Abstract
OBJECTIVES
This study investigated sex differences in coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) in patients with angina in the absence of obstructive coronary artery disease.
BACKGROUND
Coronary microvascular dysfunction is associated with worse long-term outcomes, especially in women. Coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR) are 2 methods of assessing the coronary microcirculation.
METHODS
We prospectively enrolled 117 women and 40 men with angina in the absence of obstructive coronary artery disease. We performed CFR, IMR, fractional flow reserve, and quantitative coronary angiography in the left anterior descending artery. Coronary flow was assessed with a thermodilution method by obtaining mean transit time (Tmn) (an inverse correlate to absolute flow) at rest and hyperemia.
RESULTS
All patients had minimal atherosclerosis by quantitative coronary angiography (% diameter stenosis: 23.2 ± 12.3%), and epicardial disease was milder in women (fractional flow reserve: 0.88 ± 0.04 vs. 0.87 ± 0.04; p = 0.04). IMR was similar between the sexes (20.7 ± 9.8 vs. 19.1 ± 8.0; p = 0.45), but CFR was lower in women (3.8 ± 1.6 vs. 4.8 ± 1.9; p = 0.004). This was primarily due to a shorter resting Tmn in women (p = 0.005), suggesting increased resting coronary flow, whereas hyperemic Tmn was identical (p = 0.79). In multivariable analysis, female sex was an independent predictor of lower CFR and shorter resting Tmn.
CONCLUSIONS
Despite similar microvascular function in women and men by IMR, CFR is lower in women. This discrepancy appears to be due to differences in resting coronary flow between the sexes. The effect of sex differences should be considered in interpretation of physiological indexes using resting coronary flow.
Keywords: coronary flow reserve, coronary microvascular resistance, physiology, sex
Women are more likely than men to have angina in the absence of coronary artery disease (CAD) (1–3), and coronary microvascular dysfunction has been proposed as one of the explanations of this phenomenon (4). Invasively, coronary flow reserve (CFR) is typically used to interrogate microvascular function, and low CFR has been associated with major adverse outcomes, including myocardial infarction and death (5,6). Still, CFR is affected by epicardial influences and has a variability that limits its reproducibility. The index of microcirculatory resistance (IMR), on the other hand, is a direct measure of the microcirculation that has been shown to be largely independent of variations in hemodynamic state (7–9). Data remain limited on the use of CFR and IMR as measures of the coronary microvasculature, particularly between women and men, where important sex differences in microvascular dysfunction have been reported (10). Therefore, we investigated the effect of sex differences on CFR and IMR in measuring coronary microvascular dysfunction in women and men with angina in the absence of obstructive CAD.
METHODS
STUDY POPULATION
We prospectively enrolled adult patients who were electively referred to the cardiac catheterization laboratory for coronary angiography because of a clinical suspicion of coronary ischemia based on the presence of angina, with or without an abnormal stress test. Typical angina was defined as having 3 characteristics: it had substernal chest discomfort, was provoked by exertion or emotional stress, and was relieved by rest or nitroglycerin. Atypical angina was defined as having 2 of the above characteristics. Patients with noncardiac chest pain were not included (11). Exclusion criteria included the presence of an acute coronary syndrome, prior heart transplantation, coronary artery bypass grafting, renal insufficiency (creatinine >1.5 mg/dl), abnormal ejection fraction (<55%), or presence of another likely explanation of angina, such as pulmonary hypertension, hypertrophic cardiomyopathy, or valvular heart disease. To increase the diagnostic accuracy, all coronary vasodilating drugs, including calcium-channel blockers and long-acting nitrates, were discontinued at least 48 h prior to the examination, except for sublingual nitroglycerin, which could be used if needed. A baseline coronary angiogram was performed via the femoral artery to rule out obstructive CAD (defined as >50% diameter stenosis) in the right and left coronary arteries. In patients without obstructive CAD, further invasive evaluation was conducted, including CFR, IMR, fractional flow reserve (FFR), quantitative coronary angiography (QCA), and volumetric intravascular ultrasound (IVUS). Patients were further excluded if the FFR was found to be ≤0.80, even in the absence of angiographic obstructive disease. The study was approved by Stanford’s institutional review board and informed, written consent was obtained from all patients.
