Abstract
The effect of a number of antibiotics on stop codon readthrough during protein synthesis in Escherichia coli was examined. Inhibitors which bind close to the entrance of the peptide exit tunnel on the 50S ribosomal subunit promote substantial levels of readthrough, presumably by disrupting the mechanism of peptide release.
Recently, our laboratory reported novel effects of the antibiotic chloramphenicol, a classical inhibitor of peptide bond formation, upon translational accuracy (17). In the same work, similar effects were also uncovered for members of the oxazolidinone family, the first group of completely synthetic compounds to be approved for clinical use. The precise mode(s) of action of members of this class of antibiotic is not entirely understood (18), but the likelihood is that they do not inhibit protein synthesis by the same mechanism as chloramphenicol. The very similar effects of these different inhibitors of large ribosomal subunit function on stop codon readthrough and frameshifting prompted us to examine whether other antibiotics with various modes of action might also affect fidelity.
The assay used in these experiments employed lacZ as a reporter gene, with mutations in the N terminus to introduce stop codons, in this case UAG or UGA. Expression of β-galactosidase in the absence of stop codon readthrough was low, but when translational errors affecting termination occur, enzyme activity increases. The host strain used for the reporter gene plasmid constructs was Escherichia coli strain EF41 (F− Δ[lac-pro] thi recA1). Plasmids pSG400 (GUU UAG GCC) and pSG415 (GUG AAA UGA GCC) carried mutations coding uniquely for recognition by release factor 1 (RF1) and RF2, respectively (underlined). Growth of strains in the presence or absence of antibiotics and measurement of the levels of β-galactosidase produced were carried out as previously described (17). The antibiotics examined here, which affect the 50S subunit, included representatives of the macrolide/lincosamide group; inhibitors of peptidyl transfer; and capreomycin, an antibiotic requiring both subunits for binding (3) but which causes translational infidelity (8). Nalidixic acid, an inhibitor of DNA replication (4), was used as a control.
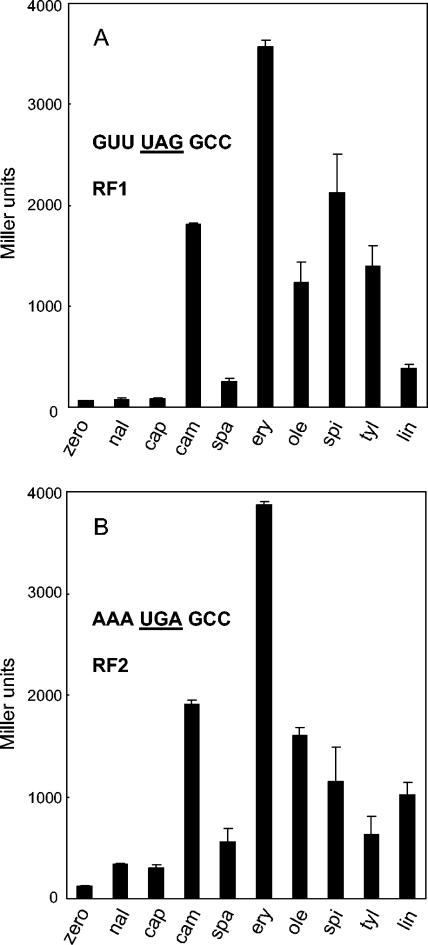
Capreomycin promotes misreading in a manner closely similar to that of members of the aminoglycoside family, with which its binding site on the 30S subunit partially overlaps (3, 8). It was, therefore, perhaps not too surprising that capreomycin had no effect upon stop codon readthrough (Fig. 1), as neither gentamicin (17) nor neomycin (data not shown) stimulate β-galactosidase production in these assays. Its binding site has not been unequivocally determined, although it would be reasonable to expect that it is near the interface of the two subunits and therefore is at some distance from the peptidyl transferase center.
FIG. 1.
Production of β-galactosidase by readthrough of stop codons for RF1 (A) and RF2 (B). E. coli strain EF41, carrying a reporter plasmid with an engineered stop codon at the N terminus of the lacZ gene, was grown in the absence (zero) or presence of nalidixic acid (nal; 5 μg/ml), capreomycin (cap; 150 μg/ml), chloramphenicol (cam; 3.5 μg/ml), sparsomycin (spa; 3 μg/ml), erythromycin (ery; 175 μg/ml), oleandomycin (ole; 1,250 μg/ml), spiramycin (spi; 400 μg/ml), tylosin (tyl; 500 μg/ml), or lincomycin (lin; 325 μg/ml) to an A600 of 0.3 to 0.6. β-Galactosidase activity was measured, as described previously (9), in a minimum of five duplicate assays.
