Abstract
Oncostatin M (OSM) is a pleiotropic cytokine and a member of the IL-6 family. It has both proinflammatory and anti-inflammatory functions and is involved in the activation of STAT3 and STAT5. Rheumatoid arthritis is an autoimmune disease that causes chronic and excessive inflammation. Rheumatoid arthritis can lead to induction of Th17 cells, which express IL-17. The aim of this study was to measure the effects of OSM on the proliferation of regulatory T cells and Th17 cells from mice. IL-2 immune complex suppressed the development of collagen-induced arthritis in mice and altered the regulatory T/Th17 cell balance by increasing OSM expression. OSM mitigated the proliferation of Th17 cells and decreased the expression of IL-17 and IL-21. It promoted the activation of suppressor of cytokine signaling 3 (SOCS3), STAT3, and STAT5. Inhibition of SOCS3, STAT3, and STAT5 lessened the OSM-induced reduction in proliferation of Th17 cells. These observations suggest that OSM can inhibit Th17 differentiation by reciprocally controlling SOCS3, STAT3, and STAT5.
Introduction
The distinct cytokines produced by Th cells play significant roles in the immune response and development of autoimmune diseases. Th17 cells are involved in the pathogenesis of several autoimmune diseases such as rheumatoid arthritis (RA) by producing IL-17, which induces chronic inflammation and tissue damage in RA patients (1). Regulatory T (Treg) cells have immunosuppressive activity by reducing inflammation, and there is evidence that Tregs play a significant role in the immune system through this immunosuppressive effect (2). An imbalance between Th17 and Treg cells and upregulation of Th17 cells in the peripheral blood of RA patients have been reported, and an imbalance between these cells is now a significant target for RA therapy (3).
The proliferation of Th17 and Treg cells is regulated by specific transcription factors. It has been suggested that STAT3 is involved in Th17 cell differentiation and that the differentiation of Th17 to Treg cells is regulated by STAT5 (4). The regulation of STAT3 activation and Th17 cell differentiation are therapeutic targets for several types of autoimmune diseases, and alterations in STAT3 and STAT5 can alter the reciprocal balance between Th17 and Treg cells (5, 6).
Oncostatin M (OSM) is a member of the IL-6 family. Interestingly, the IL-6 family includes LIF, a known proinflammatory cytokine that acts as a pleiotropic cytokine and also plays an important role in inflammatory response. Although IL-6 is primarily considered a proinflammatory cytokine, some IL-6 family members also exhibit anti-inflammatory functions (7). OSM has both proinflammatory and anti-inflammatory activities (8), and is associated with the activation of transcription factors, including the activation of STAT3 (9). OSM production is mediated by activation of STAT5: STAT5 activation induces OSM expression and deletion of STAT5 decreases OSM expression (10).
In this study, we hypothesized that OSM is associated with the regulation of STAT3 and STAT5 activation involved in the proliferation of Th17 and Treg cells. The present investigation was conducted to identify whether OSM can regulate the balance between Th17 and Treg cells. First, we investigated whether OSM could regulate Th17 cells by measuring the expression of transcription factors in vitro. Second, we evaluated the role of OSM in suppressing the proliferation of Th17 cells and the conversion of Treg into Th17 cells. To understand how OSM downregulates Th17 differentiation, we analyzed the effect of OSM on the Th17/Treg cell balance regulated by the activation of STAT3 and STAT5 during inhibition of these transcription factors.
Materials and Methods
Animals
Male DBA1/J mice (SLC, Shizuoka, Japan) at 6–8 wk of age were maintained in groups of five in polycarbonate cages in a specific pathogen-free environment. They were fed standard mouse chow (Ralston Purina, Gray Summit, MO) and water ad libitum. All experimental procedures were examined and approved by the Animal Research Ethics Committee at the Catholic University of Korea.
Immunization with collagen type II and injection of IL-2 immune complex
Collagen-induced arthritis (CIA) was induced in DBA1/J mice (n = 10 per group). Mice were immunized through the base of the tail with 100 μg of bovine collagen type II (CII) (Chondrex, Redmond, WA) in CFA or IFA (Chondrex). To study the effects of the IL-2 immune complex (IL-2IC) (IL-2/JES6-1 complexes) on CIA, IL-2IC (1.5 μg/7.5 μg; eBioscience, San Diego, CA) or saline as a control were injected i.p. three times at 2-d intervals before the first immunization.
Clinical scoring of arthritis
Mice were examined visually twice a week for the appearance of arthritis in the peripheral joints. The index of arthritis was graded using the method of Williams et al. (11) with the following five grades: grade 0: no evidence of erythema or swelling; grade 1: erythema and mild swelling confined to the midfoot (tarsals) or ankle joint; grade 2: erythema and mild swelling extending from the ankle to the midfoot; grade 3: erythema and moderate swelling extending from the ankle to the metatarsal joints; and grade 4: erythema and severe swelling encompassing the ankle, foot, and digits. The final value represented the average index from all four legs recorded by two independent observers.
