Abstract
Garenoxacin (T-3811ME, BMS-284756) is a novel, broad-spectrum des-F(6) quinolone currently under study for the treatment of community-acquired respiratory tract infections. This analysis assessed garenoxacin population pharmacokinetics and exposure-response relationships for safety (adverse effects [AE]) and antimicrobial activity (clinical cure and bacteriologic eradication of Streptococcus pneumoniae and the grouping of Haemophilus influenzae, Haemophilus parainfluenzae, and Moraxella catarrhalis). Data were obtained from three phase II clinical trials of garenoxacin administered orally as 400 mg once daily for 5 to 10 days for the treatment of community-acquired pneumonia, acute exacerbation of chronic bronchitis, and sinusitis. Samples were taken from each patient before drug administration, 2 h following administration of the first dose, and on the day 3 to 5 visit. Individual Bayesian estimates of the fu (fraction unbound), the Cmax, and the fu for the area under the concentration-time curve from 0 to 24 h (fu AUC0-24) were calculated as measurements of drug exposure by using an ex vivo assessment of average protein binding. Regression analysis was performed to examine the following relationships: treatment-emergent AE incidence and AUC0-24, Cmax, or patient factors; clinical response or bacterial eradication and drug exposure (fu Cmax/MIC, fu AUC0-24/MIC, and other exposure covariates); or disease and patient factors. Garenoxacin pharmacokinetics were described by a one-compartment model with first-order absorption and elimination. Clearance was dependent on creatinine clearance, ideal body weight, age, obesity, and concomitant use of pseudoephedrine. The volume of distribution was dependent on weight and gender. Patients with mild or moderate renal dysfunction had, on average, approximately a 16 or 26% decrease in clearance, respectively, compared to patients of the same gender and obesity classification with normal renal function. AE occurrence was not related to garenoxacin exposure. Overall, clinical cure and bacterial eradication rates were 91 and 90%, respectively, for S. pneumoniae and 93 and 92%, respectively, for the grouping of H. influenzae, H. parainfluenzae, and M. catarrhalis. The fu AUC0-24/MIC ratios were high (>90% were >200), and none of the pharmacokinetic-pharmacodynamic exposure measurements indexed to the MIC or other factors were significant predictors of clinical or bacteriologic response. Garenoxacin clearance was primarily related to creatinine clearance and ideal body weight. Although garenoxacin exposure was approximately 25% higher for patients with moderate renal dysfunction, this increase does not appear to be clinically significant as exposures in this patient population were not significant predictors of AE occurrence. Garenoxacin exposures were at the upper end of the exposure-response curves for measurements of antimicrobial activity, suggesting that 400 mg of garenoxacin once daily is a safe and adequate dose for the treatment of the specified community-acquired respiratory tract infections.
Garenoxacin (T-3811ME, BMS-284756), a novel des-F(6) quinolone that lacks the fluorine substituent at the C-6 position, is currently under study for the treatment of selected bacterial infections, including community-acquired respiratory tract infections. This agent possesses a broad spectrum of in vitro activity against both gram-positive and gram-negative bacteria, especially those associated with community-acquired respiratory tract infections, which are often associated with extracellular pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis and intracellular organisms such as Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae (12, 17).
After administration of a single 400-mg oral dose of garenoxacin to healthy adults, peak plasma drug concentrations occurred within 1 to 2 h and the elimination half-life was approximately 14 h. Administration of garenoxacin with a high-fat meal did not result in a clinically relevant change in garenoxacin exposure (14). The arithmetic mean (standard deviation [SD]) area under the concentration-time curve from time zero to infinity (AUC0-∞) values (micrograms per hour per milliliter) were 72.8 (17.7) and 64.4 (13.5) in the fasted and fed states, respectively. Garenoxacin undergoes hepatic metabolism and renal elimination via a combination of glomerular filtration and active tubular secretion; approximately 40% of the garenoxacin dose is excreted as the unchanged drug in urine. The serum protein binding of garenoxacin is 75% (A. Bello, D. Hollenbaugh, D. Gajjar, L. Christopher, and D. Grasela, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-45, p. 8, 2001), and the steady-state volume of distribution is 60 to 112 liters.
The development of therapeutic response relationships for anti-infectives requires knowledge of drug exposure (AUC0-24) and the MIC, a measurement of the sensitivity of the infecting organism to the drug. Recent studies have established that the AUC0-24/MIC ratio is an important pharmacodynamic (PD) parameter influencing quinolone efficacy. Lister demonstrated that garenoxacin exhibits PDs similar to those of clinically available quinolones (20). Eradication of S. pneumoniae from an in vitro model was achieved with fu AUC0-24/MIC ratios of approximately 29 or higher (20). Data from animal models of pneumococcal infection also suggested that achievement of an fu AUC0-24/MIC ratio of 30 to 40 for garenoxacin resulted in optimal pneumococcal killing (26; W. Craig, personal communication). In clinical and nonclinical models of infection, a free-fraction AUC/MIC ratio of 90 to 125 was predictive of efficacy for gram-negative enteric bacteria and Pseudomonas for quinolones; however, these values for H. influenzae and M. catarrhalis have not been determined (10, 11).
Knowledge of exposure-response relationships for safety of anti-infectives is also of value. Administration of single oral doses (ranging from 100 to 1,200 mg) of garenoxacin has been well tolerated in healthy volunteers. The most common adverse events (AEs) were headache, pharyngitis, dizziness, and white exudates. There did not appear to be a relationship between the garenoxacin dose and either the type or the frequency of AEs (13).
This report describes the population pharmacokinetics (PKs) and PDs of garenoxacin from data obtained in three multiple-dose phase II studies of garenoxacin safety and antimicrobial activity in patients with acute exacerbation of chronic bronchitis (ABECB), community-acquired pneumonia (CAP), or acute bacterial sinusitis (ABS).
MATERIALS AND METHODS
Study design.
Garenoxacin was studied in three phase II, multinational clinical trials, one for each indication. These studies were performed in compliance with the standards of the Institutional Review Board, Independent Ethics Committee, Code of Federal Regulations, and the principles of the Declaration of Helsinki and its amendments. The ABECB study was a randomized, double-blinded, multicenter trial designed to assess the antimicrobial activity of a 5- or 10-day course of oral garenoxacin at a dose of 400 mg administered once daily. Patients randomized to the 5-day regimen received placebo tablets on days 6 to 10. The CAP and ABS studies were open-label, multicenter trials designed to assess the antimicrobial activity of a 10-day course of oral garenoxacin at a dose of 400 mg once daily.
For the ABECB study, outpatients 18 years of age and older with a diagnosis of chronic bronchitis were eligible. Chronic bronchitis was defined as chronic cough and sputum production on most days for 3 consecutive months for more than 2 consecutive years (5). Furthermore, it was necessary that clinical evidence of ABECB be demonstrated by the production of purulent sputum and the presence of two or more of the following signs and symptoms: increased cough and/or dyspnea, increased sputum volume, or increased sputum purulence.
For the CAP study, outpatients 18 years of age and older with clinical evidence of CAP were eligible (3). The clinical diagnosis of CAP required a new infiltrate on chest X-ray, sputum production, and two or more of the following: fever (oral temperature greater than 38°C), leukocytes (greater than 10,000 white blood cells/mm3 or greater than 15% band forms), cough, chest pain, or auscultatory findings such as rales and/or evidence of pulmonary consolidation.
Patients meeting any one of the following criteria were excluded from the CAP study: requirement of long-term antibacterial therapy or hospitalization for intravenous therapy to treat the underlying infection; known or suspected active tuberculosis or infection with other mycobacteria or fungi; known or suspected Pneumocystis carinii pneumonia; receipt of chronic (longer than 2 weeks) systemic steroids at a dose of at least 10 mg of prednisone (or its equivalent) per day or other chronic immunosuppressive therapy; emphysema; primary lung abscess; infection acquired in a hospital, nursing home, or other long-term care facility; or hospitalization for any reason within the previous 14 days.