CORONARY PHYSIOLOGY MEASUREMENTS
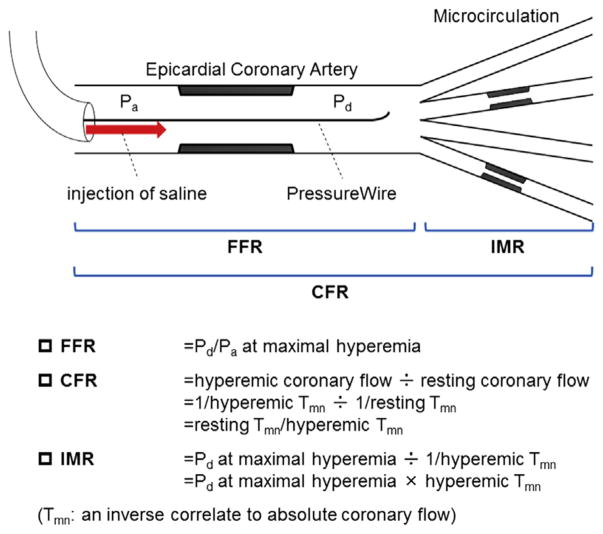
CFR, IMR, and FFR were measured in the left anterior descending artery (LAD) by methods previously described (12). Prior to wire advancement, 200 μg intracoronary nitroglycerin was administered. Following calibration, a 0.014-inch pressure-temperature sensor guidewire (PressureWire Certus, St. Jude Medical, St. Paul, Minnesota) was equalized to the guide catheter pressure with the sensor positioned at the ostium of the left coronary artery. The PressureWire was then advanced in the LAD as distally as safely possible. With commercially available software (Radi Analyzer, St. Jude Medical), the shaft of the PressureWire can act as a proximal thermistor by detecting changes in temperature-dependent electrical resistance. The sensor near the tip of the wire simultaneously measures pressure and temperature, and can thereby act as a distal thermistor. The transit time of room-temperature saline injected down a coronary artery is then determined with a thermodilution technique. The inverse value of mean transit time has been validated to correlate with absolute coronary blood flow (13,14). Approximately 3 ml of room temperature saline was injected rapidly by hand into the left coronary artery, and the resting mean transit time (Tmn) was obtained as an average of 3 injections. Thereafter, intravenous infusion of adenosine (140 μg/kg/min) was administered to induce a steady state of maximal hyperemia, followed by 3 more injections of 3 ml of room-temperature saline to calculate hyperemic Tmn. Simultaneous measurements of mean proximal coronary pressure (Pa) (by guide catheter) and mean distal coronary pressure (Pd) (by PressureWire) were also acquired in the maximal hyperemic state. FFR was calculated by the ratio of Pd/Pa at hyperemia. CFR was calculated as resting Tmn divided by hyperemic Tmn. IMR was calculated as Pd at hyperemia multiplied by hyperemic Tmn (Figure 1) (9).
FIGURE 1. Schematic of Physiological Assessment Using a Single Coronary PressureWire.
Image modified from Kobayashi and Fearon (9). CFR = coronary flow reserve; FFR = fractional flow reserve; IMR = the index of microcirculatory resistance; Pa = mean proximal coronary pressure; Pd = mean distal coronary pressure; and Tmn = mean transit time.
QCA AND IVUS ANALYSIS
All coronary angiograms and IVUS images were analyzed by an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford, California) blinded to the patient’s clinical and physiologic information. Quantitative and qualitative analyses of the LAD after an intracoronary nitroglycerin (200 μg) injection were done with an automatic edge detection system (QAngio XA 7.3, MEDIS, Leiden, the Netherlands).
IVUS recording from the distal LAD to the guide catheter was performed in a standard fashion using an automated motorized pullback (0.5 mm/s) with a commercially available 40 MHz IVUS catheter (Atlantis SR Pro 2 or OptiCross, Boston Scientific, Natick, Massachusetts). IVUS imaging was analyzed offline using a commercially available 3-dimensional reconstruction software (echoPlaque, INDEC Medical Systems, Santa Clara, California). Lumen and vessel contours were traced with 1-mm axial intervals for 50 mm length from the ostium of the LAD. For volumetric analysis, volumes (lumen and vessel) were calculated using Simpson’s rule. Thereafter, each volume was divided by axial analyzed length (volume index [VI], mm3/mm) to adjust for the subtle difference of analyzed longitudinal lengths among cases. Percent plaque volume was defined as 100 × (vessel VI – lumen VI)/vessel VI (%). Minimum lumen area was also obtained from the interpolated dataset of lumen area as a 2-dimensional IVUS index (15).
