Abstract
Mycobacterium kansasii is one of the most pathogenic and frequent nontuberculous mycobacteria isolated from humans. Patients with adverse drug reactions, resistant isolates, or suboptimal response require alternative treatment regimens. One hundred forty-eight consecutive clinical isolates of M. kansasii were tested for antimicrobial susceptibilities by the BACTEC 460 system (NCCLS) with two different inoculation protocols, one conventional and one alternative. In the alternative protocol, the inoculum 12B vial was incubated until the growth index was between 250 and 500. Four conventional antimycobacterial drugs (isoniazid, rifampin, streptomycin, and ethambutol) were studied with standard critical concentrations. The in vitro activities of linezolid, telithromycin, clarithromycin, levofloxacin, and moxifloxacin were determined by measuring radiometric MICs. All isolates tested were identified as M. kansasii genotype I and were resistant to isoniazid at a concentration of 0.4 μg/ml. One hundred twenty isolates (81.1%) were inhibited by 1 μg of isoniazid per ml. A high level of resistance to isoniazid (>10 μg/ml) was observed in six isolates (4.1%). Only five strains (3.4%) were resistant to rifampin (>1 μg/ml). All isolates studied were susceptible to streptomycin and ethambutol. The MICs at which 90% of the isolates were inhibited (in micrograms per milliliter) were as follows: linezolid, 1 (range, ≤0.25 to 2); telithromycin, >16 (range, 4 to >16); clarithromycin, 0.5 (range, ≤0.03 to 1); levofloxacin, 0.12 (range, 0.12 to 0.25); and moxifloxacin, 0.06 (range, ≤0.06 to 0.12). The susceptibility testing results with both inoculation protocols showed perfect correlation. In conclusion, all M. kansasii isolates showed decreased susceptibility to isoniazid, but resistance to rifampin was infrequent. Quinolones, especially moxifloxacin, were the most active antimicrobial agents tested, followed by clarithromycin. Linezolid also showed good activity against these microorganisms, but telithromycin's in vitro activity was poor.
Mycobacterium kansasii is one of the most pathogenic and frequent nontuberculous mycobacteria isolated from humans (3, 4, 9, 12, 25). M. kansasii produces a chronic, progressive, and cavitary lung disease similar to tuberculosis or produces disseminated infections, usually in patients infected by the human immunodeficiency virus (4, 7, 20, 24, 31).
The currently recommended treatment for M. kansasii infections includes isoniazid, rifampin, and a third agent, usually ethambutol or streptomycin, for 18 months, with at least 12 months of negative cultures (10, 18, 30). However, patients with adverse drug reactions, isolates that are resistant to one or more drugs (especially rifampin), or a poor response to initial therapy have all been documented (1, 8, 24, 29). Therefore, alternative effective antimicrobial agents for these infections are needed.
In vitro susceptibility testing of most nontuberculous mycobacteria is of little help for managing the treatment of these infections. However, there is evidence that the in vitro susceptibility of M. kansasii, based on the interpretative criteria used with Mycobacterium tuberculosis, correlates adequately with clinical response (15, 16).
The modified proportion method (agar), the BACTEC radiometric system, and the broth microdilution method have demonstrated good in vitro correlation (14, 16, 21, 27). Although the radiometric system is the fastest in vitro susceptibility testing method available for mycobacterial organisms (14-16), the standard inoculation procedure recommended by the manufacturers could be improved.
The aim of this study was to determine the susceptibilities of 148 consecutive M. kansasii strains isolated from clinical specimens to linezolid, telithromycin, clarithromycin, levofloxacin, moxifloxacin, and four conventional antimycobacterial drugs. In addition, two different inoculation procedures using the radiometric method were evaluated.
(This study was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 2003 [F. Alcaide, L. Calatayud, M. Santín, and R. Martín, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-1711, p. 208, 2003].)
MATERIALS AND METHODS
Organisms.
A total of 148 strains of M. kansasii isolated consecutively from clinical specimens (142 respiratory isolates and 6 nonrespiratory isolates) between January 1997 and December 2003 in the Hospital Universitari de Bellvitge (Barcelona, Spain) were tested for antimicrobial susceptibility. Only one isolate from each patient was studied, with 145 (98%) being initial isolates. One hundred thirty-one (88.5%) isolates were considered pathogenic according to American Thoracic Society criteria (30). M. kansasii ATCC 12478 and Staphylococcus aureus ATCC 29213 were used for quality control.