For the ABS study, outpatients 18 years of age or older with a diagnosis of acute maxillary sinusitis were eligible. Acute maxillary sinusitis was defined as facial pain or tenderness over one or both maxillary areas lasting for at least 5 days but for less than 28 days. Patients were selected for inclusion on the basis of having at least two of the following signs and symptoms: fever (oral temperature greater than 38°C), leukocytes (greater than 10,000 white blood cells/mm3 or greater than 15% band forms), nasal congestion, postnasal drainage, frequent coughing, or headache. Furthermore, patients were required to have at least one of the following physical examination findings: purulent discharge from the maxillary sinus orifice, purulent discharge from the nose, or purulent discharge present in the back of the throat. In addition, it was required that patients have at least one of the following evident in one or both maxillary sinuses following radiologic documentation of sinusitis by X-ray or computerized tomography scan: opacification, an air-fluid level, or mucosal thickening of at least 5 mm.
Patients were excluded from the ABS study for any of the following reasons: chronic presentation of the current episode of sinusitis, defined as duration of symptoms longer than 28 days; three or more episodes of acute sinusitis within the preceding 6 months; complicated sinusitis; anatomic abnormality involving the maxillary sinus ostium that would impair drainage of the sinus and might affect the response to therapy; recent sinus surgery (within 3 months prior to enrollment); nosocomial sinusitis secondary to head trauma or nasotracheal intubation; or requirement, in the opinion of the investigator, or receipt of long-term antibacterial therapy.
Patients meeting any of the following criteria were excluded from all three studies: any previously diagnosed condition that tends to mimic or complicate the course and evaluation of the infectious process, receipt of systemic antibiotic therapy within the 7-day period prior to enrollment or likelihood of receiving other systemic antibiotics during participation in the study, previously diagnosed immune function disease(s) (human immunodeficiency virus-infected subjects without AIDS were eligible for enrollment in the CAP study), cystic fibrosis, current clinically significant hepatic disease (alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST] and/or total bilirubin [TBIL] levels greater than or equal to three times the upper limit of normal), a serum creatinine level greater than 2.0 mg/dl or requiring renal dialysis, a history of a serious hypersensitivity reaction to any quinolone compound, malabsorption syndromes or other gastrointestinal disturbances affecting drug absorption, use of an investigational agent within 30 days prior to entry into the study, or pregnancy and/or breast feeding.
PK methods.
Blood samples (7 ml) for the determination of garenoxacin concentrations were scheduled to be collected from all patients before drug administration (0 h), 2 h after administration of the first dose of the study drug, and again on the day 3 to 5 visit. Additional PK data collected from these studies included garenoxacin dosing histories (times, amounts, and fed or fasted dosing state) with corresponding sample collection times and plasma garenoxacin concentrations, selected patient covariates at baseline, and record of concomitant medication use. The garenoxacin data used in the PK analysis were divided into a model development data set and a model validation data set. Within each study, 20 and 80% of the patients were randomly selected for inclusion in the model validation and development data sets, respectively.
Covariates.
The patient covariates evaluated in this analysis included age, weight (WTKG), height, race, gender, congestive heart failure status (CAP study only), ideal body weight (IBW) (30), percentage of IBW, and obesity (defined as a WTKG greater than 130% of the IBW). The laboratory measurements evaluated included alkaline phosphatase (ALP), ALT, AST, TBIL, serum creatinine, blood urea nitrogen, estimated creatinine clearance (CrCL) (6, 30), and absolute eosinophil count.
Garenoxacin doses on the days of PK sample collection were classified as fasted or fed to evaluate the effect of food on relative garenoxacin bioavailability (F; fasted F = 1). Meal type was also recorded as either none, snack, light, or heavy. Doses with missing meal information were assumed to have been administered in a fasted state.
Because the number of patients taking any one concomitant medication was generally small, the concomitant medications were grouped on the basis of previously identified types (23, 24, 31) of PK interactions and used to analyze the effects of concomitant medication use on the clearance of garenoxacin. In addition, frequently prescribed medications, defined as a medication administered to at least 50 patients (approximately 5% of the study population), were also evaluated.
Garenoxacin assay.
Plasma samples were assayed for garenoxacin concentrations by a validated liquid chromatography with tandem mass spectrometry (LC/MS/MS) assay that used an internal standard (N. H. Fukumoto, Toyama Chemical Co., Ltd., Tokyo, Japan; data on file). The range of the standard curve in plasma (0.01 to 10 μg/ml) was used to define the quantifiable limits for study curves. The precision between runs and the precision within runs for analytical quality control samples had coefficients of variation (CV) no greater than 7.3 and 4.2%, respectively, with deviations from the nominal concentrations of no more than ±5.8% across the three studies.
Population PKs. (i) Statistical methods.
All PK analyses were performed with the computer program NONMEM, version 5.0, level 1.1, with the first-order estimation method (28). For each analysis, NONMEM computed the minimum value of the objective function, a statistic that is proportional to minus twice the log likelihood of the data. In the case of hierarchical models, the change in the minimum value of the objective function produced by the inclusion of a parameter is asymptotically distributed as χ2 with the number of degrees of freedom equal to the number of parameters added to or deleted from the model. Covariates contributing at least a 3.84 change in the objective function (α = 0.05, 1 degree of freedom) were considered significant.
The goodness of fit of each model tested was assessed by evaluation of the agreement between the observed and predicted garenoxacin concentrations, reductions in the range of weighted residuals, uniformity of the distribution of weighted residuals about zero across the range of both the predicted concentrations and the time since administration of the last dose, and increases in the precision of the parameter estimates as measured by the percent standard error of the mean (standard error/parameter estimate × 100), as well as the reduction in both interindividual and residual variabilities.
(ii) Structural model development.
Plots of full-profile data collected in phase I studies conducted with healthy volunteers indicated a monoexponential decline of garenoxacin over a 24-h time period following administration of single and multiple doses ranging from 100 to 400 mg (14). Therefore, a one-compartment model with first-order absorption and elimination parameterized with the apparent oral clearance (CL/F), volume (V/F), and absorption rate constant (ka) was used to fit the phase II data. Interindividual variability of all PK parameters was described with an exponential error model. A proportional error model was used to describe the residual variability.
(iii) Covariate analyses.
Covariate analyses were conducted by a stepwise forward selection procedure. For each step, Bayesian estimates of the PK parameters were generated for each patient, and a partial residual was calculated for each parameter as the Bayesian parameter estimate minus the population mean value of the parameter. Plots of the partial residuals for each parameter versus each of the patient covariates were examined for observable trends and used to assess the functional form of the relationship between the PK parameter and the covariate. A univariate analysis of each patient covariate with an observable trend was then performed, and the most significant covariate (α = 0.05) was added to the model.
The interindividual and residual variability models were then evaluated for bias with standard goodness-of-fit plots, and other error models were used if more appropriate. This evaluation was followed by a stepwise univariate backward elimination analysis of the covariates (α = 0.001).
(iv) Evaluation of food effect.
Following the establishment of the covariate model, the effect of the presence of food at the time of dosing on the relative bioavailability (F) of garenoxacin was evaluated. The effect of food on the ka was not evaluated because the sampling strategy used provided limited information for the estimation of this parameter.
(v) Analysis of concomitant medications.
Concomitant medication classes with a minimum of 30 patients who received at least one medication in each drug class and frequently prescribed medications were evaluated as shown in Table 1. The effects of concomitant medications on garenoxacin clearance were evaluated with the stepwise procedure previously described.
TABLE 1.
Concomitant-medication statistics
| Concomitant medication or class | No. (%) of patientsa |
|---|---|
| Acetaminophen | 150 (26) |
| Acetylsalicylic acid | 63 (11) |
| Albuterol | 153 (26) |
| Guaifenesin | 83 (14) |
| Hydrocodone | 57 (10) |
| Ibuprofen | 47 (8) |
| Prednisone | 48 (8) |
| Pseudoephedrine | 82 (14) |
| Theophylline | 53 (9) |
| Renal transport inhibitors | |
| Anionic | 83 (14) |
| Cationic | 44 (8) |
| CYP2C9/19 inhibitors | 61 (11) |
| CYP2D6 inhibitors | 66 (11) |
| P-glycoprotein inhibitor | 42 (7) |
| Steroids (chronic only) | 32 (6) |
| Steroids (acute and chronic) | 67 (12) |
Patients could receive multiple concomitant medications.
