Abstract
Vaccination is the most successful immunological practice that improves the quality of human life and health. Vaccine materials include antigens of pathogens and adjuvants potentiating the effectiveness of vaccination. Vaccines are categorized using various criteria, including the vaccination material used and the method of administration. Traditionally, vaccines have been injected via needles. However, given that most pathogens first infect mucosal surfaces, there is increasing interest in the establishment of protective mucosal immunity, achieved by vaccination via mucosal routes. This review summarizes recent developments in mucosal vaccines and their associated adjuvants.
Keywords: Adjuvants, Mucosal immunity, Vaccines
Introduction
Early vaccination strategies used live or attenuated pathogens to induce adaptive immunity [1]. However, the re-activation of attenuated vaccine materials and the possible tumorigenicity of inactivated oncogenic viruses are important safety concerns [2]. Thus, non-pathogenic materials including inactivated toxins, synthetic peptides, and recombinant subunit proteins have been considered as vaccine materials [3]. However, these antigens are poor in immunogenicity and must be given with supplemental materials to potentiate the vaccination capacity [3]. Such supplemental materials are termed adjuvants and can be divided into two classes depending on their mode of action: efficient delivery of vaccine materials and/or stimulation of the immune system (Tables 1, 2) [1]. Adjuvants facilitating vaccine delivery include liposomes, nanogels, oil-in-water emulsions, and virosomes targeting the co-administered antigens to professional antigen-presenting cells (APCs) [4]. Adjuvants that stimulate the immune system include molecules binding to intracellular receptors including Toll-like receptors (TLRs), Nod-like receptors, and RIG-I–like receptors and to cytosolic DNA sensors, all of which modulate the immune response [1]. However, most adjuvants have been evaluated in the context of parenteral immunization; thus, it is not clear how well the adjuvants function in the mucosal immune compartment.
Table 1. Currently licensed adjuvants used as carriers of vaccine materials.
| Adjuvant name | Adjuvant class | Immune response | Component |
|---|---|---|---|
| Alum | Mineral salts | Antibody, Th2 response | Aluminum phosphate or aluminum hydroxide |
| MF59 | Oil-in-water emulsion | Antibody, Th1/Th2 response | Squalene, Polysorbate 80 (Tween 80), sorbitan trioleate (Span 85) |
| Virosomes | Liposomes | Antibody, Th1/Th2 response, cross-presentation | Lipids, hemagglutinin |
| AS03 | Oil-in-water emulsion | Antibody, Th1/Th2 response | Squalene, Polysorbate 80 (Tween 80), α-tocopherol |
| Montanide ISA51 | Water-in-oil emulsion | Antibody, Th1/Th2 response | Drakeol 6 VR, mannide monooleate |
Based on Rappuoli R et al. Nat Rev Immunol 2011;11:865-72 [1].
Table 2. Immunostimulatory molecules used as vaccine adjuvants.
| Adjuvant name | Target receptor | Type (component) | Immune response |
|---|---|---|---|
| Licensed adjuvant | |||
| RC529 | TLR4 | RC529 | Antibody, Th1 response |
| AS01 | TLR4 | Liposome, MPL, QS21 | Antibody, Th1 response, CD8+ T cells |
| AS04 | TLR4 | Aluminum hydroxide, MPL | Antibody, Th1 response |
| Not licensed adjuvant | |||
| Poly(I:C), Poly(IC:LC) | TLR3 | dsRNA | Type I IFN, pro-inflammatory cytokines, antibody, CD4/CD8 response |
| Imiquimod, Resiquimod, Gardiquimod | TLR7/TLR8 | ssRNA | Type I IFN, pro-inflammatory cytokines, antibody, CD4/CD8 response |
| IC31 | TLR9 | Unmethylated CpG DNA | Type I IFN, pro-inflammatory cytokines, antibody, CD8 response |
| iE-DAP, MDP | NOD1/2 | Peptidoglycan | Pro-inflammatory cytokines, antibody |
| M8, defective interfering (DI) RNA | RIG-1, MDA-5 | dsRNA | Type I IFN, pro-inflammatory cytokines, antibody, CD4/CD8 response |
| cGAMP, C-di-GMP | STING | Cyclic dinucleotide | Type I IFN, pro-inflammatory cytokines, antibody, CD8 response |
Based on Rappuoli R et al. Nat Rev Immunol 2011;11:865-72 [1].