Mycobacterial isolates were identified by conventional biochemical and culture tests, PCR-restriction fragment length polymorphism analysis of the hsp65 gene, and DNA probes (AccuProbe; GenProbe Inc., San Diego, Calif.). These gene probes were selectively applied to each positive culture to identify M. kansasii on the basis of the colony pigmentation and microscopic characteristics (cross-barring morphology) when grown in liquid medium.
Antimicrobial agents.
Four conventional antimycobacterial drugs (Becton Dickinson, Sparks, Md.) were studied with different critical concentrations: isoniazid (0.1, 0.4, 1, 5, and 10 μg/ml), rifampin (1 μg/ml), streptomycin (2 and 6 μg/ml), and ethambutol (5 and 7.5 μg/ml). The antimicrobial concentration ranges tested for MIC determination were as follows: linezolid (Pharmacia, Kalamazoo, Mich.), 0.25 to 8 μg/ml; telithromycin (Aventis Pharma, Madrid, Spain), 0.5 to 16 μg/ml; clarithromycin (Abbot Laboratories, Queenborough, United Kingdom), 0.03 to 2 μg/ml; levofloxacin (Aventis Pharma), 0.03 to 4 μg/ml; and moxifloxacin (Bayer, Wuppertal, Germany), 0.03 to 4 μg/ml. Drugs were prepared according to the manufacturer's recommendations. High concentrations of stock solutions (10,000 μg/ml) were prepared. Small volumes of these solutions were stored in sterile polypropylene vials for up to 6 months at −80°C.
Susceptibility testing.
The susceptibilities of the strains were tested with the radiometric (BACTEC) susceptibility test (Becton Dickinson), based on the interpretative criteria used with M. tuberculosis (14-16, 19, 21). Two different inoculum preparation protocols were performed and compared in parallel with all drugs studied.
(i) Conventional protocol (26).
A suspension from the initial bacterial growth on solid medium (McFarland no. 1) was subcultured (0.1 ml) into a vial of fresh BACTEC 12B medium. The growth index (GI) was measured daily (including weekends and holidays) with the BACTEC 460-TB instrument until it reached 999, and then 0.5 ml of this 12B suspension was diluted (1:20) in 9.5 ml of BACTEC diluting fluid.
(ii) Alternative protocol (proposed by us).
The 12B vial was incubated until the GI was between 250 and 500. The inoculation and incubation of the susceptibility testing vials were similar to those for the conventional protocol. The drug-containing vials were inoculated with 0.1 ml of the 12B suspension. The drug-free control vial was inoculated with 0.1 ml of a suspension diluted 1:100. Vials were incubated at 37 ± 1°C in the dark, and they were read daily (including weekends and holidays) until the GI of the vial control reached ≥30. The results were interpreted when the GI of the control vial was ≥30 between a minimum and maximum of 4 and 14 days of incubation, respectively. The MIC was defined as the lowest drug concentration that inhibited more than 99% of the bacterial population.
RESULTS
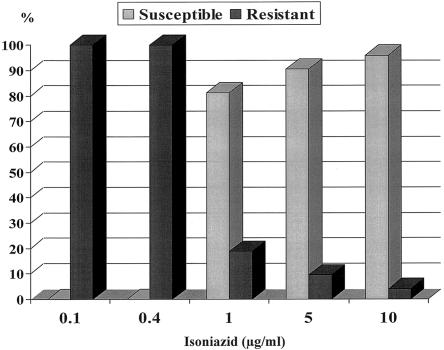
All isolates tested were identified as M. kansasii subtype I according to PCR-restriction fragment length polymorphism analysis of the hsp65 gene and were resistant to isoniazid at a concentration of ≤0.4 μg/ml (Fig. 1).
FIG. 1.
Percentages of M. kansasii isolates found to be susceptible and resistant to isoniazid at different concentrations.
One hundred twenty isolates (81.1%) were inhibited by 1 μg of isoniazid per ml. Of the 14 M. kansasii isolates that were resistant at a concentration of ≥5 μg/ml, a high level of resistance to isoniazid (>10 μg/ml) was detected in 6 isolates (4.1%). Five strains (3.4%) were resistant to rifampin (>1 μg/ml). These strains were susceptible to 1 μg of isoniazid per ml. Three of the five patients with rifampin-resistant isolates were previously treated for M. kansasii infection. All isolates studied were susceptible to 6 μg of streptomycin per ml and to 7.5 μg of ethambutol per ml.