(vi) Final model.
The final model including all significant patient covariates, food effects, and concomitant medications was then evaluated for any remaining biases in the interindividual and residual error models and for possible simplifications.
(vii) Model validation.
The final PK model including all statistically significant covariates was applied to the model validation data set. The differences between the measured and predicted garenoxacin concentrations were evaluated for bias and precision (32). As a measurement of bias, the prediction error percentage (PE%) was computed as the difference between the predicted and measured observations divided by the predicted value, and the absolute PE% (|PE%|) was computed as a measurement of precision. A bias within ±5% and a precision within 30% for most of the samples were considered adequate.
PD safety methods.
An AE was defined as any untoward medical occurrence (an unfavorable or unintended sign, including clinically significant abnormal laboratory findings, symptoms, or diseases) that appeared or worsened during the course of the study. AEs were either spontaneously reported by the patient throughout the study or determined by patient inquiry (1 to 3 days following the last dose) or examination (7 to 14 days following administration of the last dose) by the clinical investigator. An AE was considered drug related if it was classified by the investigator as certainly, probably, or possibly related to garenoxacin during the trial.
PD antimicrobial methods.
To be evaluated for clinical response, patients had to have received at least 5 days of garenoxacin therapy and could not have received any concomitant systemic antibiotic with documented activity against the pathogen, except to treat clinical failure. It was also required that the patient receive a posttreatment assessment 7 to 14 days after the end of therapy or an end-of-treatment assessment (in the case of clinical failure).
Cure was defined as resolution or improvement of all signs and symptoms present at study entry such that no additional antibiotic therapy was required on the basis of the clinical investigator's evaluation. In the ABECB study, it was also required that no new signs or symptoms of acute infection be present and that if the patient was febrile at study entry, the fever was resolved. For the CAP and ABS studies, radiographic abnormalities must have either improved or not progressed.
In all three studies, failure was defined as persistence or progression of one or more cardinal signs and/or symptoms of the treated condition after at least 3 consecutive days of study drug therapy or the development of new signs or symptoms. In the ABECB study, any of the following criteria also qualified as failure: clinical or radiological evidence of pneumonia evolution during treatment or requirement of another antibiotic for treatment of the acute episode despite resolution or improvement of signs and symptoms. For the CAP study, progression of radiographic abnormalities and death due to pneumonia were also included in the definition of failure. Finally, in the ABS study, garenoxacin therapy was considered a failure if the patient was placed on additional or alternate antibiotic therapy because of persistent, worsened, or new signs and symptoms of acute infection. Patients in all three studies were classified as “unable to determine” if extenuating circumstances precluded classification as a cure or a failure. Only patients who met all of the criteria for evaluation and were classified as either cures or failures were considered in the clinical response analysis.
To be evaluated for bacteriologic response, patients were required to meet all of the clinical availability criteria and have a pretreatment culture positive for a bacterial pathogen susceptible to the study drug. Bacteriologic response was determined at the posttreatment visit (7 to 14 days after the end of therapy) and was classified as either eradicated, presumed eradicated, persisted, or presumed persisted. Eradicated was defined as the original pathogen being absent from the culture of a good-quality sputum specimen in the ABECB and CAP studies and from a repeat sinus aspirate culture in the ABS study. If a patient did not have material available for culture and the clinical response was classified as a cure, then the bacteriologic response was classified as presumed eradicated. These classifications of eradication and presumed eradication of the organism were considered successful outcomes. Persisted was defined as continued presence of the original pathogen in the culture of a sputum specimen for the ABECB and CAP studies and in the sinus aspirate culture for the ABS study. If a patient did not have material available for culture and the clinical response was classified as a failure, then the bacteriologic response was classified as presumed persisted. The classifications of persistence and presumed persistence of the organism were considered unsuccessful outcomes.
All isolated pathogens were tested for susceptibility to garenoxacin by broth microdilution in accordance with guidelines recommended by the National Committee for Clinical Laboratory Standards (25). AUC0-24 and Cmax were calculated for each individual with the Bayesian PK parameter estimates obtained with the POST-HOC option in NONMEM and the standard one-compartment model equations for AUC0-τ (micrograms per hour per milliliter) and Cmax (micrograms per milliliter) at steady state (15). The free-fraction parameters fu AUC0-24 and fu Cmax were calculated for the present analyses as one-fourth of the total value of these parameters.
Safety analysis of AEs.
Each patient was classified as experiencing either at least one drug-related AE or none. Logistic regression was performed (SAS v6.12; SAS, Inc., Cary, N.C.) to determine whether the probability of experiencing a drug-related AE was significantly related to drug exposure measurements (Cmax or AUC0-24) or patient demographic factors. The demographic factors considered in the regression were age, WTKG, height, gender, ethnicity, CrCL, and condition treated. Discrete covariates with five or fewer patients per response category were excluded from the analysis. The regression was done with a stepwise procedure with criteria of α < 0.10 for the addition of a covariate and α < 0.05 for a covariate to remain in the model.
In addition to the analysis of any drug-related AE, all AEs were grouped into categories by body system (e.g., gastrointestinal, central nervous system, etc.). Each patient was classified as either experiencing at least one drug-related AE in the category or not experiencing any drug-related AEs in each specific body system category. Any body system with at least 10% of the patients categorized as experiencing at least one drug-related AE was analyzed with a logistic regression procedure identical to that described above for any drug-related AE. As an additional analysis, any specific AE with at least 3% of the patients categorized as having a drug-related AE was analyzed by the same logistic regression procedure.
Antimicrobial activity analysis.
The PD analysis of clinical cure and bacteriologic response was completed with data from enrolled patients with a pathogen isolated at baseline, a recorded clinical response of cure or failure, and a recorded bacteriologic response of eradicated, presumed eradicated, persisted, or presumed persisted, as well as an estimated drug exposure (fu AUC0-24 and fu Cmax) from the PK analysis, and recorded patient and disease covariates.
The purpose of the PD analysis was to assess the relationship between antimicrobial activity (clinical response and bacteriologic eradication) and patient covariates, disease covariates, and drug exposure measurements for S. pneumoniae and the grouping of the pathogens H. influenzae, H. parainfluenzae, and M. catarrhalis, more common causative organisms of community-acquired respiratory tract infections.
Patient covariates considered in the analysis were gender, ethnicity, age, WTKG, IBW, CrCL, ALT, and congestive heart failure status; disease covariates included the condition treated, the total number of pathogens per patient, the highest MIC for selected pathogens (those specific to the analysis) for a clinical response, and the highest MIC for persistent pathogens for a bacteriologic response; drug exposure measurements included the fu Cmax, fu AUC0-24, fu Cmax/MIC, fu AUC0-24/MIC, time above the MIC, and treatment duration. To correct for a highly skewed distribution, a log2 transform of MICs was used in all analyses. Discrete covariates with five or fewer patients per response category were excluded from the analysis.
Stepwise logistic regression was used to identify predictor variables with a statistically significant influence on the probability of a clinical cure and/or a bacteriologic response. The regressions were done with a stepwise procedure with criteria of α < 0.10 for the addition of a covariate and α < 0.05 for a covariate to remain in the model.
RESULTS
PK data.
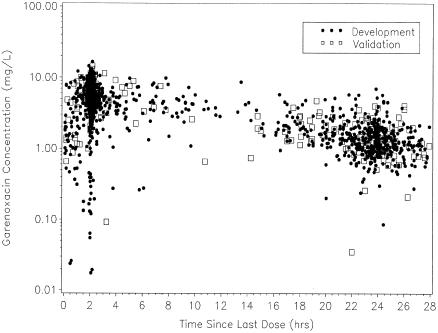
The final database consisted of 721 patients (1,908 plasma garenoxacin concentrations) with complete dosing histories and available covariate information. Patients were randomly selected by study for inclusion in the model validation data set (141 patients and 379 samples), while the remaining patients were assigned to the model development data set (580 patients and 1,529 samples). The two data sets were similar with respect to patient characteristics (Table 2) and garenoxacin concentrations and sampling times (Fig. 1).
