Abstract
Aims
There is a need for alternative strategies that might avoid recurrent admissions in patients with heart failure. home telemonitoring (HTM) to monitor patient's symptoms from a distance may be useful. This study attempts to assess changes in HTM vital signs in response to daily life activities (variations in medication, salt intake, exercise, and stress) and to establish which variations affect weight, blood pressure, and heart rate.
Methods and results
We assessed 76 patients with heart failure (mean age 76 ± 10.8 years, 75% male, mainly in NYHA class II/III and from ischaemic aetiology cause). Patients were given a calendar of interventions scheduling activities approximately twice a week before measuring their vital signs. Eating salty food or a large meal were the activities that had a significant impact on weight gain (+0.3 kg; P < 0.001 and P = 0.006, respectively). Exercise and skipping a dose of medication other than diuretics increased heart rate (+3 bpm, P = 0.001 and almost +2 bpm, P = 0.016, respectively).
Conclusions
Our HTM system was able to detect small changes in vital signs related to these activities. Further studies should assess if providing such a schedule of activities might be useful for patient education and could improve long‐term adherence to recommended lifestyle changes.
Keywords: Heart failure, Self‐management, Telemonitoring
Introduction
Heart failure (HF) is a clinical syndrome with a high prevalence, rising to more than 10% among people over the age of 70 years.1 What is more, in Europe, 24% of patients are readmitted within 12 weeks of discharge.2 Some of the most common causes of readmission are deficient treatment adherence, no or too‐late identification of disease destabilization signs, and hospital discharge without complete resolution of congestion.3 As HF prevalence keeps rising, there is an urgent need for alternative strategies that might avoid recurrent admissions, and novel technologies such as home telemonitoring (HTM) to monitor patient's symptoms from a distance may be useful.4
The effects of variations in daily routine on HTM measurements are unknown and might provide additional insights into physiological responsiveness and disease stability for individual patients. Providing patients with programmed variations in their daily routine might provide information on the sensitivity of individual patients to common interventions, such as dietary or medication compliance.
This study attempts to assess changes in HTM vital signs in response to daily life activities (variations in medication, salt intake, exercise, and stress) and to establish which variations affect weight, blood pressure (BP), and heart rate (HR).
Methods
HeartCycle is a programme that aims to improve the quality of life for cardiac patients by monitoring their condition and involving them in the daily management of their disease as well as facilitating clinicians to monitor relevant data for prescribing personalized therapies and lifestyle recommendations.5 One of its parts is the HF trial (Figure 1), which aims to investigate the HeartCycle's HF Management system (HFM), a 3rd generation HTM system that provides feedback and an educational programme about lifestyle and symptoms management. It is built on Philips' existing commercial MOTIVA product that integrates weight scales and a sphygmomanometer that measures BP and HR. The MOTIVA system has been described elsewhere.6 The trial took place in three European sites (University Hospital Castle Hill, Hull, UK; University Hospital Heidelberg, Heidelberg, Germany; University Hospital Germans Trias i Pujol, Barcelona, Spain) and was conducted in four phases. The present study focuses on Phase C of HeartCycle's HF trial and tries to assess how variations of everyday routine alter vital signs.
Figure 1.
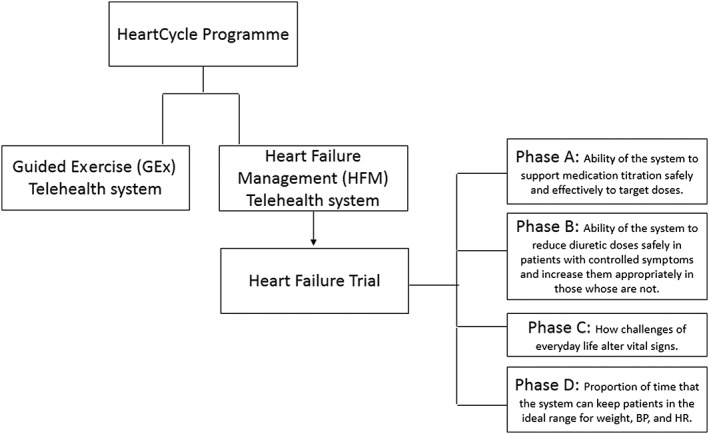
Heart failure trial phases of the HeartCycle programme.
