol Tube: Memory Nitinol Tube Specifications. http://www.memry.com/products-services/material/tube. [Google Scholar]
- 14.Mubarak SJ, Hargens AR, Owen CA, Garetto LP, Akeson WH. The wick catheter technique for measurement of intramuscular pressure: a new research and clinical tool. J Bone Joint Surg. Am. 1976;58A:1016–1020. [PubMed] [Google Scholar]
- 15.Poncet P. Applications of superelastic nitinol tubing; International Conference on Shape Memory and Super Elastic Technologies; 1994. [Google Scholar]
- 16.Sejersted OM, Hargens AR. Intramuscular pressures for monitoring different tasks and muscle conditions. In: Gandevia SC, editor. Fatigue: Neural and Muscular Mechanisms. New York, NY: Plenum Press; 1995. pp. 339–350. [DOI] [PubMed] [Google Scholar]
- 17.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl. Physiol. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 18.Styf J. Compartment Syndromes: Diagnosis, Treatment, and Complications. Boca Raton: CRC Press; 2004. p. 301. [Google Scholar]
- 19.Ward SR, Davis J, Kaufman KR, Lieber RL. Relationship between muscle stress and intramuscular pressure during dynamic muscle contractions. Muscle Nerve. 2007;36:313–319. doi: 10.1002/mus.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winters TM, Sepulveda GS, Cottler PS, Kaufman KR, Lieber RL, Ward SR. Correlation between isometric force and intramuscular pressure in rabbit tibialis anterior muscle with an intact anterior compartment. Muscle Nerve. 2009;40:79–85. doi: 10.1002/mus.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]