TABLE 2.
Patient characteristics by data set
| Variable | Development (n = 580) | Validation (n = 141) |
|---|---|---|
| Age (yr), mean (SD), range | 49.5 (17), 18-88 | 49.6 (17), 18-88 |
| Ht (cm), mean (SD), range | 169 (9.9), 125-198 | 170 (10.4), 139-193 |
| WTKG (kg), mean (SD), range | 79.3 (21.3), 34-178 | 79.6 (22.7), 38-181 |
| IBW (kg), mean (SD), range | 64.2 (11.8), 20.9-96.9 | 64.6 (12.4), 33.4-91.6 |
| % of IBW, mean (SD), range | 125 (33.2), 67.5-298 | 126 (39.4), 66.5-N340 |
| CrCL (ml/min), mean (SD), range | 86.9 (29.5), 14.5-205 | 89.3 (29.8), 30.8-180 |
| Gender, n (%) | ||
| Male | 288 (50) | 73 (52) |
| Female | 292 (50) | 68 (48) |
| Ethnicity, n (%) | ||
| Black | 32 (6) | 10 (7) |
| Caucasian | 492 (85) | 117 (83) |
| Hispanic | 56 (10) | 14 (10) |
| CHFa status, n (%) | ||
| Absent | 571 (98) | 134 (95) |
| Present | 9 (2) | 7 (5) |
| Obesity, n (%) | ||
| Obese | 196 (34) | 45 (32) |
| Nonobese | 384 (66) | 96 (68) |
CHF, congestive heart failure.
FIG. 1.
Observed garenoxacin concentrations versus the time since administration of the last dose for the model development and validation data sets (development n = 1,529; validation n = 379).
Basic structural model.
A one-compartment model with first-order absorption and elimination was fitted to the model development data set. Interindividual variability of ka, CL/F, and V/F was described with an exponential error model. A proportional error model was used to describe the residual variability. The resulting fit demonstrated that the PK parameters ka, CL/F, and V/F were estimated with good precision; however, the model was not able to adequately estimate the interindividual variability. Given that the protocol-specified sampling sequence provided limited data during the absorption phase, the interindividual variability of ka was removed from the model. The resulting parameters for this model were estimated with acceptable accuracy and precision (Table 3), and the goodness-of-fit plots indicated a reasonably unbiased fit of the data.
TABLE 3.
Parameter estimates and percent standard error of the mean for the base model
| Parameter | Final estimate of population mean | % SEM | Final estimate of magnitude of interindividual variability (%CV) | % SEM |
|---|---|---|---|---|
| ka (l/hr) | 3.15 | 34.3 | ||
| CL/F (ml/min) | 81.5 | 1.9 | 33.9 | 13.6 |
| V/F (liters) | 71.8 | 1.8 | 29.9 | 15.9 |
| Residual variability (%CV) | 27.6 | 11.6 |
Covariate analyses.
During forward selection, the covariates IBW (P < 0.00001), CrCL (P < 0.00001), obesity (P < 0.00001), age (P < 0.00003), gender (P < 0.0399), and TBIL levels of ≥1.1 mg/dl (P < 0.01055) and an interaction term between CrCL and TBIL were found to be significant predictors of clearance. The covariates WTKG (P < 0.00001), gender (P < 0.00001), and race (P < 0.00684) were found to be significant predictors of volume.
On the basis of examination of the diagnostic plots, there were no biases in the interindividual or residual error models. The plots also indicated that the interindividual variability terms for clearance and volume were not correlated.
During backward elimination, the effects of gender (P > 0.04139), the interaction term for CrCL and TBIL (P > 0.02469), and the effect of TBIL (P > 0.04866) on clearance, as well as the effect of race (P > 0.00684) on volume, were removed from the model in a stepwise fashion in the order presented.
Evaluation of food effect.
The population mean estimate of relative drug bioavailability was 98.2% for doses administered in the fed dosing state. This finding indicated that the presence of food did not statistically significantly alter (P = 0.8516) bioavailability.
Analysis of concomitant medications.
During the forward selection analysis of the effect of concomitant medications on garenoxacin clearance, pseudoephedrine and guaifenesin, as well as the P-glycoprotein inhibitor and CYP2D6 inhibitor drug classes, were statistically significant (α = 0.05). As a result of backward elimination, the effects of guaifenesin (P > 0.01064) and the CYP2D6 inhibitor (P > 0.01325) and P-glycoprotein inhibitor (P > 0.00894) drug classes on garenoxacin clearance were removed from the covariate model in the order listed, leaving only pseudoephedrine in the model.
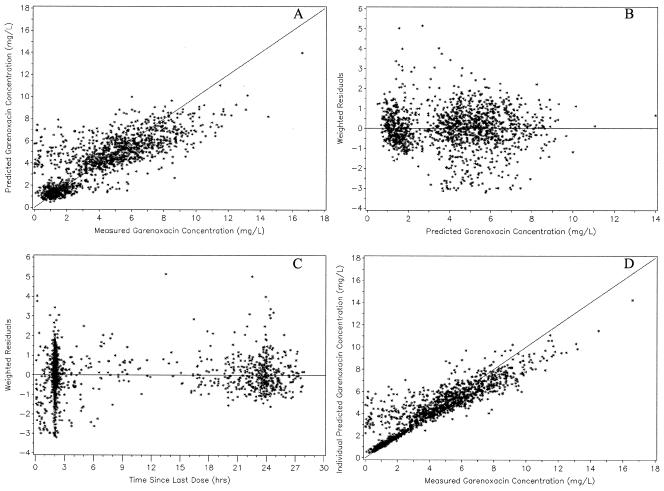
Final model.
The final population PK model for garenoxacin is shown in Table 4, and the goodness-of-fit plots for the final model are shown in Fig. 2. The final model indicated that garenoxacin clearance was influenced by CrCL, IBW, age, obesity, and concomitant use of pseudoephedrine and that the volume of distribution of garenoxacin was influenced by WTKG and gender. The equations used for computing the population mean CL/F and volume of distribution of garenoxacin are CL̃jk (milliliters per minute) = [83.4 · (CRCLj/86.9)0.436 + 0.764 · (IBWj − 64.2) + 10.9 · OBESEj + 0.301 · (AGEj − 49.5)] · (1 − 0.144 · PSEUjk) and Ṽj (liters) = 67.1 · (WTKGj/79.3)0.635 + 17.7 · SEXMj, where CL̃jk is the typical clearance value of the jth patient at the kth time, CRCLj is the CrCL (milliliters per minute) of the jth patient, IBWj is the IBW (kilograms) of the jth patient, OBESEj is 1 if the jth patient had a percentage of the IBW of >130% and 0 otherwise, AGEj is the age (years) of the jth patient, PSEUjk is 1 if the kth sample was collected from the jth patient in the presence of concomitantly administered pseudoephedrine and 0 otherwise, Ṽj is the typical volume value for the jth patient, WTKGj is the WTKG (kilograms) of the jth patient, and SEXMj is 1 if the jth patient is male and 0 if the jth patient is female.
TABLE 4.
Parameter estimates and percent standard error of the mean for the final model
| Parameter | Final estimate of population mean | % SEM | Final estimate of magnitude of interindividual variability (%CV) | % SEM |
|---|---|---|---|---|
| ka (l/h) | 2.41 | 27.3 | ||
| CL/Fa coefficient (ml/min) | 83.4 | 1.9 | 25.5 | 15.0 |
| V/Fb coefficient (liters) | 67.1 | 1.9 | 18.8 | 31.0 |
| WTKG-V power | 0.635 | 8.8 | ||
| IBW-CL slope | 0.764 | 15.8 | ||
| CRCL-CL power | 0.436 | 13.0 | ||
| Additive shift in V for males | 17.7 | 10.1 | ||
| Additive shift in CL for obesity | 10.9 | 25.3 | ||
| Age-CL slope | 0.301 | 33.9 | ||
| Proportional shift in CL for concomitant pseudoephedrine use | −0.144 | 37.4 | ||
| Residual variability (%CV) | 27.6 | 11.4 |
The typical value of the apparent CL/F for nonobese patients at the median values of age (49.5 years), CrCL, (86.9 ml/min), and IBW (64.2 kg).