CD4+ T cell isolation and differentiation into Th17 or Treg cells
To purify CD4+ T cells, splenocytes were incubated with CD4-coated magnetic beads and isolated on MACS columns for cell separation (Miltenyi Biotec, San Diego, CA). CD4+ T cells were stimulated with plate-bound anti-CD3 mAb at 0.5 μg/ml and anti-CD28 mAb at 1 μg/ml (BD Pharmingen, CA) for 3 d under either a Th17 cell-polarizing condition (anti-IFN-γ at 2 μg/ml, anti-IL-4 at 2 μg/ml, anti-IL-2 at 2 μg/ml, TGF-β at 2 ng/ml, and IL-6 at 20 ng/ml) or Treg cell-polarizing condition (anti-IFN-γ at 2 μg/ml, anti-IL-4 at 2 μg/ml, and TGF-β at 5 ng/ml) in the presence or absence of recombinant mouse OSM at 10 ng/ml (all from R&D Systems, Minneapolis, MN).
Treg conversion into Th17 cells
The culture medium was washed from the cultures of differentiated Treg cells. These cells were restimulated under a Th17 cell-polarizing condition (plate-bound anti-CD3 mAb at 0.5 μg/ml, anti-CD28 mAb at 1 μg/ml, anti-IFN-γ at 2 μg/ml, anti-IL-4 at 2 μg/ml, TGF-β at 2 ng/ml, and IL-6 at 20 ng/ml) in the presence or absence of recombinant OSM at 10 ng/ml for 3 d.
Suppressor of cytokine signaling 3 small interfering RNA transfection
Small interfering RNA (siRNA) constructs for suppressor of cytokine signaling 3 (SOCS3) siRNA and nontargeting siRNA (Dharmacon) were obtained using siGENOME SMARTpool reagents (Dharmacon, Lafayette, CO). Transfection of siRNA was performed using an Amaxa 4D-nucleofector X unit according to the manufacturer’s recommendations with program DN-100 (Lonza, Cologne, Germany).
ELISA
The concentrations of IL-17 and IL-21 in culture supernatants were measured by sandwich ELISA. Anti-mouse IL-17 mAb and anti-mouse IL-21 mAb (R&D Systems) were added to 96-well plates (Nunc, Roskilde, Denmark) and the plates were incubated at 4°C overnight. Wells were treated with blocking solution (PBS containing 1% bovine BSA and 0.05% Tween 20). Samples and the standard recombinant IL-17 and IL-21 (R&D Systems) were added to the 96-well plates and the plates were incubated with biotinylated IL-17 and IL-21 polyclonal Ab (R&D Systems). After the incubation, the plates were washed, and a ExtrAvidin–alkaline phosphate (Sigma Aldrich, St. Louis, MO) diluted 2000-fold was added. The reaction was allowed to proceed. After washing the plates, p-nitrophenyl phosphate disodium salt (Pierce Chemical Company, Rockford, IL) diluted in diethanolamine buffer was added to each well, and the absorbance was measured at 405 nm on an ELISA microplate reader (Molecular Devices, Sunnyvale, CA).
Intracellular staining for flow cytometry
Before cell staining, differentiated CD4+ T cells were stimulated with PMA at 25 ng/ml and ionomycin at 250 ng/ml (both from Sigma) in the presence of GolgiStop (BD Pharmingen) for 4 h. To analyze intracellular cytokines, cells were stained with anti-mouse CD4–PerCP, anti-mouse CD25–allophycocyanin, anti-mouse IL-17–FITC, and anti-mouse Foxp3–PE (eBiosciences) followed by fixation and permeabilization with a Foxp3 staining buffer kit according to the manufacturer’s instructions. All data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Confocal microscopy
For confocal staining, spleen tissues or CD4 T cells were stained with anti-mouse CD4–PE, anti-mouse Foxp3–FITC, anti-mouse CD25–allophycocyanin, anti-mouse IL-17–FITC or –PE, and anti-mouse IL-21–FITC (all from eBiosciences). Stained sections were analyzed using a confocal microscopy system (LSM 510 Meta; Carl Zeiss, Thornwood, NY).