The susceptibility testing results for linezolid, telithromycin, clarithromycin, levofloxacin, and moxifloxacin are shown in Table 1. The strains were classified in two groups according to their isoniazid susceptibilities (MIC): ≤1 μg/ml (81.1%) and >1 μg/ml (18.9%). The MICs at which 50% of the isolates were inhibited (MIC50s) and the MIC90s of the antimicrobial agents tested were similar in the two groups. All isoniazid-resistant strains were inhibited at 0.12 μg of moxifloxacin per ml, 0.25 μg of levofloxacin per ml, 1 μg of clarithromycin per ml, and 2 μg of linezolid per ml or less.
TABLE 1.
In vitro activities of linezolid, telithromycin, clarithromycin, levofloxacin, and moxifloxacin against M. kansasii isolates with two different levels of resistance to isoniazid
| Isoniazid concn, μg/ml (n) | Parameter | Value (μg/ml) for:
|
||||
|---|---|---|---|---|---|---|
| Linezolid | Telithromycin | Clarithromycin | Levofloxacin | Moxifloxacin | ||
| ≤1 (120) | MIC50 | 1 | 16 | 0.12 | 0.12 | 0.06 |
| MIC90 | 1 | >16 | 0.5 | 0.12 | 0.06 | |
| Range | ≤0.25-2 | 4->16 | ≤0.03-1 | 0.12-0.25 | ≤0.06-0.12 | |
| >1 (28) | MIC50 | 1 | 16 | 0.12 | 0.12 | 0.06 |
| MIC90 | 1 | >16 | 0.5 | 0.12 | 0.06 | |
| Range | ≤0.25-2 | 4->16 | ≤0.03-1 | 0.12-0.25 | ≤0.06-0.12 | |
The susceptibility testing results with both inoculation protocols had a correlation of 100% with all isolates. With the alternative proposed protocol (a GI of between 250 and 500 in the inoculum vial), the control vial GI was ≥30 after the fourth day of incubation. Therefore, the susceptibility testing could be properly interpreted for each isolate studied. The results with the alternative protocol were reported between 5 and 8 days after inoculation, and the mean turnaround times were 2 days shorter than the mean times with the conventional protocol.
DISCUSSION
This is the first in vitro susceptibility study of M. kansasii in which a genotyping of isolates has been performed. To date, seven subtypes (I to VII) have been identified by PCR-restriction fragment length polymorphism of the hsp65 gene (22, 28). This heterogeneity may have important pathogenic, clinical, and epidemiological implications (2, 28). M. kansasii subtype I seems to be by far the most frequent and pathogenic subtype isolated from humans in many geographic areas of the world (2, 25, 28, 33). Since all isolates tested in this study were identified as M. kansasii subtype I, the results obtained provide useful microbiological data for the clinical management of these infections.
In agreement with previous reports, all isolates of M. kansasii studied showed decreased susceptibility to isoniazid. Consequently, as the NCCLS recommends (21), susceptibility testing of isoniazid in these microorganisms should use critical concentrations higher than those for M. tuberculosis (≥1 μg/ml). Although only one-fifth of the isolates showed significant in vitro resistance to isoniazid (≥5 μg/ml), they might represent a possible therapeutic problem in some circumstances.
Streptomycin and ethambutol showed good in vitro activity with the two (low and high) critical concentrations used. Resistance to rifampin was rare. However, the great majority of M. kansasii strains tested (98%) in this study were initial clinical isolates from each patient, and resistance to rifampin, isoniazid, and ethambutol may be acquired during treatment (29). Given that rifampin is the cornerstone of the current treatment, the primary testing of this drug should be recommended.
Although some new or secondary antimycobacterial agents have been tested with M. kansasii isolates (5, 6, 11, 17, 23, 32), data on the effects of many of these drugs on this microorganism are limited. In the present evaluation with a large number of strains, quinolones (especially moxifloxacin) were the most active antimicrobial agents studied. Clarithromycin and linezolid also showed good activity against these microorganisms, whereas the in vitro activity of telithromycin was poor. In addition, the M. kansasii isolates for which MICs of isoniazid were >1 μg/ml did not show decreased susceptibility to the new antimicrobial agents tested. These in vitro data therefore suggest that moxifloxacin followed by levofloxacin, clarithromycin, and linezolid may represent a good therapeutic alternative in M. kansasii infections. However, controlled clinical trials are needed to validate the potential usefulness of these drugs and their safety in clinical practice. Preliminary data with a short-course and intermittent treatment regimen with clarithromycin have recently been reported, with good results in a small number of M. kansasii-infected patients (13).