The typical value of the apparent volume of distribution for female patients at the median WTKG (79.3 kg).
FIG. 2.
Predicted garenoxacin concentrations versus observed garenoxacin concentrations (A), weighted residuals versus predicted garenoxacin concentrations (B), weighted residuals versus the time since administration of the last dose (C), and individual predicted garenoxacin concentrations versus observed garenoxacin concentrations (D) for the final model (n = 1,529).
In Fig. 1, there were many concentrations that were low (<2 mg/liter) relative to most of the data collected less than 6 h postdose. These concentrations were overpredicted by the final model, indicating that patient covariates did not explain this variability. Therefore, the unusual low concentrations may have been the result of protocol adherence issues. The model also exhibited a small degree of underprediction for concentrations greater than 8 mg/liter.
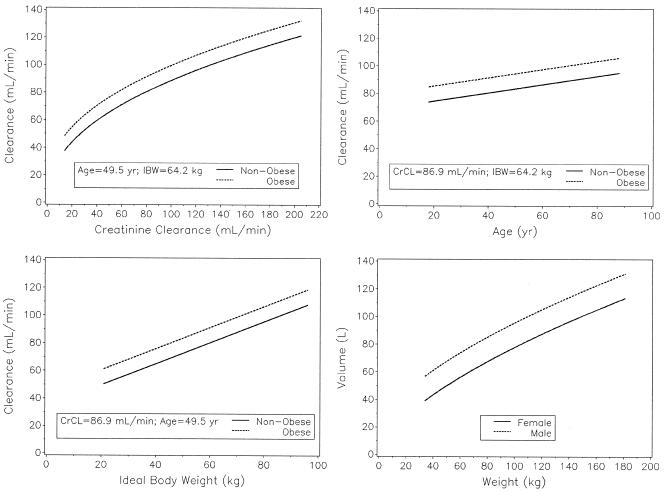
An extrapolation of the equations is shown graphically in Fig. 3, varying one covariate over the studied range while all of the other covariates are held at the median values. As the plot indicates, age contributes very little change while the mean clearance changes by 84 and 57 ml/min over the broad range of CrCLs and IBWs studied, respectively. For the population studied, the individual CL predictions had a mean (SD) of 84 (22) ml/min and a range of 31 to 158 ml/min. This corresponds to a mean (SD) AUC0-τ of 85 (25) mg · h/liter with a range of 42 to 215 mg · h/liter.
FIG. 3.
Population mean parameter values estimated over the range of each significant covariate studied.
Model validation.
The mean and median PE% for the development data set were −1.59 and −1.23%, respectively (Ta-ble 5). While there was a slight underprediction bias, the PE% distribution was very symmetrical, with the exception of a few samples with a predicted concentration much less than the observed value (PE%, <−110%). The distribution also showed that the prediction error was within ±30% for most of the samples. The mean and median |PE%| for the development data set were 29.4 and 21.1%, respectively. Evaluation of the prediction errors indicated that the size of the prediction error is slightly larger for samples collected more than 20 h after administration of the last dose.
The mean and median PE% for the model validation data set were −8.9 and −3.8%, respectively. This indicates that the final model resulted in a slightly larger underprediction bias when extrapolated to a new population. However, this bias is still relatively small and more than 50% of the samples had a PE% within ±30%. The mean and median |PE%| for the model validation data set were 32.5 and 22.1%, respectively. The full distribution showed a high degree of skewness, indicating that the median is more reflective of the data. These results indicate that the accuracy of the model remained reasonable and very similar to the accuracy for the development data set.
PD analyses. (i) Safety analysis of AEs.
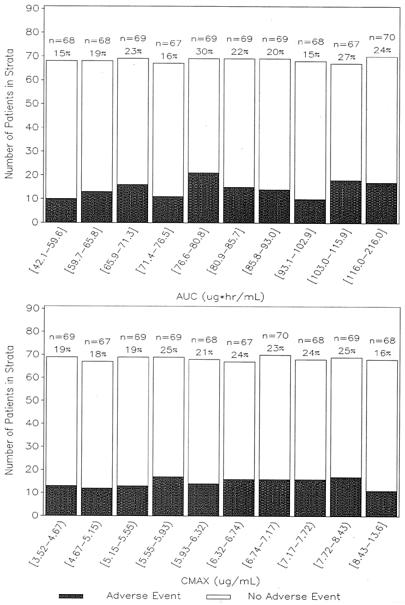
The population of patients used in the AE analysis consisted of 684 patients with both AE and PK data available. Of the 684 patients, 337 were male and 347 were female; their mean (SD) age was 49.3 (17.0) years, their mean (SD) WTKG was 79.3 (21.6) kg, and their mean (SD) CrCL was 87.7 (29.6) ml/min. Approximately 84% of the patients were Caucasian, 6% were black, and 10% were Hispanic. Furthermore, approximately 38% of the patients were treated for ABECB, 27% were treated for CAP, and 35% were treated for ABS. The overall incidence of drug-related AEs was 21%. The range of AUC0-24 values observed was 42.1 to 216.0 μg · h/ml and the range of Cmax values was 3.5 to 13.6 μg/ml.
Histograms of the frequency of drug-related AEs versus AUC and Cmax (shown in Fig. 4) did not indicate any relationships. Stepwise logistic regression showed neither AUC0-24 nor Cmax to be a statistically significant predictor of the occurrence of a drug-related AE within the range of exposures studied. Patients with CAP were more likely to experience a drug-related AE (probability, 0.29) than were patients without CAP (P = 0.0044, odds ratio = 1.77, probability, 0.18).
FIG. 4.
Histogram of drug-related AE occurrence versus garenoxacin AUC (top) and Cmax (bottom). The symbol [ indicates equal to, and the symbol) indicates less than. The values above the bars are the number of patients in the range specified and the percentage of patients with a drug-related AE.
The digestive system was the only body system for which at least 10% of the patients experienced a drug-related AE. The logistic regression showed neither AUC0-24 nor Cmax to be a statistically significant predictor of a drug-related digestive system AE within the range of exposures studied.
Diarrhea and nausea were the only drug-related AEs that occurred in at least 3% of the patients. The logistic regression analyses, for both diarrhea and nausea, found no significant predictors. This was consistent with, and further supported, the finding that the occurrences of AEs were not related to garenoxacin exposure within the range studied.
(ii) Antimicrobial activity analysis.
MIC and PK data were available for 96 patients who presented with an infection with S. pneumoniae and were eligible for clinical evaluation. There were 52 males and 44 females with a mean (SD) age of 47.9 (17.5) years, a mean (SD) WTKG of 74.4 (16.7) kg, and a mean (SD) CrCL of 87.3 (31.6) ml/min. Approximately 78% of the patients were Caucasian, 9% were black, 12% were Hispanic, and 1% were classified as other. Furthermore, approximately 38% of the patients were treated for ABECB, 21% were treated for CAP, and 41% were treated for ABS. Ninety-one of these patients were evaluable for the bacteriologic response analysis of this pathogen.
Two hundred sixteen patients who presented with infections associated with H. influenzae, H. parainfluenzae, or M. catarrhalis had MIC and PK data and were eligible for clinical response evaluation. Of these 216 patients, 115 were male and 101 were female; their mean (SD) age was 51.4 (17.2) years, their mean (SD) WTKG was 78.2 (22.2) kg, and their mean (SD) CrCL was 87.8 (30.2) ml/min. Approximately 89% were Caucasian, 6% were black, and 5% were Hispanic. Also, approximately 48% were treated for ABECB, 30% were treated for CAP, and 22% were treated for ABS. Two-hundred seven of these patients were evaluable for the bacteriologic response analysis of these pathogens.