Real-time quantitative PCR
The mRNA expression levels were estimated using a LightCycler 2.0 instrument (Roche Diagnostic, Mannheim, Germany) with version 4.0 software. All reactions were performed with LightCycler FastStart DNA Master SYBR Green I (Takara, Shiga, Japan) following the manufacturer’s instructions. Relative mRNA levels were normalized to that of β-actin. The following primers were used: STAT3, 5′-CCG TCT GGA AAA CTG GAT AAC TTC-3′ (sense) and 5′-CCT TGT AGG ACA CTT TCT GCT GC-3′ (antisense), STAT5a, 5′-GTG AAG CGC TCA ACA TGA AA-3′ (sense) and 5′-ACT GGG ACC AGG ACA CAG AC-3′ (antisense), STAT5b, 5′-TGT GGA TAC AGG CTC AGC AG-3′ (sense) and 5′-TGG GTG GCC TTA ATG TTC TC-3′ (antisense), SOCS3, 5′-CCT TTG ACA AGC GGA CTC TC-3′ (sense) and 5′-GCC AGC ATA AAA ACC CTT CA-3′ (antisense), IL-17A, 5′-CCT CAA AGC TCA GCG TGT CC-3′ (sense) and 5′-GAG CTC ACT TTT GCG CCA AG-3′ (antisense), RAR-related orphan receptor γ (RORγT), 5′-TGT CCT GGG CTA CCC TAC TG-3′ (sense), 5′-GTG CAG GAG TAG GCC ACA TT-3′ (antisense), IL-21, 5′-AAG ATT CCT GAG GAT CCG AGA AG-3′ (sense) and 5′-GCA TTC GTG AGC GTC TAT AGT GTC-3′ (antisense), Foxp3 5′-GGC CCT TCT CCA GGA CAG A-3′ (sense) and 5′-GCT GAT CAT GGC TGG GTT GT-3′ (antisense), and β-actin, 5′-GTA CGA CCA GAG GCA TAC AGG-3′ (sense) and 5′-GAT GAC GAT ATC GCT GCG CTG-3′ (antisense).
Western blot analysis
Isolated CD4+ T cells from wild-type spleen tissues were cultured with OSM at 1 or 10 ng/ml and anti-mouse CD3 at 0.5 μg/ml for 1 or 24 h. Protein samples were separated by SDS gel electrophoresis and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). The membranes were incubated with primary Abs against phosphorylated (p)-STAT3 Y705, STAT3, p-STAT5, STAT5, and SOCS3 (all from Cell Signaling, Danvers, MA), and β-actin (Sigma). The membranes were washed to remove nonspecific binding and then incubated with HRP-conjugated secondary Ab.
Statistical analysis
All data are expressed as mean ± SD and were analyzed using SPSS 10.0 for Windows (IBM, Armonk, NY). Numerical data were compared between groups using one-way ANOVA and the nonparametric Mann–Whitney U test. Differences in the mean values between groups were analyzed using ANOVA with Bonferroni correction as a post hoc test. A p value <0.05 was considered to be significant.
Results
IL-2IC induces OSM and SOCS3 activation
It has been reported that the IL-2IC can suppress CIA through the IL-2–STAT5 signaling pathways (12). To determine whether CIA development was inhibited by IL-2IC treatment, DBA1/J mice were immunized intradermally with CII on day 0 and boosted with an intradermal injection of CII on day 14. PBS or IL-2IC was injected i.p. into DBA1/J mice on days 0, 2, and 4. As expected, IL-2IC treatment reduced the severity and incidence of arthritis (Fig. 1A).
FIGURE 1.
Effects of OSM in IL-2IC–treated CIA mice. DBA1/J mice were immunized intradermally with CII on day 0 and boosted by intradermal injection with CII on day 14. PBS (n = 10) or IL-2IC (n = 10) was injected i.p. into mice on day 0 (first injection), day 2 (second injection), and day 4 (third injection). (A) Arthritis severity was determined based on the mean arthritis index score and incidence score. (B) Confocal images of spleen tissues stained for CD4 (red), CD25 (blue), IL-17 (green), and Foxp3 (green). Positive cells are indicated in the graphs (right). (C) mRNA levels of OSM in spleen cells analyzed by real-time PCR. (D) Expression levels of STAT3, STAT5a, STAT5b, and SOCS3 in spleen cells were measured by real-time PCR. Data are presented as mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.005).
Next, we used confocal staining to examine the expression of CD4+IL-17+ T cells and CD4+CD25+Foxp3+ Treg cells in spleen tissue. CIA mice treated with IL-2IC showed markedly reduced expression of CD4+IL-17+ T cells compared with mice treated with PBS. By contrast, the number of CD4+CD25+Foxp3+ Treg cells was significantly higher (p < 0.05) in IL-2IC–treated CIA mice than in PBS-treated mice (Fig. 1B).