TLR, Toll-like receptor; IFN, interferon.
The Mucosal Immune System
Mucosal surfaces cover 400 m2 of the body including the gastrointestinal, urogenital, and respiratory tracts [5]. Mucosae are continuously exposed to microbiota and antigens. The gastrointestinal mucosa is especially prone to the development of tolerogenic microenvironments, where luminal antigens may persist. The mucosal immune system has both inductive and effector sites differing in terms of their anatomical and functional characteristics [6]. The major mucosal immune inductive sites include gut-associated lymphoid tissue (GALT) and the nasopharyngeal-associated lymphoid tissue (NALT). GALT includes Peyer's patches, mesenteric lymph nodes, and isolated lymphoid follicles, while NALT includes tonsils/adenoids, inducible bronchus-associated lymphoid tissue, cervical lymph nodes, and hilar lymph nodes. Mucosal immune inductive sites are covered by follicle-associated epithelium (FAE), which is composed of enterocytes and M cells.
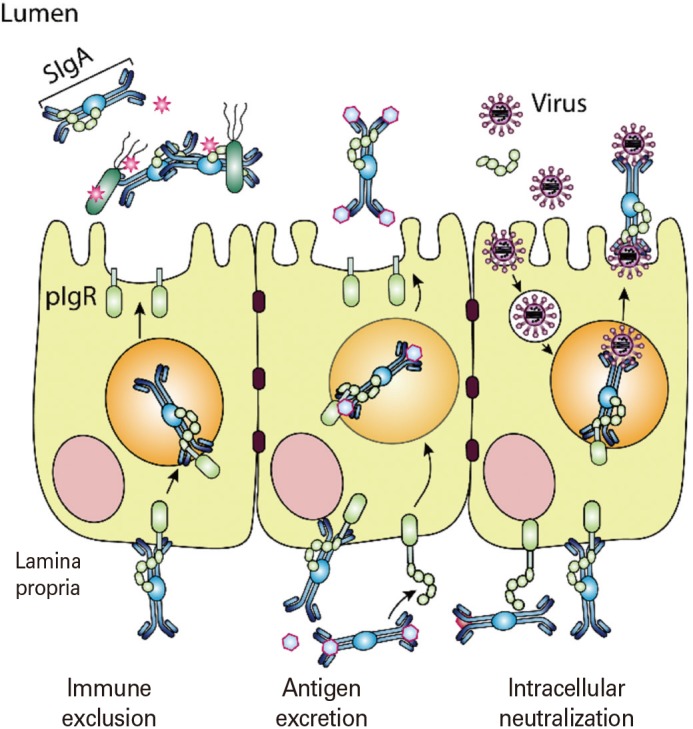
M cells are specialized epithelial cells for antigen uptake [7]. These cells are overlaid by a thin mucus layer and possess short irregular microvilli [8]. M cells can transfer antigens via transcytosis to APCs located in pockets within M cell clusters [9]. Dendritic cells that come in contact with antigens transcytosed through M cells enter the interfollicular T cell zone to activate naïve T cells [10]. Finally, effector T cells move to the B cell follicles of germinal centers (GCs) and secrete cytokines capable of promoting IgA class-switch recombination [11]. In mucosal immune effector sites such as the lamina propria of the gut, the upper respiratory tract, and the female reproductive tract, IgA+ plasma cells terminally differentiate to release secretory IgA (SIgA), the most important immune effector molecule in the mucosa. SIgA is transported across mucosal epithelial cells via a polymeric Ig receptor (pIgR) (Fig. 1) [12]. SIgA is a major immune effector at mucosal surfaces that acts via three mechanisms: antigen excretion, immune exclusion, and intracellular antigen neutralization (Fig. 2) [13]. Antigen excretion by SIgA features the binding of SIgA to pathogen-derived antigens, thus inhibiting pathogen–epithelial cell contact. SIgA exerts immune exclusion by eliminating antigens via secretion of an IgA–antigen complex, and invading pathogens can also be eliminated by complex formation with IgA-joining (J) chain-pIgR. SIgA inhibits the binding of pathogens and/or pathogenic antigens to specific receptors by neutralizing and eventually removing the pathogenic antigens.