The clinical and bacteriologic response rates of patients with S. pneumoniae infections were 91 and 90%, respectively, and 99% of the patients achieved fu AUC0-24/MIC ratios of >200 (Table 6). A similar pattern of fu AUC0-24/MIC ratios was exhibited in the H. influenzae, H. parainfluenzae, or M. catarrhalis pathogen group. Values for fu AUC0-24/MIC ratios were also high in these patients, with approximately 91% of the patients achieving an fu AUC0-24/MIC ratio in excess of 200 for both the clinical and bacteriologic response analysis populations. The clinical cure and bacteriologic eradication rates for this group of pathogens were 93 and 92%, respectively.
TABLE 6.
Distribution of fu AUC0-24/MIC ratios stratified by clinical and bacteriologic responses
| fu AUC0-24/MIC ratio | Clinical response
|
Bacteriologic response
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Cure | Failure | % Cure | No. of patients | Eradicated | Persisted | % Eradicated | |
| S. pneumoniae | ||||||||
| 0-200 | 1 | 1 | 0 | 100 | 1 | 1 | 0 | 100 |
| 201-400 | 36 | 35 | 1 | 97.2 | 35 | 33 | 2 | 94.3 |
| 401-600 | 21 | 19 | 2 | 90.5 | 20 | 18 | 2 | 90.0 |
| 601-800 | 21 | 18 | 3 | 85.7 | 19 | 17 | 2 | 89.5 |
| 801-1,000 | 11 | 10 | 1 | 90.9 | 11 | 10 | 1 | 90.9 |
| >1,000 | 6 | 4 | 2 | 66.7 | 5 | 3 | 2 | 60.0 |
| Total | 96 | 87 | 9 | 90.6 | 91 | 82 | 9 | 90.1 |
| H. influenzae, H. parainfluenzae, M. catarrhalis | ||||||||
| 0-200 | 20 | 19 | 1 | 95.0 | 16 | 13 | 3 | 81.2 |
| 201-400 | 26 | 25 | 1 | 96.2 | 26 | 24 | 2 | 92.3 |
| 401-600 | 22 | 21 | 1 | 95.5 | 20 | 20 | 0 | 100 |
| 601-800 | 23 | 22 | 1 | 95.7 | 23 | 21 | 2 | 91.3 |
| 801-1,000 | 13 | 11 | 2 | 84.6 | 13 | 12 | 1 | 92.3 |
| >1,000 | 112 | 103 | 9 | 92.0 | 109 | 100 | 9 | 91.7 |
| Total | 216 | 201 | 15 | 93.1 | 207 | 190 | 17 | 91.8 |
For the clinical and bacteriologic response analysis of the S. pneumoniae patient population, the logistic regression indicated that none of the patient factors, disease covariates, or exposure measures were significant predictors of failure. The logistic regression for the H. influenzae, H. parainfluenzae, or M. catarrhalis patient population showed fu AUC0-24 to be a significant predictor of clinical failure and bacteriologic persistence (P = 0.0309 and P = 0.0077, respectively). Although it is counterintuitive, for these models, the odds ratios of 1.07 and 1.082, respectively, indicate that for every unit increase in fu AUC0-24 there was a 7% greater chance of clinical failure and an 8.2% greater chance of bacteriologic persistence. Other selected covariates tested in the clinical and bacteriologic response analyses, along with their associated P values, are shown in Tables 7 and 8.
TABLE 7.
Selected covariate results from univariate logistic regression analysis of clinical cure
| Covariate |
P value
|
|
|---|---|---|
| S. pneumoniae (n = 96) | H. influenzae, H. parainfluenzae, M. catarrhalis (n = 216) | |
| Patient factors | ||
| Age (yr) | 0.4193 | 0.9316 |
| Gender | 0.5409 | 0.5877 |
| CrCL (ml/min) | 0.3881 | 0.7028 |
| WTKG | 0.6313 | 0.6365 |
| Disease covariates | ||
| Condition treated | 0.6412 | 0.3467 |
| MIC | —a | 0.8954 |
| Exposure measurements | ||
| fu AUC0-24 | 0.8531 | 0.0309 |
| fu Cmax | 0.9246 | 0.1465 |
| fu AUC0-24/MIC ratio | 0.0510 | 0.8350 |
| fu Cmax/MIC ratio | 0.0530 | 0.7605 |
—, MIC was excluded from the clinical cure analysis of the S. pneumoniae patient population since for 94% of the patients the value was either 0.03 or 0.06 μg/ml.
TABLE 8.
Selected covariate results from univariate logistic regression analysis of bacteriologic eradication
| Covariate |
P value
|
|
|---|---|---|
| S. pneumoniae (n = 91) | H. influenzae, H. parainfluenzae, M. catarrhalis (n = 207) | |
| Patient factors | ||
| Age (yr) | 0.5145 | 0.7102 |
| Gender | 0.6007 | 0.5678 |
| CrCL (ml/min) | 0.3667 | 0.3578 |
| WTKG | 0.8430 | 0.5747 |
| Disease covariates | ||
| Condition treated | 0.5536 | 0.7855 |
| MIC | —a | 0.1922 |
| Exposure measures | ||
| fu AUC0-24 | 0.7994 | 0.0077 |
| fu Cmax | 0.8258 | 0.0749 |
| fu AUC0-24/MIC ratio | 0.1557 | 0.9439 |
| fu Cmax/MIC ratio | 0.2560 | 0.8366 |
—, MIC was excluded from the bacteriologic eradication analysis of the S. pneumoniae patient population since for 95% of the patients the value was either 0.03 or 0.06 μg/ml.
DISCUSSION
PKS.
The base model population mean estimates of clearance and volume of distribution for this patient population were approximately 23 and 35% lower than the noncompartmental parameter values previously reported following administration of a single 400-mg dose to healthy volunteers under fasted conditions (CL/F = 93 ml/min; V/F = 112 liters) (14). When making this comparison, it is important to note that the demographic characteristics of the healthy volunteers were quite different from those of the patients in the phase II clinical trial program. Therefore, the differences in clearance and volume observed may be attributed to the differences in the characteristics of the populations studied. For the final model, the mean of the typical value estimates of clearance and volume for nonobese males less than 35 years of age with normal renal function were more similar (99 ml/min and 84.1 liters, respectively).
The analyses of patient covariates showed that IBW, CrCL, obesity, and age were associated with statistically significant effects on clearance; however, gender and race were not predictors of clearance. These analyses also showed that WTKG and gender were statistically significant covariates of volume. The addition of IBW, CrCL, obesity, age, and pseudoephedrine resulted in decreases of 2.8, 3.6, 1.2, 0.6, and 0.3%, respectively. Collectively, these factors reduced the interindividual variability of clearance to 26% (relative to 34% prior to covariates). These findings show that the variability in garenoxacin clearance was primarily explained by CrCL and IBW.
In the absence of urinary excretion data, independent estimates of renal clearance and nonrenal clearance are not possible. Therefore, in these analyses, CrCL served as a predictor of total clearance of garenoxacin and the magnitude of its predictive nature is not limited to renal clearance. The significance of age and IBW as predictors of total garenoxacin clearance differed from their contribution to the estimation of CrCL. The addition of age to the model helps offset the underprediction of total clearance that could occur on the basis of CrCL alone. This underprediction of total clearance is most likely a reflection of the potential for a slower decline in liver function relative to renal function during the aging process (29). Hence, as age increases, the percentage of total clearance attributed to renal clearance is lower, resulting in the model requiring another covariate to offset this phenomenon. The addition of IBW to the model likely adjusts for the increase in total clearance (e.g., larger liver mass) associated with a higher IBW. It has been shown that the modified method of Cockcroft and Gault underestimates CrCL for obese patients (9). Therefore, the increase in clearance for obese patients may result from underestimation of their CrCL.