Next, we used microarray analysis to identify which molecules were upregulated or downregulated in the CIA mice. OSM and SOCS3 were highly upregulated in IL-2IC–treated mice (data not shown). Based on these results, we hypothesized that the gene expression of OSM would be upregulated in splenocytes of IL-2IC–treated CIA mice compared with the controls. The gene expression level of OSM was significantly increased (p < 0.05) in the splenocytes of IL-2IC–treated CIA mice compared with the controls. The mRNA levels of STAT5 and SOCS3 were also significantly increased (p < 0.05). By contrast, the gene expression of STAT3 was significantly decreased (p < 0.05) in splenocytes of IL-2IC–treated CIA mice compared with the controls (Fig. 1C, 1D).
Regulation of Th17 activation by OSM
The structure and function of OSM are similar to those of LIF, which has recently been reported to inhibit the differentiation of Th17 and Treg cells (13). It is known that IL-2IC can induce Treg proliferation and STAT5 activation (12). We hypothesized that IL-2IC would reduce the severity of CIA through reciprocal regulation of Th17 and Treg cell differentiation. To determine whether OSM affected Th17 cell differentiation, we isolated CD4+ T cells from mouse spleens, cultured the cells for 3 d in the Th17 condition, and measured the expression of IL-17 and IL-21, the main cytokines produced by Th17 cells with important roles in autoimmunity (14). ELISA results showed that OSM decreased the expression of IL-17 and IL-21 in a dose-dependent manner (Fig. 2A). Flow cytometry showed that OSM also reduced Th17 differentiation (Fig. 2B). We observed the expression of IL-17 and IL-21 in Th17 cells (Fig. 2C). The mRNA levels of RORγT, IL-17, and IL-21 were downregulated significantly in these cells (Fig. 2D).
FIGURE 2.
Inhibition of Th17 cell differentiation by OSM. Isolated CD4+ T cells from DBA1/J mice were stimulated with OSM under the Th17-polarizing condition for 3 d. (A) The intracellular levels of IL-17 and IL-21 in Th17 cells were measured by ELISA. (B) Flow cytometry. (C) Immunohistological analysis of cytokine expression levels of IL-17 and IL-21. Th17 cells were stained with CD4 (white), IL-17 (red), IL-21 (green), and DAPI. (D) Expression levels of IL-17, RORγT, and IL-21 analyzed using real-time PCR. Data are presented as mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.005).
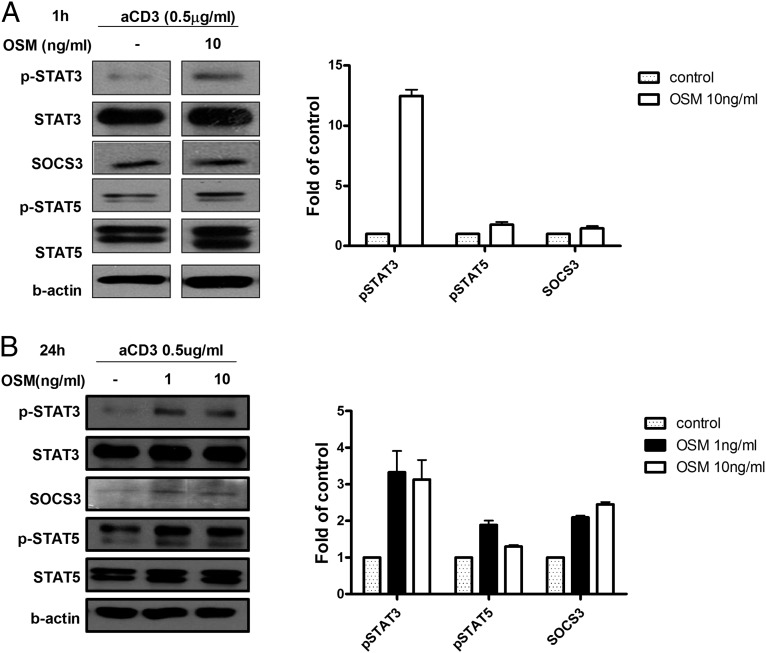
OSM induced p-STAT3, p-STAT5, and SOCS3 signaling in T cells
Because OSM is a member of the IL-6 family (8), we wondered how it inhibits Th17 differentiation. To identify the pathway of OSM involved in the inhibition of Th17 differentiation, we isolated CD4+ T cells from mouse spleens, stimulated them with anti-CD3, and harvested them for Western blot analysis. OSM increased the levels of p-STAT3, p-STAT5, and SOCS3 in a dose-dependent manner (Fig. 3A). The expression levels of p-STAT3, p-STAT5, and SOCS3 activated by OSM were maintained for 24 h (Fig. 3B).
FIGURE 3.
CD4+ T cells were collected from DBA1/J mice and then incubated with membrane-bound anti-CD3 Ab in the presence or absence of OSM at 1 or 10 ng/ml for 1 or 24 h. (A and B) Expression levels of p-STAT3, SOCS3, and p-STAT5 were measured by Western blotting at the times indicated. The expression of β-actin was used to normalize the data, and the data are expressed as relative fold changes in OSM, STAT3, STAT5a, STAT5b, and SOCS3 level. The black lines indicate where parts of the image were joined. Representative results are shown in the right panel. Data are presented as the mean ± SD of three independent experiments.