Next, two classical inhibitors of peptidyl transfer were examined, chloramphenicol and sparsomycin. The dramatic stimulatory effect of chloramphenicol on UAG readthrough (Fig. 1A) has already been reported (17), although in the E. coli strain used here the effect is even more pronounced, as the concentration of drug employed was almost 10-fold higher than that used in the previous study. Conversely, the stimulation by sparsomycin was very much less, with only a modest elevation of β-galactosidase levels compared to those seen with nalidixic acid. These two antibiotics are both potent inhibitors of peptidyl transfer, and sparsomycin can block the binding of chloramphenicol on polysomes (10), so their differing effects on stop codon readthrough were unexpected. Cocrystals of each of the drugs with 50S subunits have, however, been visualized by X-ray crystallography (6, 13), and these visualizations offer insights. Sparsomycin in the Haloarcula marismortui crystal structure lies, as expected, at the heart of the peptidyl transferase center, interacting with the CCA end of the P-site-bound substrate required for the antibiotic to bind (5). This site essentially overlaps that visualized for chloramphenicol in the Deinococcus radiodurans structure (14) at the active-site hydrophobic crevice of the peptidyl transferase center, and it is entirely consistent with their competitive binding. On the other hand, in the H. marismortui crystal, chloramphenicol was located at the entrance to the peptide exit channel (6) at a site almost completely overlapping that of erythromycin. H. marismortui, an archaeon, is naturally highly resistant to chloramphenicol, so the visualization of chloramphenicol bound in an ordered fashion was somewhat surprising. Biochemical and genetic evidence suggest, however, that the binding site at the entrance to the exit tunnel may be real, as footprinting and resistance mutations also agree (6 and references cited therein). The ability of chloramphenicol to enhance the premature release of short oligopeptidyl tRNAs in vitro (12), probably by disturbing the correct positioning of the nascent peptide, provides further support for its influence at the peptide exit channel. Thus, early experiments indicating two binding sites for chloramphenicol on the large subunit (2) with approximately fivefold different affinities may well be correct.
Representatives of the macrolide group of antibiotics examined included erythromycin and oleandomycin, each with 14-member lactone rings, and spiramycin and tylosin, with 16-member rings. The stimulation of β-galactosidase production by erythromycin was even greater than that reported for linezolid when both are compared to that of chloramphenicol (17) (Fig. 1), but all macrolides tested caused extensive stop codon readthrough. Lincomycin, a representative of the lincosamides that has a binding site overlapping that of erythromycin (3), also caused readthrough. Large-subunit cocrystal structures of erythromycin (13), spiramycin and tylosin (5), and other macrolide/ketolide derivatives (1, 5, 6, 12, 13) have been visualized in both H. marismortui and D. radiodurans 50S subunits. Broadly, and in line with their modes of action (16), these antibiotics bind either at the mouth of the exit tunnel, close to the peptidyl transferase centre, or at various positions further into the tunnel.
In conclusion, the common property of antibiotics which stimulate stop codon readthrough in these assays is that they bind at or within the peptide exit tunnel (assuming that the lower affinity binding site is the one responsible for the stimulatory effect of chloramphenicol). Two potential ways in which readthrough could be enhanced are either by weakening RF binding or by inhibition of hydrolysis of the nascent peptide, allowing a ternary complex time to deliver a tRNA for successful peptidyl transfer. RF-binding contacts have been visualized around the exit tunnel by cryoelectron microscopy (7, 11), so antibiotic binding may cause sufficient structural distortion to interfere with factor binding. Equally, structural changes may be enough to disrupt peptide release without affecting peptide bond formation, as it now appears that the catalysis of these two mechanisms may be effected in very different ways (15, 19). Further exploration of the ways in which these antibiotics affect the proper termination of translation, coupled with more high-resolution cocrystal structures, will provide detailed insights into ribosome function at the peptidyl transferase centre.
Acknowledgments
We are grateful to Antonio Jimenez for his generous gift of sparsomycin; all other antibiotics were purchased from Sigma.
This research was supported by NIH grant GM 19756 to A.E.D.
REFERENCES
- 1.Berisio, R., F. Schluenzen, J. Harms, A. Bashan, T. Auerbach, D. Baram, and A. Yonath. 2003. Structural insight into the role of the ribosomal tunnel in cellular recognition. Nat. Struct. Biol. 10:366-370. [DOI] [PubMed] [Google Scholar]
- 2.Contreras, A., and D. Vazquez. 1977. Cooperative and antagonistic interactions of peptidyl-tRNA and antibiotics with bacterial ribosomes. Eur. J. Biochem. 74:539-547. [DOI] [PubMed] [Google Scholar]
- 3.Cundliffe, E. 1981. Antibiotic inhibitors of ribosome function, p. 402-547. In E. F. Gale, E. Cundliffe, P. E. Reynolds, M. H. Richmond, and M. J. Waring (ed.), The molecular basis of antibiotic action. Wiley, London, United Kingdom.