The model was further explored to examine the influence of the significant patient covariates on the PKs of garenoxacin. Patients were first classified into groups on the basis of their CrCL estimates. The median covariates of CrCL, WTKG, IBW, and age for the three groups, subset by gender and obesity, were then used to estimate the typical AUC and Cmax as shown in Table 9. The typical clearance value for females is approximately 18 ml/min slower than that for males in the same renal function and obesity group. Comparison of these estimates further shows that patients with mild and moderate renal dysfunction have approximately 14- and 25-ml/min decreases in clearance, respectively, compared to patients of the same gender and obesity classification with normal renal function. Changes in garenoxacin exposure for patients with renal dysfunction do not appear to be clinically significant given that increased garenoxacin exposure and renal function were not found to be significant predictors of drug-related AEs in this population.
TABLE 9.
Estimated population mean PK parameters at selected covariate values
| Renal function categoryb | Gender | Obesity | n | Covariate Valuea
|
PK parameter value
|
||||
|---|---|---|---|---|---|---|---|---|---|
| CrCL (ml/min) | IBW (kg) | Age (yr) | WTKG (kg) | AUCc (μg · h/ml) | Cmax (mg/liter) | ||||
| Moderate (30-50 ml/min) | Male | Nonobese | 22 | 44 | 71 | 77 | 73 | 88.3 | 6.12 |
| Obese | 5 | 45 | 66 | 75 | 92 | 80.8 | 5.52 | ||
| Female | Nonobese | 15 | 43 | 50 | 74 | 54 | 115 | 8.69 | |
| Obese | 17 | 45 | 45 | 67 | 73 | 104 | 7.49 | ||
| Mild (51-80 ml/min) | Male | Nonobese | 67 | 67 | 69 | 67 | 75 | 79.9 | 5.77 |
| Obese | 19 | 65 | 67 | 60 | 100 | 74.3 | 5.18 | ||
| Female | Nonobese | 61 | 71 | 52 | 49 | 60 | 99.7 | 7.83 | |
| Obese | 52 | 68 | 52 | 55 | 81 | 85.3 | 6.58 | ||
| Normal (>80 ml/min) | Male | Nonobese | 133 | 107 | 77 | 41 | 80 | 67.7 | 5.24 |
| Obese | 41 | 105 | 76 | 46 | 116 | 60.9 | 4.51 | ||
| Female | Nonobese | 84 | 96 | 58 | 35 | 61 | 85.5 | 7.29 | |
| Obese | 61 | 99 | 59 | 42 | 99 | 71.7 | 5.67 | ||
The covariate values represent the median patient covariates for each renal function category, subset by gender and obesity, rounded to the nearest integer.
There was an insufficient number of patients (n = 3) with severe renal function (15 to 29 ml/min) to perform this comparison.
CL (milliliters per minute) = (400 mg·103)/AUC (micrograms per hour per milliliter)·60.
The only statistically significant drug interaction was a 14.4% decrease in clearance associated with the concomitant use of pseudoephedrine (P < 0.00001). On the basis of the small magnitude of this effect, it is unlikely that the resulting increase in exposure to garenoxacin due to the concomitant use of pseudoephedrine would represent a safety or efficacy concern. The mechanism of this PK interaction has not been clearly elucidated, and no significant interaction between pseudoephedrine and any currently marketed fluoroquinolones has been reported to date (33). The renal clearance of pseudoephedrine has been reported to be approximately 511 to 532 ml/min for a 70-kg adult (2), indicating that the drug is renally excreted by active tubular secretion in addition to glomerular filtration (estimated to be 120 ml/min for a healthy adult). Since quinolones such as levofloxacin, ciprofloxacin, gatifloxacin, gemifloxacin, and garenoxacin (Bristol-Myers Squibb, Princeton, N.J.; data on file) are also renally excreted by a combination of glomerular filtration and active tubular secretion (2), pseudoephedrine may compete for the active secretion of these drugs.
Model validation indicated that the model exhibited a slight underprediction bias (approximately 4%). However, the model was fairly accurate, with 50% of the measured garenoxacin concentrations within 22% of the predicted value. Additionally, the magnitude of the prediction error was not correlated with any of the covariates studied, indicating that the model should accurately predict differences in the PKs of the various patient subpopulations identified.
PDS.
Consideration of exposure-response relationships for drug safety is an issue of increasing interest in clinical drug development (19). In the present analysis, there was no statistically significant relationship between the incidence of a drug-related AE and garenoxacin exposure measured as Cmax or AUC. The overall AE rate was 21%, a finding consistent with results reported from a retrospective review of 23 clinical trials in the U.S. ciprofloxacin database that indicated that drug-related AEs were reported in 24% of ciprofloxacin-treated patients aged <65 years and in 17% of patients aged >65 years (16). In this analysis, at least 3% of the patients experienced diarrhea and nausea, which is similar to the incidence reported for moxifloxacin (4).
For quinolone antibacterial agents, the relationship between drug exposure and antimicrobial effects has been well elucidated. The fu AUC0-24/MIC ratio is the PD measurement that generally has the strongest correlation with outcome in nonclinical models of infection (animal and in vitro) and in infected patients (10, 11, 18, 27) for these agents.
The PD goal of therapy differs for gram-positive and gram-negative microorganisms. Existing clinical and nonclinical data for quinolone antimicrobials suggest that an fu AUC0-24/MIC ratio of >90 to 125 correlates with optimal microbiological response to therapy in patients infected with gram-negative enteric pathogens and Pseudomonas aeruginosa (7, 10, 11), whereas in patients infected with gram-positive microorganisms, such as S. pneumoniae, an fu AUC0-24/MIC ratio of >25 to 30 correlates with an optimal microbiological response to therapy (1, 18, 21, 22).
In previous analyses, Lister demonstrated in an in vitro model of pneumococcal infection that, for garenoxacin, fu AUC0-24/MIC ratios of ≥29 were associated with total eradication of S. pneumoniae from the model regardless of the garenoxacin MIC. Lower fuAUC0-24/MIC ratios failed to eliminate S. pneumoniae from the model and, in some instances, regrowth occurred and viable counts increased to that of drug-free controls (20). Moreover, Craig et al. conducted a study to identify both the PK-PD measurement predictive of efficacy and the magnitude of the measurement required for optimal in vivo activity in a neutropenic-mouse thigh infection model. The PK-PD measurement predictive of efficacy for garenoxacin was the fu AUC0-24/MIC ratio; the fu AUC0-24/MIC ratio required to achieve a 1-log or greater decrease in bacterial counts was 32.2 (Craig, personal communication). In an earlier analysis by our group evaluating the PK-PD of gatifloxacin and levofloxacin against S. pneumoniae in patients with community-acquired respiratory tract infections, patients with fu AUC0-24/MIC ratios of 33.7 or greater had a 1.0 probability of a microbiological response to therapy, whereas patients with lesser exposures had only a 0.64 probability of a positive response (1). Although the optimal PK-PD targets for H. influenzae, H. parainfluenzae, and M. catarrhalis have yet to be elucidated for quinolone antibiotics, an fu AUC0-24/MIC ratio of >30 is expected to be an appropriate goal. Studies of beta-lactams and macrolides have shown that the PK-PD target for H. influenzae is similar to that for pneumococci and much less than the target for gram-negative bacilli (8).
In the present analyses, the correlation between the fu AUC0-24/MIC ratio and the clinical or microbiologic response was not significant for any pathogen. However, all of the patients with an infection associated with pneumococci and 91% of the patients with the other pathogens analyzed had fu AUC0-24/MIC ratios of >30 and >200, respectively. This observation highlights the most important reason why the fu AUC0-24/MIC ratio was not predictive of the clinical or microbiological response in both pathogen group analyses. Specifically, the magnitude of the fu AUC0-24/MIC ratios observed in this patient population resulted in few failures, which in turn precluded the elucidation of an exposure-response relationship (i.e., the observed fu AUC0-24/MIC ratios were on the upper, flat portion of the exposure-response curve).