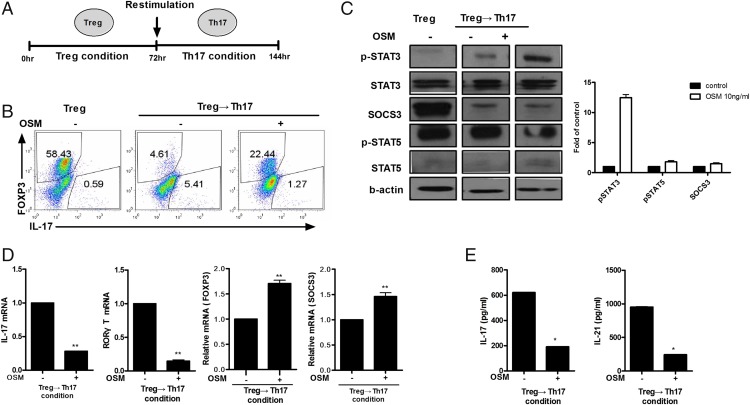
OSM suppression of Treg cell conversion to Th17 cells
Stimulation with IL-6 and TGF-β can initiate the redifferentiation from Treg to Th17 cells (15). To determine whether OSM can inhibit the redifferentiation from Treg to Th17 cells, we isolated CD4+ T cells from mouse spleens, and cultured these cells under the Treg differentiation condition for 72 h and then another 72 h under the Th17 differentiation condition (Fig. 4A). Flow cytometry showed that OSM decreased the redifferentiation from Treg to Th17 cells (Fig. 4B). Western blot analysis showed that the expression levels of p-STAT3 and SOCS3, but not p-STAT5, were increased by OSM (Fig. 4C). OSM significantly decreased (p < 0.05) the gene expression levels of IL-17 and RORγT but significantly increased (p < 0.05) the gene expression levels of Foxp3 and SOCS3 (Fig. 4D). ELISA showed that OSM significantly decreased the protein levels of IL-17 and IL-21 (p < 0.05) (Fig. 4E).
FIGURE 4.
Control of T cell plasticity by OSM. CD4+ T cells were stimulated in the Treg-polarizing condition and then 3 d later restimulated in the Th17-polarizing condition in the presence of OSM at 10 ng/ml and cultured for a further 3 d. (A) Diagram of the Th17 conversion protocol. (B) IL-17 and Foxp3+ T cells were analyzed by intracellular staining for cytokines and flow cytometry. (C) p-STAT3, p-STAT5, and SOCS3 levels were measured by Western blotting. Representative results are shown in the right panel. (D) mRNA expression levels of IL-17, RORγT, Foxp3, and SOCS3 were determined by real-time PCR. (E) IL-17 and IL-21 levels in culture supernatants were measured using ELISA. Data are presented as mean ± SD of three independent experiments (*p < 0.05, **p < 0.01).
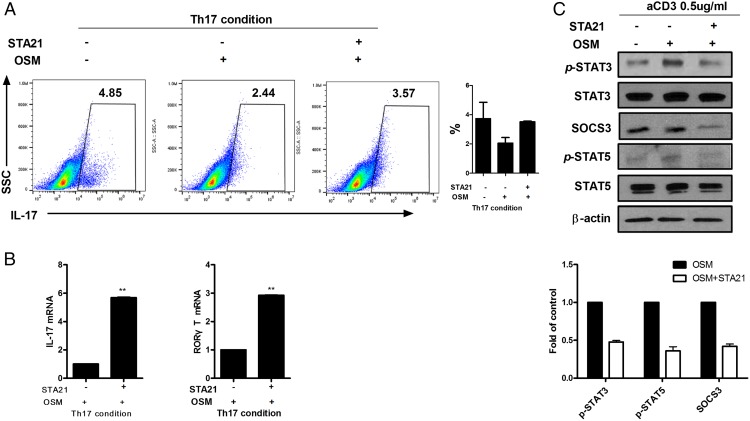
STAT3-dependent inhibition of Th17 cell differentiation
We next used STA-21 as a STAT3 inhibitor to investigate whether the OSM-increased STAT3 expression could promote SOCS3 and regulate Th17 cell differentiation. CD4+ T cells isolated from mouse spleens were cultured in the presence of 10 ng/ml OSM and in the absence or presence of 10 μM STA-21 under the Th17 cell-differentiating condition. Flow cytometry showed that OSM reduced Th17 cell differentiation but STA-21 given with OSM increased Th17 cell differentiation (Fig. 5A). The mRNA levels of IL-17 and RORγT were increased significantly (p < 0.05) by STA-21 treatment (Fig. 5B). Western blot analysis demonstrated that the expressions levels of p-STAT3, SOCS3, and p-STAT5 were reduced by STAT3 inhibition (Fig. 5C).