Fig. 1. Schematic diagram of mucosal immune induction. The luminal antigens transcytosed by M cells encounter dendritic cells (DCs) in the subepithelial dome of Peyer's patch. DCs loaded with the antigens move into the interfollicular T cell zone and induce the effector T cells. Antigen-specific effector CD4+ T cells expressing CD40 ligand induce the IgA+ plasmablasts. FDC, follicular dendritic cell; TCR, T-cell receptor; TGF β, transforming growth factor β; IL, interleukin; AID, activation-induced cytidine deaminase; CSR, class switch recombination; CXCL13, CXC chemokine ligand 13; CXCR5, CXC chemokine receptor 5.
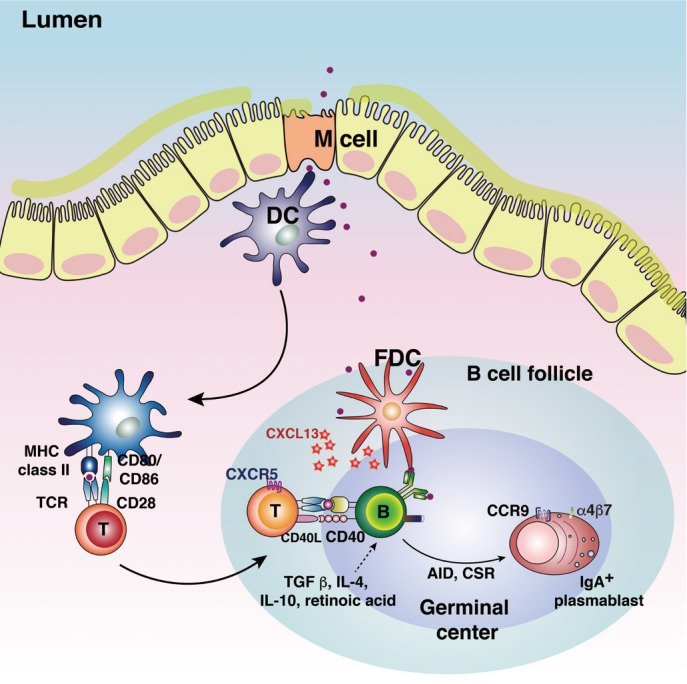
Fig. 2. The role played by secretory antibodies in the mucosal compartment. Secreted antibodies can protect mucosal surfaces by immune exclusion, antigen excretion, and intracellular neutralization. Immune exclusion is that secretory IgA (SIgA) interact with antigens and block their attachment to epithelial cells. The SIgAs bind to antigen and remove from the lamina propria through antigen excretion. The intracellular pathogen can also be eliminated by intracellular neutralization.
Mucosal Vaccines and Delivery Routes
Mucosal vaccination can induce antigen-specific humoral and cell-mediated immune responses in both the systemic and mucosal compartments [14]. Additionally, such vaccination efficiently induces long-lasting B- and T-cell memory [15]. Importantly, the characteristics of mucosal immune response induction depend on the vaccine delivery route chosen (Fig. 3). For example, oral delivery (a traditional form of mucosal vaccination) can induce production of antigen-specific SIgA in the gastrointestinal tract, salivary glands, and mammary glands [14]. Currently, the licensed human live attenuated vaccines for rotavirus, poliovirus, Salmonella Typhi, and cholera are delivered orally (Table 3) [14,16]. Intranasal vaccines such as FluMist, a live attenuated influenza virus vaccine, generate SIgA in the upper and lower respiratory, gastric, and genital tracts [17]. Upon sublingual vaccination, antigen-specific immune responses are induced in the gastrointestinal and the upper and lower respiratory tracts [14]. Although a few human mucosal vaccines are licensed, safety issues remain; the current vaccines are live attenuated or non-living whole-cell vaccines (Table 3). Subunit vaccines lacking entire pathogens are considered to be safer next-generation vaccines. However, several issues must be addressed when developing subunit mucosal vaccines, including poor immunogenicity, degradation of vaccine materials in the harsh mucosal environment, delivery of vaccine materials to mucosal immune inductive tissue, and modulation of the mucosal immune environment such that oral tolerance does not develop.