For the clinical response in the H. influenzae, H. parainfluenzae, or M. catarrhalis patient population, an increasing fu AUC0-24 was identified to be related to a statistically significant increase in the probability of clinical failure. A similar pattern was observed for fu AUC0-24 and the likelihood of bacteriologic persistence. These results may appear counterintuitive but are a consequence of the large number of subjects with relatively low fu AUC0-24 values and a low corresponding clinical failure rate. In contrast, there were fewer subjects with higher fu AUC0-24 values, causing the few failure cases in that fuAUC0-24 range to be highly influential in the logistic regression. When the patient with the highest fu AUC0-24 in the failure group is excluded from the analysis, the relationship is no longer statistically significant. In addition, when the MIC is included in the relationship and the fu AUC0-24/MIC ratio is examined as a possible predictor of clinical failure or bacteriological persistence, there is also no longer a statistical significance, thereby indicating that this was a spurious finding.
This PK-PD investigation illustrates the utility of population modeling as a means of evolving an effective dosage strategy during drug development. The population PK analysis, in conjunction with the AE analysis, indicates that while patients with renal impairment have an approximately 25% higher exposure level, a dosage adjustment should not be required. Overall, these PK-PD analyses support the 400-mg once-daily garenoxacin regimen as a safe and effective treatment of community-acquired respiratory tract infections.
TABLE 5.
Summary statistics for PE%a and |PE%|
| Data set and variable | Mean (SD) | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|
| Developmentb | ||||||
| PE% | −1.59 (40.8) | −272.85 | −22.4 | −1.23 | 20.41 | 99.66 |
| |PE%| | 29.39 (28.4) | 0.01 | 10.4 | 21.12 | 40.21 | 272.85 |
| Validationc | ||||||
| PE%a | −8.87 (45.5) | −229.95 | −28.6 | −3.76 | 17.77 | 97.73 |
| |PE%| | 32.45 (33) | 0.19 | 10.64 | 22.09 | 42.84 | 229.95 |
PE% = (population predicted concentration − observed concentration)· 100/population predicted concentration.
1,529 samples.
379 samples.
Acknowledgments
Financial support for this analysis was provided by Bristol-Myers Squibb Company, Princeton, N.J.
REFERENCES
- 1.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolone against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Society of Health-System Pharmacists, Inc. 2002. American hospital formulary service drug information, p. 1304. Bethesda, Md.
- 3.American Thoracic Society. 1993. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity and initial antimicrobial therapy. Am. Rev. Respir. Dis. 1481418-1426. [DOI] [PubMed] [Google Scholar]
- 4.Bayer Corporation. 2001. Avelox (moxifloxacin) product information. Bayer Corporation, West Haven, Conn.
- 5.Celli, B. R., G. L. Snider, J. Heffner, et al. 1995. American Thoracic Society statement—standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 152:S77-S120. [PubMed] [Google Scholar]
- 6.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W.A. 2002. Pharmacodynamics of antimicrobials: general concepts and applications, p. 10. In C. H. Nightingale, T. Murakawa, and P. G. Ambrose (ed.). Antimicrobial pharmacodynamics in theory and clinical practice. Marcel Dekker, Inc., New York, N.Y.
- 9.Dionne, R. E., L. A. Bauer, G. A. Gibson, W. O. Griffen, and R. A. Blouin. 1981. Estimating creatinine clearance in morbidly obese patients. Am. J. Hosp. Pharm. 38:841-844. [PubMed] [Google Scholar]
- 10.Drusano, G. L., D. E. Johnson, M. Rosen, and H. C. Stadiford. 1993. Pharmacodynamics of a fluoroquinolone antimicrobial in a neutropenic rat model of Pseudomonas sepsis. Antimicrob. Agents Chemother. 37:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung-Tomc, J. C., B. Minassian, B. Kolek, E. Huczko, L. Aleksunes, T. Stickle, T. Washo, E. Gradelski, L. Valera, and D. P. Bonner. 2000. Antibacterial spectrum of a novel des-fluoro(6) quinolone, BMS-284756. Antimicrob. Agents Chemother. 44:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajjar, D. A., A. Bello, Z. Ge, L. Christopher, D. M. Grasela. 2003. Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects. Antimicrob. Agents Chemother. 47:2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajjar, D. A., S. C. Sukoneck, A. Bello, Z. Ge, L. Christopher, and D. M. Grasela. 2002. Effect of a high fat meal on the pharmacokinetics of the des-6(F) quinolone BMS-284756. Pharmacotherapy 22:160-165. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi, M., D. Perrier. 1982. Pharmacokinetics, 2nd edition. Marcel Dekker, Inc., New York, N.Y.
- 16.Heyd, A., and D. Haverstock. 2000. Retrospective analysis of the safety profile of oral and intravenous ciprofloxacin in a geriatric population. Clin. Ther. 22:1239-1250. [DOI] [PubMed] [Google Scholar]
- 17.Hoellman, D. B., L. M. Kelly, M. R. Jacobs, and P. C. Applebaum. 2001. Comparative antianaerobic activity of BMS-284756. Antimicrob. Agents Chemother. 45:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacy, M. A., W. Lu, X. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesko, L. J., M. Rowland, C. C. Peck, and T. F. Blaschke. 2000. Optimizing the science of drug development: opportunities for better candidate selection and accelerated evaluation in humans. Pharm. Res. 17:1335-1344. [DOI] [PubMed] [Google Scholar]
- 20.Lister, P. D. 2003. Impact of AUC0-24/MIC ratios on the pharmacodynamics of the des-F(6) quinolone garenoxacin (BMS-284756) is similar to other fluoroquinolones. J. Antimicrob. Chemother. 51:199-202. [DOI] [PubMed] [Google Scholar]
- 21.Lister, P. D. 2002. Pharmacodynamics of gatifloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model: impact of area under the curve/MIC ratios on eradication. Antimicrob. Agents Chemother. 46:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. 1999. J. Antimicrob. Chemother. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 23.Matzke, G. R., and S. P. Millikin. 1992. Influence of renal function and dialysis on drug disposition, p. 1-49. In W. J. Jusko, W. E. Evans, and J. J. Schentag (ed.), Applied pharmacokinetics: principles of therapeutic drug monitoring, 3rd ed. Applied Therapeutics, Inc., Vancouver, Wash.
- 24.Michalets, E. L. 1998. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 18:84-112. [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1998. Performance standards for antimicrobial susceptibility testing. Standard M100-S8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Nicolau, D. P., H. M. Mattoes, M. Banevicius, D. Xuan, C. H. Nightingale. 2003. Pharmacodynamics of a novel des-F(6)-quinolone, BMS-284756, against Streptococcus pneumoniae in the thigh infection model. Antimicrob. Agents Chemother. 47:1630-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noel, G. J., J. Natarajan, S. Chien, T. L. Hunt, D. B. Goodman, R. Abels. 2003. Effects of three fluoroquinolones on QT interval in healthy adults after single doses. Clin. Pharmacol. Ther. 73:292-303. [DOI] [PubMed] [Google Scholar]
- 28.NONMEM Project Group. 1994. Part 5, p. 138-139. In S. L. Beal and L. B. Sheiner (ed.), NONMEM user's guide. University of California at San Francisco.
- 29.Ozdemir, V., J. Fourie, U. Busto, and C. A. Naranjo. 1996. Pharmacokinetic changes in the elderly. Clin. Pharmacokinet. 31:372-385. [DOI] [PubMed] [Google Scholar]
- 30.Peck, C. C., and M. G. Murphy. 1989. Bedside estimation of ideal body weight, p. 80. In C. C. Peck and M. G. Murphy (ed.), Bedside clinical pharmacokinetics: simple techniques for individualizing drug therapy. Applied Therapeutics, Inc., Vancouver, Wash.
- 31.Piscitelli, S.C., and K. A. Struble. 2001. Drug interactions with antiretrovirals for HIV infection, p. 40. In S. C. Piscitelli and K. A. Rodvold (ed.), Drug interactions in infectious diseases. Humana Press, Totowa, N.J.
- 32.Sheiner, L. B., and S. L. Beal. 1989. Scientific commentary: some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 9:503-511. [DOI] [PubMed] [Google Scholar]
- 33.Stahlmann, R., and H. Lode. 2000. Safety overview: toxicity, adverse effects and drug interactions, p. 398-442. In V. Andriole (ed.), The quinolones, 3rd edition. Academic Press, San Diego, Calif.