FIGURE 5.
Suppression of Th17 cells by OSM through STAT3 activation. (A and B) CD4+ T cells were cultured in the Th17-polarizing condition for 3 d in the absence or presence of OSM (10 ng/ml) or STA-21 (10 μM). (A) Intracellular staining for CD4+IL-17+ T cells was analyzed by flow cytometry. (B) Expression levels of IL-17 and RORγT were measured using real-time PCR. (C) CD4+ T cells were incubated with membrane-bound anti-CD3 Ab with or without OSM (10 ng/ml) or STA-21 (10 μM) for 1 h. The levels of p-STAT3, p-STAT5, and SOCS3 were measured by Western blotting. Data are presented as mean ± SD of three independent experiments (**p < 0.01).
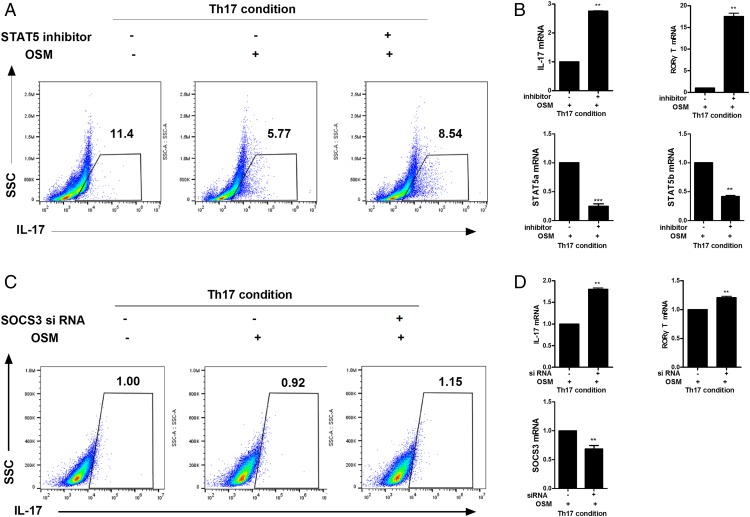
Role of SOCS3/STAT5 activated by OSM in Th17 cells
To determine whether STAT5 and SOCS3 activated by OSM could decrease the proliferation of Th17 cells, CD4+ T cells were isolated and cultured with 100 nM SOCS3 siRNA or 10 ng/ml STAT5 inhibitor in the presence of 10 ng/ml OSM under the Th17 differentiating condition. Flow cytometry analysis revealed that STAT5 inhibition and SOCS3 siRNA increased Th17 cell proliferation, which had been decreased by OSM treatment (Fig. 6A, 6C). Real-time PCR showed that the gene expression levels of IL-17 and RORγT were increased significantly (p < 0.05) by STAT5 inhibition and SOCS3 siRNA. We also observed that mRNA levels of STAT5a, STAT5b, and SOCS3 were decreased significantly by STAT5 inhibition and SOCS3 siRNA (Fig. 6B, 6D).
FIGURE 6.
Role of OSM in the inhibition of Th17 differentiation via STAT5 and SOCS3. (A and B) Isolated CD4+ T cells were activated under the Th17-polarizing condition in the presence or absence of OSM (10 ng/ml) or STAT5 inhibitor (10 ng/ml) for 3 d. (A) The proportion of IL-17–producing CD4+ T cells was analyzed by flow cytometry. (B) The gene expression levels of IL-17 and RORγT in Th17 cells were measured by real-time PCR. (C and D) CD4+ T cells were isolated from spleens of normal mice and stimulated with anti-CD3 mAb followed by transfection with SOCS3 siRNA or control siRNA using a nucleofector kit. After transfection, the cells were stimulated under the Th17-polarizing condition for 72 h; (C) CD4+IL-17+ T cell populations were analyzed by flow cytometry. (D) mRNA expression levels of IL-17 and RORγT were analyzed using real-time PCR. Data are presented as mean ± SD of three independent experiments (**p < 0.01, ***p < 0.005).
Discussion
OSM is a pleiotropic cytokine that plays a role in the inflammatory response (8). It has recently been found to be involved in the expression of transcription factors associated with inflammatory and immune responses mediated by T cells (9, 10). However, few studies have reported the effects of OSM on the proliferation of Th17 and Treg cells. In this study, we analyzed the inhibitory effects of OSM on Th17 cell differentiation and identified a new mechanism involved in determining the balance between Th17 and Treg cells.