Fig. 3. Mucosal immunization routes and the regions affected. The mucosal IgA responses are differentially induced according to the routes of mucosal immunization. Oral vaccination is effective for the immune induction in the gastrointestinal tract, salivary glands, and mammary glands. Intranasal vaccination is effective for the immune induction in respiratory, gastric and genital tracts.
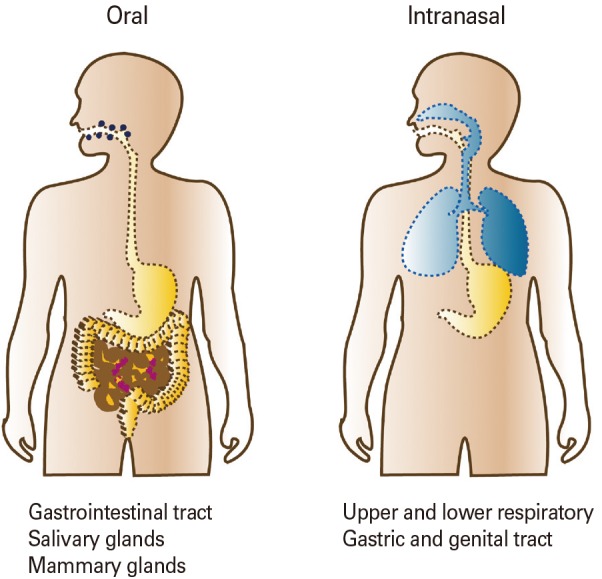
Table 3. Currently licensed mucosal vaccines.
| Pathogen | Trade name | Composition | Dosage | Immunological mechanism | Efficacy |
|---|---|---|---|---|---|
| Rotavirus | Rotarix, RotaTeg | Live attenuated, monovalent or pentavalent rotaviruses | Oral, 3 doses | Mucosal IgA and systemic neutralizing IgG | Over 70%-90% against severe disease |
| Poliovirus | Orimune, OPV, Poliomyelitis vaccine | Live attenuated trivalent, bivalent, and monovalent polioviruses | Oral, 3 doses | Mucosal IgA and systemic IgG | Over 90% in most of the world |
| Salmonella Typhi | Vivotif, Ty21A | Live attenuated S. Typhi bacteria | Oral, 3-4 doses | Mucosal IgA, systemic IgG, and CTL responses | Variable, but more than 50% |
| Vibrio cholera | Dukoral, ORC-Vax, Shanchol | Inactivated V. cholera O1 classical and El Tor biotypes with or without CTB | Oral, 2-3 doses | Antibacterial, toxin-specific, and LPS-specific IgA | Strong herd protection over 85% |
| Influenza type A and B virus | FluMist | Live viral reassortant with trivalent mix of H1, H3, and B strains of hemagglutinin and neuraminidase genes in an attenuated donor strain | Intranasal in young children, 2 doses | Hemagglutinin- and neuraminidase-specific mucosal IgA and systemic IgG responses | > 85% in children, variable in adults |
Mucosal Vaccine Adjuvants
The mucosa is continuously exposed to various antigens and microbiota and tightly regulates the influx of luminal antigens. Therefore, special delivery systems are required for development of successful mucosal vaccines [18]. M cells are the ideal targets of mucosal vaccine materials. Not only are the cells localized to the FAE of mucosal immune inductive sites, but many APCs are located nearby and/or under pockets of M cells. Although antigen uptake by M cells was previously thought to be non-specific, many recent studies have shown that a specific antigen delivery mechanism is involved [19]. GP2, a protein expressed specifically by M cells, drives transcytosis of FimH+ bacteria into such cells. Therefore, M cell-specific markers can be utilized for antigen delivery to mucosal immune inductive sites [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] (Table 4). For example, an M cell-specific antibody, NKM 16-2-4, recognizes the α(1,2)-fucose-containing carbohydrate moiety of M cells and can be used to enhance delivery of an associated antigen [33]. Additionally, an M cell-targeting ligand, Co1, also targets antigens to M cells by interacting with the complement 5a receptor, inducing an antigen-specific immune response [23,40]. Thus, M cell–targeting of vaccine materials will play a pivotal role in successful mucosal vaccination.