STATISTICAL ANALYSIS
All analyses were performed using SPSS software, version 21 (SPSS Inc., Chicago, Illinois). Categorical variables are presented as counts and percentages. Pearson’s chi-square test or Fisher’s exact test was used for comparisons of categorical variables, as appropriate. Continuous variables are presented as mean and SD. Normality of the continuous variables was confirmed with the Shapiro-Wilk test. Depending on the result of Levene’s test for homoscedasticity, variables with normal distribution were compared with the Student t test or Welch t test, as appropriate. If the normality test failed, variables were compared using the Mann-Whitney U test. After the Box-Cox transformation, multiple regression analysis was performed to identify the independent predictors of CFR and resting Tmn. Among individual variables listed in Table 1, except for the physiological indexes, variables with p < 0.10 in Spearman’s correlation analysis were considered for inclusion into multivariable models to determine the independent predictors. Because vessel VI and lumen VI were highly correlated with each other due to the small amount of plaque in this patient population (Spearman’s rank correlation coefficient = 0.88; p < 0.001), only vessel VI was included in the multiple regression analysis. An overall difference among 4 subgroups (based on sex and median value of vessel size) was determined by 1-way analysis of variance test. If the overall difference was significant, differences between the individual subgroups were estimated using the Turkey-HSD or Games-Howell test, as appropriate. A 2-sided p value <0.05 was considered significant.
TABLE 1.
Comparisons of Patient Clinical Characteristics and Invasive Indexes
| Women (n = 117) | Men (n = 40) | p Value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, yrs | 53.7 ± 11.3 | 53.5 ± 13.5 | 0.91 |
| Diabetes mellitus | 28 (23.9) | 10 (25.0) | 0.89 |
| Hypertension | 63 (53.8) | 14 (35.0) | 0.04* |
| Dyslipidemia | 63 (53.8) | 28 (70.0) | 0.07 |
| Smoking | 31 (26.7) | 16 (40.0) | 0.12 |
| Family history | 61 (52.1) | 9 (22.5) | 0.001† |
| Body mass index, kg/m2 | 29.0 ± 7.4 | 28.1 ± 4.6 | 0.91 |
| Hormonal status | |||
| Pre-menopausal | 54 (46.2) | – | |
| Peri-menopausal | 55 (47.0) | – | |
| Post-menopausal | 8 (6.8) | – | |
|
| |||
| Medications | |||
| Long-acting nitrate | 16 (13.7) | 7 (17.5) | 0.56 |
| Short-acting nitrate | 34 (29.1) | 13 (32.5) | 0.68 |
| Calcium-channel blocker | 17 (14.5) | 7 (17.5) | 0.65 |
| β-blocker | 51 (43.6) | 16 (40.0) | 0.69 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 17 (14.5) | 5 (12.5) | 0.75 |
| Statin | 66 (56.4) | 24 (60.0) | 0.69 |
| Aspirin | 75 (64.1) | 30 (75.0) | 0.21 |
| Clopidogrel | 5 (4.3) | 2 (5.0) | 0.85 |
| Diuretic agents | 19 (16.2) | 3 (7.5) | 0.17 |
|
| |||
| Clinical demographics | |||
| Chest pain characteristics | |||
| Typical | 73 (62.4) | 19 (47.5) | 0.10 |
| Atypical | 44 (37.6) | 21 (52.5) | |
| Stress test results | |||
| No test | 4 (3.4) | 2 (5.0) | 0.13 |
| Normal | 33 (28.2) | 5 (12.5) | |
| Ischemic | 80 (68.4) | 33 (82.5) | |
|
| |||
| Physiological indexes | |||
| CFR | 3.8 ± 1.6 | 4.8 ± 1.9 | 0.004† |
| IMR | 20.7 ± 9.8 | 19.1 ± 8.0 | 0.45 |
| FFR | 0.88 ± 0.04 | 0.87 ± 0.04 | 0.04* |
|
| |||
| QCA indexes | |||
| Reference diameter, mm | 2.59 ± 0.56 | 2.80 ± 0.61 | 0.03* |
| Minimal lumen diameter, mm | 2.01 ± 0.49 | 2.03 ± 0.54 | 0.75 |
| % diameter stenosis | 21.8 ± 12.0 | 27.2 ± 12.2 | 0.02* |
|
| |||
| IVUS indexes (n = 142) | n = 106 | n = 36 | |
| 3-dimensional | |||
| Vessel VI, mm3/mm | 12.3 ± 3.1 | 13.9 ± 2.8 | 0.003† |
| Lumen VI, mm3/mm | 9.2 ± 2.7 | 9.6 ± 2.7 | 0.42 |
| % plaque volume | 25.0 ± 8.5 | 31.3 ± 10.4 | 0.002† |
| 2-dimensional | |||
| Minimum lumen area, mm2 | 5.1 ± 2.0 | 4.7 ± 1.6 | 0.56 |
Values are n mean ± SD or n (%).