The most notable observation in this study was that OSM suppressed Th17 cell differentiation but induced Treg cell differentiation by activating SOCS3, STAT3, and STAT5. To our knowledge, this is the first evidence that OSM may be a regulator of the Th17/Treg cell balance. Previous reports have shown that STAT3 activation can contribute to the proliferation of Th17 cells (4, 16), that activation of STAT5 can induce Treg cell differentiation and inhibit Th17 cell differentiation (4, 17), and that SOCS3 can reduce STAT3 activation and the proliferation of Th17 cells (18, 19). OSM can lead to the activation of STAT3 and STAT5 (20), and STAT3 activated by OSM can increase the expression of SOCS3, a negative regulator of STAT3 (21). In this study, OSM reduced the extent of Th17 cell differentiation and upregulated the activation of SOCS3, STAT3, and STAT5. The inhibitory effect of OSM on Th17 differentiation was diminished by inhibition of these transcription factors. Our results indicate that OSM decreases Th17 cell differentiation and IL-17 production, which can induce Treg differentiation, and suggest a novel mechanistic strategy to modulate the proliferation of Th17 and Treg cells via OSM.
Although OSM belongs to the IL-6 family with proinflammatory actions, it also has anti-inflammatory activity (8). The IL-6 family, including OSM, plays an anti-inflammatory role, and LIF, another member of the IL-6 family, also has anti-inflammatory activity (22). Activation of STAT3 and STAT5 is associated with OSM expression (9, 10). In this study, we found that OSM suppressed Th17 cell differentiation but induced Treg cell differentiation through the conversion of Treg into Th17 cells. Interestingly, we also found that this effect was dependent on the OSM-induced activation of SOCS3, STAT3, and STAT5. Although OSM did not induce p-STAT5 expression in the Th17-polarizing condition, the OSM-induced increase in SOCS3 level may reduce IL-17 production. Because STAT3 can induce IL-2R activation and STAT3 inhibition can reduce STAT5 activation through IL-2R expression (23, 24), Th17 cell differentiation was increased by STA21 given with OSM. These results suggest that OSM is a cytokine with dual functions: it inhibits the proliferation of Th17 cells by inducing the activation of SOCS3 and STAT5.
Previously, we have demonstrated that the therapeutic activity of IL-2IC is mediated through IL-2/STAT5-signaling pathways in a CIA model (12). In this study, we observed that IL-2IC treatment improved CIA by inhibiting Th17 expression. However, IL-2IC induced Treg cells, and OSM expression was increased in IL-2IC–treated mice. We also found that OSM reduced the mRNA levels of RORγT, IL-17, and IL-21. Th17 differentiation and the expression levels of IL-17 and IL-21 were decreased by OSM treatment in vitro. RORγT can mediate STAT3 upregulation and increase the proliferation of IL-17– and IL-21–expressing Th17 cells (25). These results suggest that OSM attenuates CIA by reducing Th17 differentiation. In this study, OSM decreased Th17 cell differentiation but induced Treg cell differentiation by activating SOCS3, STAT3, and STAT5. However, IL-2IC treatment caused more than OSM induction in vivo. Further study will be needed to find the reciprocal balance between STAT3 and STAT5 mediated by OSM in vivo. In addition, the experiment using OSM-knockout mice made the activity of OSM more apparent.
Th17 and Treg cells play key roles in the pathogenesis of several inflammatory diseases including RA. IL-17 is known as a pathogenic factor in many autoimmune disorders such as RA. The activity of Th17 cells and IL-17 production correlate with RA progression. However, Foxp3 expressed by Tregs has immunosuppressive activity by inducing an anti-inflammatory response (26–28). Therefore, regulating the proliferation of Th17 and Treg cells may improve several inflammatory diseases including RA. Further studies using OSM treatment are needed to determine whether OSM has therapeutic effects on inflammatory disease. Our findings on the regulatory effects of OSM on the proliferation of Th17 and Treg cells may shed light on the role of these cells in regulating inflammation. This study suggests that OSM may reduce Th17 progression by activating SOCS3 and STAT5, and that OSM has potential as a therapeutic target molecule against T cell-mediated inflammatory disease.
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0016 and HI14C3417), and by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A2A01051677).