Table 4. M cell–specific molecules and their ligands.
| Ligand | Receptors on M cells | Reference |
|---|---|---|
| Ulex europaeus 1 (UEA-1) | α1,2 fucose | [21] |
| Aleuria auranitia (AAL) | α-L-fucose | [20] |
| Galectin-9 | N-glycans/repeated oligosaccharide | [22] |
| Peptide Co1 (SFHQLPARSPLP) | C5aR | [23] |
| Cathelicidin LL-37 | P2X7 receptor, | [25] |
| Formyl peptide receptor 2 | [24] | |
| Antibody NKM 16-2-4 | α1,2 fucose-containing carbohydrate | [33] |
| Antibody LM112 | Sialyl Lewis A | [29] |
| Antibody 3G7-H9 | Glycoprotein 2 | [27] |
| σ1 protein (reovirus) | α2,3 sialic acid | [39] |
| Invasion (Yersinia) | β1 integrin | [26] |
| Long polar fimbriae (Escherichia coli, Salmonella) | Unknown | [29] |
| FimH (E. coli, Salmonella) | Glycoprotein 2/Uromodulin | [36] |
| OmpH (Yersinia) | C5aR | [23] |
| LPS | TLR-4 | [28] |
| Lipoteichoic acid | TLR-2 | [37] |
| Phosphorylcholine moiety of LPS | PFAR | [38] |
| Hsp60 of Brucella abortus | Cellular prion protein | [32] |
| Lipid A domain of LPS (gram-negative bacteria) | ANXA5 | [35] |
| Bacterial peptidoglycan | PGLRP-1 | [34] |
| SIgA | Unknown | [30] |
| c-term domain of enterotoxin (Clostridium perfringens) | Claudin 4 | [31] |
In the tolerogenic mucosal environment, adjuvants with immunostimulatory capacities enhance immune induction (Table 5) [37,41,42,43,44,45,46,47,48,49]. When TLR agonists such as Pam3CSK4, poly(I:C), MPL, or CpG-ODN were given either nasally or orally, in combination with vaccine materials, both systemic and mucosal antigen-specific immune responses were enhanced [3]. In addition, some immunostimulatory adjuvants improve the quality of the immune response. Cholera toxin (CT) is an effective mucosal vaccine adjuvant because it interacts with the GM1 ganglioside. However, the use of CT in this context raises a safety concern. Thus, CTA1-DD, which contains a mutant GM1 ganglioside-targeting A subunit of CT and the D-fragment of Staphylococcus aureus protein A to activate follicular dendritic cells (FDCs) closely associated with GCs, has been developed. CTA1-DD effectively promotes the induction of high-affinity B-cell clones and long-lived memory B cells and plasma cells [50]. Another mucosal vaccine adjuvant is the oil-in water emulsion MF59, which is currently licensed for human use. Although the mechanism of action remains unclear, MF59 not only enhances recruitment of innate immune cells via release of ATP and antigen uptake, but it also increases the adjunctive capacities of B cells by enhancing GC actions via activation of follicular helper T cells [51]. Finally, cathelicidin LL-37 is an immunostimulatory adjuvant that targets antigens to M cells. LL-37 increases antigen delivery to such cells and activates FDCs by interacting with the formyl peptide receptor 2 [24]. This enhances the induction of antigen-specific immune responses in both the systemic and mucosal compartments.