- 4.Emmerson, A. M., and A. M. Jones. 2003. The quinolones: decades of development and use. J. Antimicrob. Chemother. 51(Suppl. 1):13-20. [DOI] [PubMed] [Google Scholar]
- 5.Hansen, J. L., J. A. Ippolito, N. Ban, P. Nissen, P. B. Moore, and T. A. Steitz. 2002. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell. 10:117-128. [DOI] [PubMed] [Google Scholar]
- 6.Hansen, J. L., P. B. Moore, and T. A. Steitz. 2003. Structures of five antibiotics bound at the peptidyl transferase center of the large ribosomal subunit. J. Mol. Biol. 330:1061-1075. [DOI] [PubMed] [Google Scholar]
- 7.Klaholz, B. P., T. Pape, A. V. Zavialov, A. G. Myasnikov, E. V. Orlova, B. Vestergaard, M. Ehrenberg, and M. van Heel. 2003. Structure of the Escherichia coli ribosomal termination complex with release factor 2. Nature 421:90-94. [DOI] [PubMed] [Google Scholar]
- 8.Marrero, P., M. J. Cabanas, and J. Modolell. 1980. Induction of translational errors (misreading) by tuberactinomycins and capreomycins. Biochem. Biophys. Res. Commun. 97:1047-1052. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor, M., H. U. Göringer, and A. E. Dahlberg. 1992. A ribosomal ambiguity mutation in the 530 loop of E. coli 16S rRNA. Nucleic Acids Res. 20:4221-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pestka, S. 1974. Antibiotics as probes of ribosome structure: binding of chloramphenicol and erythromycin to polyribosomes; effect of other antibiotics. Antimicrob. Agents Chemother. 5:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat, U. B. S., A. V. Zavialov, J. Sengupta, M. Valle, R. A. Grassucci, J. Linde, B. Vestergaard, M. Ehrenberg, and J. Frank. 2003. A cryo-electron microscopic study of ribosome-bound termination factor RF2. Nature 421:87-90. [DOI] [PubMed] [Google Scholar]
- 12.Rheinberger, H.-J., and K. H. Nierhaus. 1990. Partial release of AcPhe-Phe-tRNA from ribosomes during poly(U)-dependent poly(Phe) synthesis and the effects of chloramphenicol. Eur. J. Biochem. 193:643-650. [DOI] [PubMed] [Google Scholar]
- 13.Schlünzen, F., R. Zarivach, J. Harms, A. Bashan, A. Tocilj, R. Albrecht, A. Yonath, and F. Franceschi. 2001. Structural basis for the interaction of antibiotics with the peptidyl transferase center in eubacteria. Nature 413:814-821. [DOI] [PubMed] [Google Scholar]
- 14.Schlünzen, F., J. M. Harms, F. Franceschi, H. A. S. Hansen, H. Bartels, Zarivach, R., and A. Yonath. 2003. Structural basis for the antibiotic activity of ketolides and azalides. Structure 11:329-338. [DOI] [PubMed] [Google Scholar]
- 15.Sievers, A., M. Beringer, M. V. Rodnina, and R. Wolfenden. 2004. The ribosome as an entropy trap. Proc. Natl. Acad. Sci. USA 101:7897-7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenson, T., M. Lovmar, and M. Ehrenberg. 2003. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 330:1005-1014. [DOI] [PubMed] [Google Scholar]
- 17.Thompson, J., M. O'Connor, J. A. Mills, and A. E. Dahlberg. 2002. The protein synthesis inhibitors, oxazolidinones and chloramphenicol, cause extensive translational inaccuracy in vivo. J. Mol. Biol. 322:273-279. [DOI] [PubMed] [Google Scholar]
- 18.Xiong, L., P. Kloss, S. Douthwaite, N. M. Andersen, S. Swaney, D. L. Shinabarger, and A. S. Mankin. 2000. Oxazolidinone resistance mutations in 23S rRNA of Escherichia coli reveal the central region of domain V as the primary site of drug action. J. Bacteriol. 182:5325-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youngman, E. M., J. L. Brunelle, A. B. Kochaniak, and R. Green. 2004. The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release. Cell 117:589-599. [DOI] [PubMed] [Google Scholar]