- CIA
- collagen-induced arthritis
- CII
- collagen type II
- IL-2IC
- IL-2 immune complex
- OSM
- oncostatin M
- RA
- rheumatoid arthritis
- siRNA
- small interfering RNA
- SOCS3
- suppressor of cytokine signaling 3
- Treg
- regulatory T cell.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.van Hamburg J. P., Asmawidjaja P. S., Davelaar N., Mus A. M., Colin E. M., Hazes J. M., Dolhain R. J., Lubberts E. 2011. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 63: 73–83. [DOI] [PubMed] [Google Scholar]
- 2.Corthay A. 2009. How do regulatory T cells work? Scand. J. Immunol. 70: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu Q., Cai B., Huang Z. C., Shi Y. Y., Wang L. L. 2012. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol. Int. 32: 2731–2736. [DOI] [PubMed] [Google Scholar]
- 4.O’Shea J. J., Paul W. E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327: 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son H. J., Lee J., Lee S. Y., Kim E. K., Park M. J., Kim K. W., Park S. H., Cho M. L. 2014. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014: 973986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J. S., Kwok S. K., Lim M. A., Kim E. K., Ryu J. G., Kim S. M., Oh H. J., Ju J. H., Park S. H., Kim H. Y., Cho M. L. 2014. STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis Rheumatol. 66: 918–929. [DOI] [PubMed] [Google Scholar]
- 7.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M., Miyajima A. 2003. Oncostatin M, a multifunctional cytokine. Rev. Physiol. Biochem. Pharmacol. 149: 39–52. [DOI] [PubMed] [Google Scholar]
- 9.Fossey S. L., Bear M. D., Kisseberth W. C., Pennell M., London C. A. 2011. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer 11: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoermann G., Cerny-Reiterer S., Perné A., Klauser M., Hoetzenecker K., Klein K., Müllauer L., Gröger M., Nijman S. M., Klepetko W., et al. 2011. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am. J. Pathol. 178: 2344–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams R. O., Feldmann M., Maini R. N. 1992. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 89: 9784–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S. Y., Cho M. L., Oh H. J., Ryu J. G., Park M. J., Jhun J. Y., Park M. K., Stone J. C., Ju J. H., Hwang S. Y., et al. 2012. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology 137: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao W., Yang Y., Wang Z., Liu A., Fang L., Wu F., Hong J., Shi Y., Leung S., Dong C., Zhang J. Z. 2011. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity 35: 273–284. [DOI] [PubMed] [Google Scholar]
- 14.Harrington L. E., Hatton R. D., Mangan P. R., Turner H., Murphy T. L., Murphy K. M., Weaver C. T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 15.Kleinewietfeld M., Hafler D. A. 2013. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 25: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X. O., Panopoulos A. D., Nurieva R., Chang S. H., Wang D., Watowich S. S., Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282: 9358–9363. [DOI] [PubMed] [Google Scholar]
- 17.Laurence A., Tato C. M., Davidson T. S., Kanno Y., Chen Z., Yao Z., Blank R. B., Meylan F., Siegel R., Hennighausen L., et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26: 371–381. [DOI] [PubMed] [Google Scholar]
- 18.Carow B., Rottenberg M. E. 2014. SOCS3, a major regulator of infection and inflammation. Front. Immunol. 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bixler S. L., Sandler N. G., Douek D. C., Mattapallil J. J. 2013. Suppressed Th17 levels correlate with elevated PIAS3, SHP2, and SOCS3 expression in CD4 T cells during acute simian immunodeficiency virus infection. J. Virol. 87: 7093–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hintzen C., Haan C., Tuckermann J. P., Heinrich P. C., Hermanns H. M. 2008. Oncostatin M-induced and constitutive activation of the JAK2/STAT5/CIS pathway suppresses CCL1, but not CCL7 and CCL8, chemokine expression. J. Immunol. 181: 7341–7349. [DOI] [PubMed] [Google Scholar]
- 21.Stross C., Radtke S., Clahsen T., Gerlach C., Volkmer-Engert R., Schaper F., Heinrich P. C., Hermanns H. M. 2006. Oncostatin M receptor-mediated signal transduction is negatively regulated by SOCS3 through a receptor tyrosine-independent mechanism. J. Biol. Chem. 281: 8458–8468. [DOI] [PubMed] [Google Scholar]
- 22.Banner L. R., Patterson P. H., Allchorne A., Poole S., Woolf C. J. 1998. Leukemia inhibitory factor is an anti-inflammatory and analgesic cytokine. J. Neurosci. 18: 5456–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellery J. M., Nicholls P. J. 2002. Possible mechanism for the alpha subunit of the interleukin-2 receptor (CD25) to influence interleukin-2 receptor signal transduction. Immunol. Cell Biol. 80: 351–357. [DOI] [PubMed] [Google Scholar]
- 24.Akaishi H., Takeda K., Kaisho T., Shineha R., Satomi S., Takeda J., Akira S. 1998. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int. Immunol. 10: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 26.Waite J. C., Skokos D. 2012. Th17 response and inflammatory autoimmune diseases. Int. J. Inflam. 2012: 819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakae S., Nambu A., Sudo K., Iwakura Y. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171: 6173–6177. [DOI] [PubMed] [Google Scholar]
- 28.Wing K., Sakaguchi S. 2010. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11: 7–13. [DOI] [PubMed] [Google Scholar]