Table 5. Mucosal adjuvants.
| Composition | Target | T-cell–mediated immune response | Mucosal IgA | Reference | |||
|---|---|---|---|---|---|---|---|
| Th1 | Th2 | Th17 | CTL | ||||
| MDP | TLR-2 | + | + | − | − | + | [37] |
| MPL | TLR-4 | + | − | − | + | + | [45] |
| Flagellin | TLR-5 | + | − | − | + | ++ | [47] |
| CT | GM1 | − | + | + | + | ++++++ | [42] |
| CTA1-DD | Ig heavy chain | + | + | + | + | +++++ | [41] |
| Quillaja saponins fraction | DCs | + | + | − | + | ++ | [44] |
| Cationic DDA | ND | + | − | − | + | ++ | [43] |
| Chitosan | Tight junctions | − | + | − | − | ++ | [48] |
| IL-1 | IL-1R | + | + | − | − | +++ | [46] |
| IL-12 | IL-12R | + | − | − | + | + | [49] |
Adapted from Lycke N. Nat Rev Immunol 2012;12:592-605 [14] and Kim SH and Jang YS. Exp Mol Med 2014;46:e85 [16].
+ or - in this table means the strength of induced immune response.
CTL, cytotoxic T lymphocytes; MDP, muramyl dipeptide; TLR, Toll-like receptor; MPL, monophosphoryl lipid A; CT, cholera toxin; DC, dendritic cell; DDA, dimethyldioctadecylammonium; ND, not determined; IL, interleukin.
Conclusion
Recently, the need for mucosal vaccines has become recognized. Such vaccines offer several advantages including safety, convenience of vaccination, economical production, induction of mucosal immune responses, and enhanced memory B- and T-cell induction. However, several hurdles must be overcome in the development of practical subunit mucosal vaccines, including poor immunogenicity, degradation of vaccine materials in a harsh mucosal environment, delivery of vaccine materials to mucosal immune inductive tissue, and modulation of the mucosal immune environment to ensure that oral tolerance does not develop. These obstacles will be overcome by developing effective mucosal adjuvants that target M cells and are immunostimulatory.
Footnotes
No potential conflict of interest relevant to this article was reported.
This study was supported by 2014K1B1A1073861 through the National Research Foundation (NRF) funded by the Korean Ministry of Science, ICT, & Future Planning and by HI15C3039 through the Korea Health Industry Development Institute (KHIDI) funded by the Korean Ministry of Health and Welfare.
References
- 1.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwok R. Vaccines: the real issues in vaccine safety. Nature. 2011;473:436–438. doi: 10.1038/473436a. [DOI] [PubMed] [Google Scholar]
- 3.Gutjahr A, Tiraby G, Perouzel E, Verrier B, Paul S. Triggering intracellular receptors for vaccine adjuvantation. Trends Immunol. 2016;37:573–587. doi: 10.1016/j.it.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 5.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 7.Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008;52:2–12. doi: 10.1111/j.1574-695X.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohno H. Intestinal M cells. J Biochem. 2016;159:151–160. doi: 10.1093/jb/mvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann N Y Acad Sci. 2012;1247:97–116. doi: 10.1111/j.1749-6632.2011.06378.x. [DOI] [PubMed] [Google Scholar]
- 12.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 13.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 14.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Jang YS. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014;46:e85. doi: 10.1038/emm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012;8:689–693. doi: 10.4161/hv.19568. [DOI] [PubMed] [Google Scholar]
- 18.Lamichhane A, Azegamia T, Kiyonoa H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711–6723. doi: 10.1016/j.vaccine.2014.08.089. [DOI] [PubMed] [Google Scholar]
- 19.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark MA, Jepson MA, Simmons NL, Hirst BH. Differential surface characteristics of M cells from mouse intestinal Peyer’s and caecal patches. Histochem J. 1994;26:271–280. [PubMed] [Google Scholar]
- 21.Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine. 1998;16:536–541. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 22.Hirabayashi J, Hashidate T, Arata Y, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Jung DI, Yang IY, et al. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol. 2011;41:3219–3229. doi: 10.1002/eji.201141592. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Lee HY, Jang YS. Expression of the ATP-gated P2X7 receptor on M cells and its modulating role in the mucosal immune environment. Immune Netw. 2015;15:44–49. doi: 10.4110/in.2015.15.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SH, Yang IY, Kim J, Lee KY, Jang YS. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur J Immunol. 2015;45:1402–1413. doi: 10.1002/eji.201444988. [DOI] [PubMed] [Google Scholar]