p < 0.05.
p < 0.01.
CFR = coronary flow reserve; FFR = fractional flow reserve; IMR = index of microcirculatory resistance; QCA = quantitative coronary angiography; VI = volume index.
RESULTS
A total of 157 patients, including 117 women (74.5%) and 40 men (25.5%), were enrolled in the present study. IVUS pullback images of the LAD were analyzable in 142 of 157 patients (90.4%). The patients were relatively young (mean age 53.7 ± 11.9 years) and had minimal to no epicardial disease (% diameter stenosis by QCA 23.2 ± 12.3 and FFR 0.88 ± 0.04). Women were significantly more likely than men to have hypertension and a family history of early-onset CAD. There was no significant difference in baseline medical treatments between the sexes (Table 1).
COMPARISONS OF INVASIVE INDEXES BETWEEN WOMEN AND MEN
Comparisons of invasive indexes between women and men are summarized in Table 1. QCA and IVUS consistently showed that vessel size was significantly smaller in women (p = 0.03 for reference diameter by QCA and p = 0.003 for vessel VI by IVUS). However, because of less epicardial disease in women (p = 0.02 for % diameter stenosis by QCA and p = 0.002 for % plaque volume by IVUS), lumen size was similar between the sexes (p = 0.75 for minimal lumen diameter by QCA, p = 0.42 for lumen VI by IVUS, and p = 0.56 for minimum lumen area by IVUS). By physiological assessment, women had a higher FFR value (0.88 ± 0.04 vs. 0.87 ± 0.04; p = 0.04), also suggesting less epicardial disease severity.
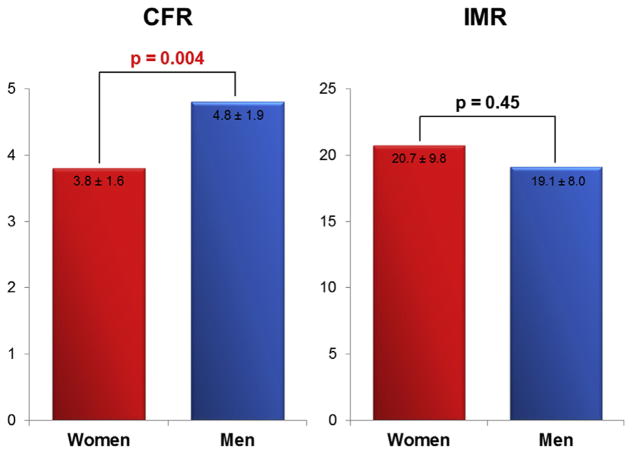
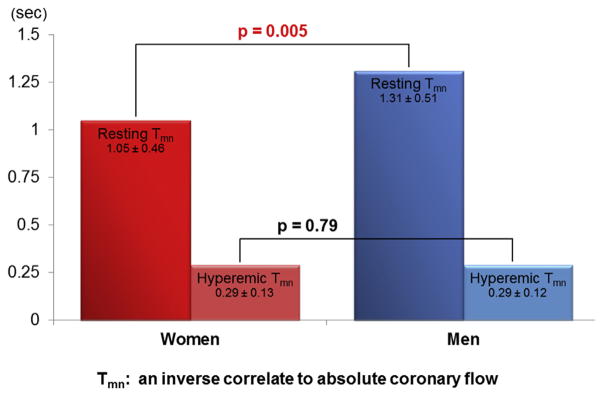
With regard to measures of coronary microvascular dysfunction, IMR was similar between women and men (20.7 ± 9.8 vs. 19.1 ± 8.0; p = 0.45), whereas CFR was lower in women (3.8 ± 1.6 vs. 4.8 ± 1.9; p = 0.004) (Figure 2). To further elucidate the mechanism of this discrepancy, resting and hyperemic Tmn were compared between women and men. Notably, resting Tmn was significantly shorter in women (1.05 ± 0.46 s vs. 1.31 ± 0.51 s; p = 0.005), whereas hyperemic Tmn was identical (0.29 ± 0.13 s vs. 0.29 ± 0.12 s; p = 0.79) (Figure 3). While 10 of 117 women had a CFR ≤2.0, no men had a CFR ≤2.0 (8.5% vs. 0%; p = 0.048). With respect to IMR, 33 of 117 women and 6 of 40 men had an IMR ≥25 (28.2% vs. 15%; p = 0.10). Regardless