In addition, a new inoculation protocol using radiometric susceptibility testing in M. kansasii is proposed. The in vitro susceptibility results showed perfect correlation with both inoculation protocols. However, the alternative modified protocol offers certain advantages over the standard one: (i) it saved material and hand labor because dilution with diluting fluid was not necessary, and (ii) the results were available sooner than with the standard protocol, thus allowing faster interpretation. In conclusion, the new proposed protocol is a simple, fast, accurate, and cost-saving procedure that can be implemented in the routine susceptibility testing of M. kansasii by using the radiometric method.
Acknowledgments
We are grateful to M. Tutusaus and M. Juncal for their technical assistance.
REFERENCES
- 1.Ahn, C. H., R. J. Wallace, Jr., L. C. Steele, and D. T. Murphy. 1987. Sulfonamide-containing regimens for disease caused by rifampin-resistant Mycobacterium kansasii. Am. Rev. Respir. Dis. 135:10-16. [DOI] [PubMed] [Google Scholar]
- 2.Alcaide, F., I. Ritcher, C. Bernasconi, B. Springer, C. Hagenau, R. Schulze-Röbbecke, E. Tortoli, R. Martín, E. C. Böttger, and A. Telenti. 1997. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J. Clin. Microbiol. 35:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittner, M. J., E. A. Horowitz, T. J. Safranek, and L. C. Preheim. 1996. Emergence of Mycobacterium kansasii as the leading mycobacterial pathogen isolated over a 20-year period at a Midwestern veterans affairs hospital. Clin. Infect. Dis. 22:1109-1110. [DOI] [PubMed] [Google Scholar]
- 4.Bloch, K. C., L. Zwerling, M. J. Pletcher, J. A. Hahn, J. L. Gerberding, S. M. Ostroff, D. J. Vugia, and A. L. Reingold. 1998. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann. Intern. Med. 129:698-704. [DOI] [PubMed] [Google Scholar]
- 5.Brown, B. A., R. J. Wallace, Jr., and G. O. Onyi. 1992. Activities of clarithromycin against eight slowly growing species of nontuberculous mycobacteria, determined by using a broth microdilution MIC system. Antimicrob. Agents Chemother. 36:1987-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliot, B. A., C. J. Crist, L. B. Mann, R. W. Wilson, and R. J. Wallace, Jr. 2003. In vitro activity of linezolid against slowly growing nontuberculous mycobacteria. Antimicrob. Agents Chemother. 47:1736-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canueto-Quintero, J., F. J. Caballero-Granado, M. Herrero-Romero, A. Dominguez-Castellano, P. Martin-Rico, E. V. Verdu, D. S. Santamaria, R. C. Cerquera, M. Torres-Tortosa, et al. 2003. Epidemiological, clinical, and prognostic differences between the diseases caused by Mycobacterium kansasii and Mycobacterium tuberculosis in patients infected with human immunodeficiency virus: a multicenter study. Clin. Infect. Dis. 37:584-590. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1996. Impact of HIV protease inhibitors on the treatment of HIV-infected tuberculosis patients with rifampin. Morb. Mortal. Wkly. Rep. 45:921-925. [PubMed] [Google Scholar]
- 9.Chobot, S., J. Malis, H. Sebakova, M. Pelikan, O. Zatloukal, P. Palicka, and D. Kocurova. 1997. Endemic incidence of infections caused by Mycobacterium kansasii in the Karvina district in 1968-1995 (analysis of epidemiological data-review). Cent. Eur. J. Public Health 5:164-173. [PubMed] [Google Scholar]
- 10.Evans, S. A., A. Colville, A. J. Evans, A. J. Crisp, and I. D. A. Johnston. 1996. Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax 51:1248-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie, S. H., and O. Billington. 1999. Activity of moxifloxacin against mycobacteria. J. Antimicrob. Chemother. 44:393-395. [DOI] [PubMed] [Google Scholar]
- 12.Good, R. C., and D. E. Snider, Jr. 1980. Isolation of nontuberculous mycobacteria in the United States, 1980. J. Infect. Dis. 146:829-833. [DOI] [PubMed] [Google Scholar]
- 13.Griffith, D. E., B. A. Brown-Elliott, and R. J. Wallace, Jr. 2003. Thrice-weekly clarithromycin-containing regimen for treatment of Mycobacterium kansasii lung disease: results of a preliminary study. Clin. Infect. Dis. 37:1178-1182. [DOI] [PubMed] [Google Scholar]
- 14.Heifets, L. B. 1991. Drug susceptibility in the chemotherapy of mycobacterial infection. CRC Press, Inc., Boca Raton, Fla.