- 26.Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 28.Keely S, Glover LE, Weissmueller T, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell. 2010;21:538–546. doi: 10.1091/mbc.E09-07-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Kyd JM, Cripps AW. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26:6221–6224. doi: 10.1016/j.vaccine.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 31.Lo DD, Ling J, Eckelhoefer AH. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses. BMC Biotechnol. 2012;12:7. doi: 10.1186/1472-6750-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakato G, Hase K, Suzuki M, et al. Cutting Edge: Brucella abortus exploits a cellular prion protein on intestinal M cells as an invasive receptor. J Immunol. 2012;189:1540–1544. doi: 10.4049/jimmunol.1103332. [DOI] [PubMed] [Google Scholar]
- 33.Nochi T, Yuki Y, Matsumura A, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204:2789–2796. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osanai A, Sashinami H, Asano K, Li SJ, Hu DL, Nakane A. Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection. Infect Immun. 2011;79:858–866. doi: 10.1128/IAI.00466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rand JH, Wu XX, Lin EY, Griffel A, Gialanella P, McKitrick JC. Annexin A5 binds to lipopolysaccharide and reduces its endotoxin activity. MBio. 2012;3:pii: e00292-11. doi: 10.1128/mBio.00292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato S, Kaneto S, Shibata N, et al. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer's patch M cells. Mucosal Immunol. 2013;6:838–846. doi: 10.1038/mi.2012.122. [DOI] [PubMed] [Google Scholar]
- 37.Shafique M, Wilschut J, de Haan A. Induction of mucosal and systemic immunity against respiratory syncytial virus by inactivated virus supplemented with TLR9 and NOD2 ligands. Vaccine. 2012;30:597–606. doi: 10.1016/j.vaccine.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 38.Tyrer P, Foxwell AR, Cripps AW, Apicella MA, Kyd JM. Microbial pattern recognition receptors mediate M-cell uptake of a gram-negative bacterium. Infect Immun. 2006;74:625–631. doi: 10.1128/IAI.74.1.625-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- 40.Kim SH, Seo KW, Kim J, Lee KY, Jang YS. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010;185:5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 41.Agren LC, Ekman L, Lowenadler B, Lycke NY. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997;158:3936–3946. [PubMed] [Google Scholar]
- 42.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19:2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 44.Eliasson DG, Helgeby A, Schon K, et al. A novel non-toxic combined CTA1-DD and ISCOMS adjuvant vector for effective mucosal immunization against influenza virus. Vaccine. 2011;29:3951–3961. doi: 10.1016/j.vaccine.2011.03.090. [DOI] [PubMed] [Google Scholar]
- 45.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson AL, Johnson BT, Sempowski GD, et al. Maximal adjuvant activity of nasally delivered IL-1alpha requires adjuvant-responsive CD11c(+) cells and does not correlate with adjuvant-induced in vivo cytokine production. J Immunol. 2012;188:2834–2846. doi: 10.4049/jimmunol.1100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 48.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52:139–144. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 49.Winstone N, Wilson AJ, Morrow G, et al. Enhanced control of pathogenic Simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J Virol. 2011;85:9578–9587. doi: 10.1128/JVI.05060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agren L, Sverremark E, Ekman L, et al. The ADP-ribosylating CTA1-DD adjuvant enhances T cell-dependent and independent responses by direct action on B cells involving anti-apoptotic Bcl-2- and germinal center-promoting effects. J Immunol. 2000;164:6276–6286. doi: 10.4049/jimmunol.164.12.6276. [DOI] [PubMed] [Google Scholar]
- 51.Mastelic Gavillet B, Eberhardt CS, Auderset F, et al. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J Immunol. 2015;194:4836–4845. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]