of sex, there was a significant relationship between resting and hyperemic Tmn (Spearman’s rank correlation coefficient = 0.58; p < 0.001 in women; and Spearman’s rank correlation coefficient = 0.53; p = 0.001 in men), such that a shorter resting Tmn positively correlated with a shorter hyperemic Tmn, and a longer resting Tmn positively correlated with a longer hyperemic Tmn.
FIGURE 2. Comparison of CFR and IMR Between Women and Men.
CFR is significantly lower in women than men. On the contrary, IMR, a direct measure of microcirculation, is similar. Abbreviations as in Figure 1.
FIGURE 3. Comparison of Tmn Between Women and Men.
Resting Tmn is significantly shorter in women than men. On the contrary, hyperemic Tmn was similar. These findings suggest resting coronary flow is higher in women than men, whereas hyperemic coronary flow is similar. Tmn = mean transit time.
MULTIVARIABLE PREDICTORS
The results of multiple regression analysis to investigate the independent predictors of CFR and resting Tmn are summarized in Table 2. Female sex was independently associated with both a lower CFR and shorter resting Tmn. Smaller vessel size as assessed by IVUS (vessel VI) and by QCA (reference diameter) was also independently associated with a lower CFR and shorter resting Tmn, respectively. With respect to IMR and hyperemic Tmn, because age was the only predictor identified on univariate analysis (p = 0.005 for IMR and p = 0.045 for hyperemic Tmn), multiple regression analysis was not performed.
TABLE 2.
Multivariable Predictors of CFR and Resting Tmn
| CFR | Resting Tmn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| B | Standard Error | 95% CI | β | p Value | B | Standard Error | 95% CI | β | p Value | |
| Female | −0.240 | 0.092 | −0.422 to −0.057 | −0.211 | 0.011* | −0.244 | 0.084 | −0.410 to −0.078 | −0.229 | 0.004† |
|
| ||||||||||
| Vessel VI | 0.038 | 0.013 | 0.012 to 0.063 | 0.239 | 0.004† | |||||
|
| ||||||||||
| Reference diameter | 0.193 | 0.063 | 0.069 to 0.317 | 0.243 | 0.003† | |||||
|
| ||||||||||
| ACEI/ARB | −0.279 | 0.098 | −0.472 to −0.085 | −0.220 | 0.005† | |||||
p < 0.05.
p < 0.01.
ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CFR = coronary flow reserve; CI = confidence interval; Tmn = mean transit time; VI = volume index.