- 15.Inderlied, C. B., and K. A. Nash. 1996. Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biologic fluids, p. 127-175. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 16.Inderlied, C. B., and G. E. Pfyffer. 2003. Susceptibility test methods: mycobacteria, p. 1149-1177. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 17.Jacobs, M. R. 1999. Activity of quinolones against mycobacteria. Drugs. 58(Suppl. 2):19-22. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson, M. A., and W. M. Isenberg. 1989. Mycobacterium kansasii diffuse pulmonary infection in a patient with acquired immune deficiency syndrome: successful therapy with an antituberculous regimen. Am. J. Clin. Pathol. 91:236-238. [DOI] [PubMed] [Google Scholar]
- 19.Lee, C., and L. B. Heifets. 1987. Determination of minimal inhibitory concentrations of antituberculosis drugs by radiometric and conventional methods. Am. Rev. Respir. Dis. 136:349-352. [DOI] [PubMed] [Google Scholar]
- 20.Lillo, M., S. Orengo, P. Cernoch, and R. L. Harris. 1990. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev. Infect. Dis. 12:760-767. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Document M24-A. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 22.Richter, E., S. Niemann, S. Rusch-Gerdes, and S. Hoffner. 1999. Identification of Mycobacterium kansasii by using a DNA probe (AccuProbe) and molecular techniques. J. Clin. Microbiol. 37:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez Díaz, J. C., M. López, M. Ruíz, and G. Royo. 2003. In vitro activity of new fluoroquinolones and linezolid against nontuberculous mycobacteria. Int. J. Antimicrob. Agents 47:416-417. [DOI] [PubMed] [Google Scholar]
- 24.Santín, M., and F. Alcaide. 2003. Mycobacterium kansasii disease among patient infected with human immunodeficiency virus type 1: improved prognosis in the era of highly active antiretroviral therapy. Int. J. Tuberc. Lung Dis. 7:673-677. [PubMed] [Google Scholar]
- 25.Santín, M., F. Alcaide, M. A. Benítez, A. Salazar, C. Ardanuy, D. Podzamczer, G. Rufí, J. Dorca, R. Martín, and F. Gudiol. 2004. Incidence and molecular typing of Mycobacterium kansasii in a defined geographical area in Catalonia, Spain. Epidemiol. Infect. 132:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddiqi, S. H. 1995. BACTEC 460 TB system. Product and procedure manual, revision D. Becton Dickinson Microbiology Systems, Sparks, Md.
- 27.Steadham, J. E., S. K. Stall, and J. L. Simmank. 1985. Use of the BACTEC system for drug susceptibility testing of Mycobacterium tuberculosis, M. kansasii, and M. avium complex. Diagn. Microbiol. Infect. Dis. 3:33-40. [DOI] [PubMed] [Google Scholar]
- 28.Taillard, C., G. Greub, R. Weber, G. E. Pfyffer, T. Bodmer, S. Zimmerli, R. Frei. S. Bassetti, P. Rohner, J-C. Piffaretti, E. Bernasconi, J. Bille, A. Telenti, and G. Prod'hom. 2003. Clinical implications of Mycobacterium kansasii species heterogeneity: Swiss national survey. J. Clin. Microbiol. 41:1240-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace, R. J., Jr., D. Dunbar, B. A. Brown, G. Onyi, R. Dunlap, C. H. Ahn, and D. T. Murphy. 1994. Rifampin-resistant Mycobacterium kansasii. Clin. Infect. Dis. 18:736-743. [DOI] [PubMed] [Google Scholar]
- 30.Wallace, R. J., Jr., J. Glassroth, D. E. Griffith, K. N. Olivier, J. L. Cook, and F. Gordin. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156(Suppl.):S1-S25.9279284 [Google Scholar]
- 31.Witzig, R. S., B. A. Fazal, R. M. Mera, D. M. Mushatt, P. M. Dejace, D. L. Greer, and N. E. Hyslop, Jr. 1995. Clinical manifestations and implications of coinfection with Mycobacterium kansasii and human immunodeficiency virus type 1. Clin. Infect. Dis. 21:77-85. [DOI] [PubMed] [Google Scholar]
- 32.Yew, W. W., L. J. Piddock, M. S. Li, D. Lyon, C. Y. Chan, and A. F. Cheng. 1994. In-vitro activity of quinolones and macrolides against mycobacteria. J. Antimicrob. Chemother. 34:343-351. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., L. B. Mann, R. W. Wilson, B. A. Brown-Elliott, V. Vincent, Y. Iinuma, and R. J. Wallace, Jr. 2004. Molecular analysis of Mycobacterium kansasii isolates from the United States. J. Clin. Microbiol. 42:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]