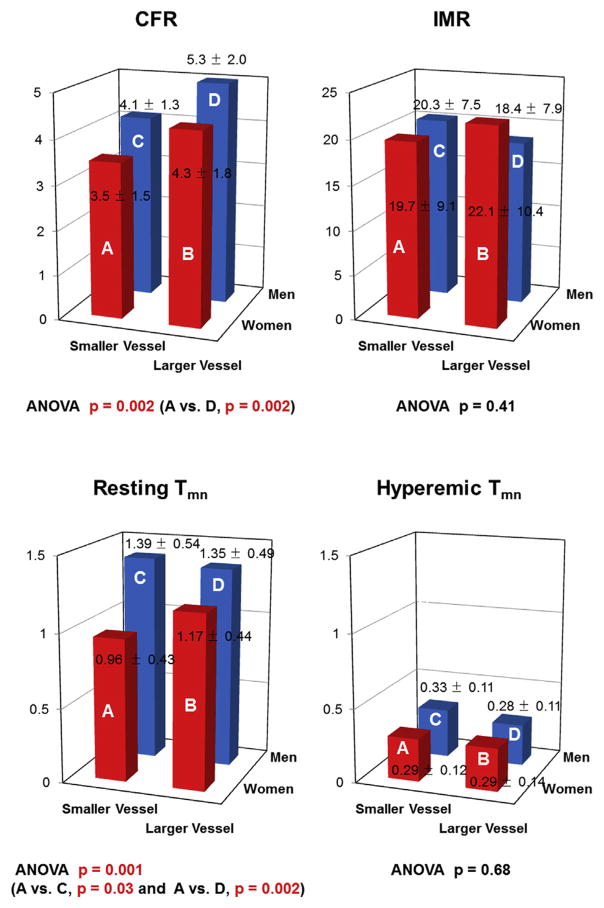
When patients were divided into 4 subgroups according to sex and median value of vessel size, CFR and resting Tmn showed significant differences among the subgroups, whereas IMR and hyperemic Tmn did not (Figure 4). Among women, differences in hormonal status (pre-, peri-, or post-menopausal) had no significant effect on any coronary physiologic parameters.
FIGURE 4. Comparison of Physiological Indexes According to Patient Sex and Vessel Size.
ANOVA = analysis of variance; other abbreviations as in Figure 1.
DISCUSSION
We studied the effect of sex differences on invasive measures of coronary microvascular dysfunction (CFR and IMR) in women and men with angina in the absence of obstructive CAD, and had several important findings. 1) Although CFR is lower in women than men, IMR is similar between the sexes. 2) Resting Tmn is significantly shorter in women, whereas hyperemic Tmn is equivalent between the sexes, accounting for the lower CFR in women. 3) Patient sex is independently associated with CFR and resting Tmn. These findings strongly suggest that resting flow status has a major effect on CFR, and that previous reports on sex differences in CFR may reflect higher resting flow in women rather than more significant microvascular disease. In our study, IMR, a direct measure of the microvasculature that is not dependent on resting flow, was not different between the sexes, suggesting that there is no sex difference in coronary microvascular dysfunction.
DISCREPANCY OF CFR AND IMR BETWEEN WOMEN AND MEN
CFR, defined as hyperemic coronary flow divided by resting coronary flow, was originally proposed to evaluate the functional significance of an intermediate coronary artery stenosis (16). Because CFR interrogates flow status of both the epicardial artery and the microcirculation, CFR has become an accepted measure of microvascular function in patients without epicardial disease. However, CFR has been shown to have several limitations. Reports of CFR are not always derived from the same measuring technique. CFR can be measured with the thermodilution technique (CFRthermo) or with a Doppler velocity wire (CFRDoppler), although the latter technique is not felt to correlate as well with absolute flow-derived CFR (CFRflow), tending to underestimate the true CFR (17). In addition, CFR is affected by resting vascular tone, as well as changes in hemodynamics, including heart rate, blood pressure, and contractility. However, IMR eliminates the effect of variability in resting hemodynamic parameters by being derived at maximal hyperemia (8).
Women with angina in the absence of obstructive CAD have been found to have low CFR values, and this has been attributed to significant microvascular dysfunction. Most of the data have come from the WISE (Women’s Ischemia Syndrome Evaluation) study, but the WISE study only evaluated women (4,5), so little is known about similarly presenting men or differences between the sexes. One study that compared the sexes found lower CFR in women compared with men (10), similar to our findings. However, we also found that women had shorter resting Tmn compared with men, which resulted in a lower CFR, and that when we evaluated the microvascular function by IMR, there was no difference between women and men.
DIFFERENCE IN RESTING CORONARY PHYSIOLOGY BETWEEN WOMEN AND MEN
Even though resting Tmn has an inherent limitation of susceptibility to epicardial influences, the inverse of resting Tmn has been demonstrated to be associated with absolute coronary flow (13,14). In this context, the shorter resting Tmn in women compared with men in the present study suggests higher coronary flow at rest in women. This phenomenon of higher myocardial blood flow at rest in women has been demonstrated by noninvasive positron emission tomography studies as well (18). Why women have higher resting coronary flow is unclear and deserves further investigation. We did not find any evidence that higher resting coronary flow is associated with age or hormonal status. It may be that higher resting coronary flow in women is secondary to a lower resting vascular tone, which has been shown to differ between women and men (19).
Because a low CFR in women has been associated with worse outcomes, it is presumably detecting a pathological vascular disturbance, which may or may not have to do with the microvascular bed. Although lower CFR in women is presumably secondary to a shorter resting Tmn, it may also be considered that the lower CFR is due to a poorer augmentation of coronary flow from rest to hyperemia. However, since hyperemic Tmn and IMR was similar between women and men, poor augmentation of coronary flow from rest to hyperemia is unlikely an explanation for lower CFR in women. Moreover, we found that resting Tmn and hyperemic Tmn were significantly positively correlated in both women and men. However, female sex was independently associated with both lower CFR and shorter resting Tmn, further demonstrating that sex differences are an important factor for coronary physiology at rest.
Finally, although women had smaller vessel size than men, smaller vessel size itself was an independent predictor of both CFR and resting Tmn, so the relationship of lower CFR and shorter resting Tmn cannot simply be attributed to women having smaller vessels. Instead, it would appear that smaller vessels have higher resting coronary flow, regardless of sex, although further investigation is needed.
CLINICAL IMPLICATIONS
IMR has emerged as a direct measure of the coronary microvasculature that is highly reproducible and largely independent of hemodynamic perturbations (7–9). It eliminates the resting flow variable, which our study has shown differs between the sexes. Higher resting flow in women, but similar hyperemic flows in women and men, produces a lower CFR in women. Although this lower CFR has been attributed to worse microvascular dysfunction in women, our data put this assertion into question. We found that although CFR was lower in women, IMR was similar between the sexes, suggesting no difference in microvascular dysfunction. This implies that the difference in CFR between the sexes is not necessarily related to the presence or absence of microvascular dysfunction, but is a result of the measurement itself, and that CFR may be a less optimal measure of microvascular function in women. Whether or not a higher resting flow in particular women has its own implications in terms of symptoms or outcomes may warrant further study.
Both CFR and IMR have prognostic value (20–22), and CFR has been more extensively studied in women with angina in the absence of obstructive CAD. However, CFR data are limited in similarly presenting men, and this is the first study to evaluate IMR as a measure of microvascular function between the sexes. Further work is needed to ultimately determine the best measure of microvascular dysfunction in this important and underserved cohort of women and men who are experiencing angina in the absence of obstructive CAD.
STUDY LIMITATIONS
First, because we investigated only the LAD, our results cannot be generalized to the entire coronary territory. However, our approach minimized the known variability of invasive indexes among different coronary territories (23,24). Second, we did not measure absolute coronary flow, but instead used Tmn, which is a validated surrogate of absolute coronary flow. Third, differences in vessel length between the women and men could have resulted in differences in the distal position of the PressureWire sensor, which may have in turn affected resting Tmn. However, there are not necessarily known differences in vessel length between the sexes, and hyperemic Tmn was similar between the sexes, suggesting that such an effect is unlikely. Fourth, the effect of vasodilatory drugs could still be present at the time of coronary catheterization, even though we discontinued those drugs at least 48 h before procedures. This duration has been recommended in guidelines for spasm provocation testing, (25) and is longer than previous studies, which employed 24 h (26). If there was any residual effect from these drugs, it would not be expected to differ between the sexes. Fifth, this study was not designed to assess the superiority of CFR versus IMR, which would require testing these measures against a gold standard; thus, no conclusions can be drawn regarding the superiority of one measure over the other in the evaluation of microvascular function. Sixth, we do not yet have long-term follow-up data of our patients, so we cannot determine how IMR compares with CFR in predicting cardiovascular outcomes in this patient population. Finally, although the proportion of women was high in this study, the proportion is consistent with the known sex-based prevalence of angina in the absence of obstructive CAD (1,2).
CONCLUSIONS
Although CFR is lower in women compared with men, IMR, a more direct measure of coronary microvascular dysfunction, is similar between the sexes. This discrepancy appears to be due to increased resting coronary flow (shorter resting Tmn) in women compared with men. The effect of sex differences should be considered in the interpretation of physiological indexes using resting coronary flow.
PERSPECTIVES.
WHAT IS KNOWN?
Women are more likely than men to have angina in the absence of CAD, and coronary microvascular dysfunction, as assessed by CFR, has been proposed as one of the explanations of this phenomenon.
WHAT IS NEW?
IMR is similar between women and men, suggesting that there is no sex difference in microvascular dysfunction. CFR is lower in women, but this is primarily due to a shorter resting mean transit time (an inverse correlate of absolute coronary flow), suggesting increased resting coronary flow.
WHAT IS NEXT?
Further studies are warranted to test whether increased resting coronary flow itself has a prognostic implication.
Acknowledgments
This study is supported by the National Institutes of Health (K23 HL092233-05, PI: Dr. Tremmel). Dr. Fearon has received research grants from St. Jude Medical and Medtronic; has received honoraria from Medtronic; and has served on the advisory board for HeartFlow. Dr. Tremmel has received honoraria from St. Jude Medical, Boston Scientific, Terumo, Medtronic, and Recor.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CFR
coronary flow reserve
- FFR
fractional flow reserve
- IMR
index of microcirculatory resistance
- IVUS
intravascular ultrasound
- LAD
left anterior descending artery
- Pa
mean proximal coronary pressure
- Pd
mean distal coronary pressure
- QCA
quantitative coronary angiography
- Tmn
mean transit time
- VI
volume index
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Author Disclosures: This study is supported by the National Institutes of Health (K23 HL092233-05, PI: Dr. Tremmel). Dr. Fearon has received research grants from St. Jude Medical and Medtronic; has received honoraria from Medtronic; and has served on the advisory board for HeartFlow. Dr. Tremmel has received honoraria from St. Jude Medicall, Boston Scientific, Terumo, Medtronic, and Recor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–84. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–8. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 3.Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155:375–81. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease: results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 5.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortigiani L, Rigo F, Gherardi S, et al. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106:1703–8. doi: 10.1016/j.amjcard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–32. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 8.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–61. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y, Fearon WF. Invasive coronary microcirculation assessment–current status of index of microcirculatory resistance. Circ J. 2014;78:1021–8. doi: 10.1253/circj.cj-14-0364. [DOI] [PubMed] [Google Scholar]
- 10.Han SH, Bae JH, Holmes DR, Jr, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–69. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983;1:574–5. doi: 10.1016/s0735-1097(83)80093-x. [DOI] [PubMed] [Google Scholar]
- 12.Fearon WF, Nakamura M, Lee DP, et al. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA Study) Circulation. 2003;108:1605–10. doi: 10.1161/01.CIR.0000091116.84926.6F. [DOI] [PubMed] [Google Scholar]
- 13.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–6. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, De Bruyne B, Smith L, et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105:2482–6. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 15.Hirohata A, Nakamura M, Waseda K, et al. Changes in coronary anatomy and physiology after heart transplantation. Am J Cardiol. 2007;99:1603–7. doi: 10.1016/j.amjcard.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33:87–94. doi: 10.1016/0002-9149(74)90743-7. [DOI] [PubMed] [Google Scholar]
- 17.Fearon WF, Farouque HM, Balsam LB, et al. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003;108:2198–200. doi: 10.1161/01.CIR.0000099521.31396.9D. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. [PubMed] [Google Scholar]
- 19.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 20.Fearon WF, Low AF, Yong AS, et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–41. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Hoef TP, Bax M, Meuwissen M, et al. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6:207–15. doi: 10.1161/CIRCINTERVENTIONS.112.000168. [DOI] [PubMed] [Google Scholar]
- 22.Meuwissen M, Chamuleau SA, Siebes M, et al. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;71:291–7. doi: 10.1002/ccd.21331. [DOI] [PubMed] [Google Scholar]
- 23.Kern MJ, Bach RG, Mechem CJ, et al. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28:1154–60. doi: 10.1016/S0735-1097(96)00327-0. [DOI] [PubMed] [Google Scholar]
- 24.Marroquin OC, Holubkov R, Edmundowicz D, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J. 2003;145:628–35. doi: 10.1067/mhj.2003.95. [DOI] [PubMed] [Google Scholar]
- 25.JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745–62. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 26.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Intv. 2012;5:646–53. doi: 10.1016/j.jcin.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]