Consecutive patients were asked to participate in the HeartCycle HTM programme. Inclusion criteria included the following: (1) a clinical diagnosis of HF, (2) requiring treatment with at least 40 mg/day of furosemide or equivalent, (3) evidence of advanced or unstable disease (mainly admission to hospital for or complicated by HF currently or within the previous 60 days or out‐patients with persistent NYHA III/IV symptoms), (4) an elevated NT‐proBNP (>1000 pg/mL if in sinus rhythm or >2000 pg/mL if not). Patients unwilling to comply with the protocol, with rapidly reversible causes of HF (such as severe anaemia, thyrotoxicosis, or admission with rapid atrial fibrillation with good ventricular function), with inability in the investigators' opinion to operate the HTM system, unable to communicate in the local language, aged <18 years and vulnerable groups were excluded.
Patients were asked to report symptoms and measure weight, HR, and BP on a daily basis. After a period of stabilization and diuretic optimization (Phase A and B, respectively), patients entered the 2 month follow‐up Phase C. Patients were given a calendar of interventions scheduling activities approximately twice a week; activities included taking salty food, skipping a dose of diuretic or other HF medication, listening to loud and irritating noise or relaxing music, drinking tea or coffee, taking a large meal, being hungry, exercise, or bathing. Patients were asked to measure their vital signs after these extra activities. All patients signed an informed consent. The study complied with the requirements of the declaration of Helsinki and was approved by the ethics committee of each participating hospital.
Statistical differences between the day before the action and the day of the action were assessed using mean comparison for paired data. Statistical analyses were performed with spss 15 (SPSS Inc., Chicago, IL, USA). A two‐sided P‐value of <0.05 was considered significant.
Results
A total of 123 patients were enrolled in the HF Trial after confirmation of the inclusion criteria and informed consent signing. Of the 100 patients that finally started the first phase of the trial, 76 reached Phase C (Figure 2). They (n = 76) had a mean age of 76 ± 10.8 years, 75% were male, with mean NT‐proBNP values of 4729 ± 5339 pg/mL, mainly in NYHA class II/III (31.6%/65.8%) and from ischaemic aetiology cause (55.3%). Other frequent HF aetiologies were idiopathic cardiomiopathy (27.6%) and valvular and hypertensive origin (2.6% and 1.3%).
Figure 2.
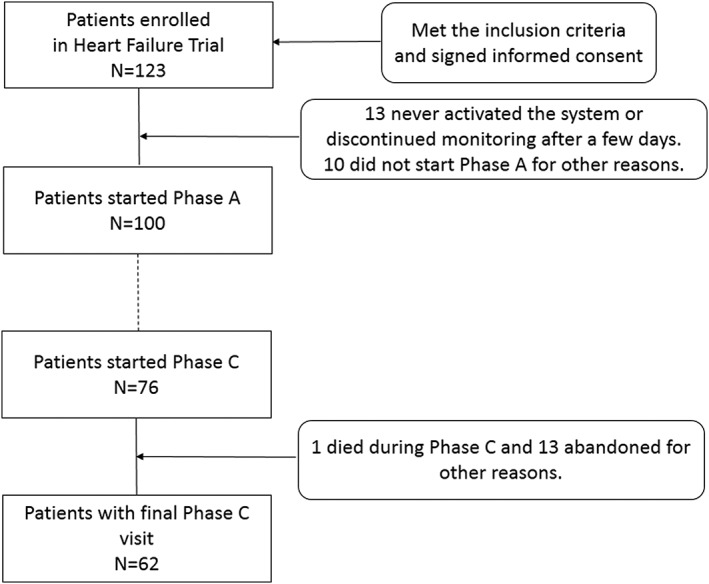
Study flow chart.
Main results of the 1200 activities performed by the 60 patients that fulfilled Phase C final visit are collected in Table 1 (two patients were non‐evaluable because of missing data). Eating salty food or a large meal were the activities that had a significant impact on weight gain (+0.3 kg; P < 0.001 and P = 0.006, respectively). Skipping a medication dose other than diuretics also showed a tendency on increasing patient's weight (P = 0.059). In contrast, weight gain after skipping a dose of diuretic had a P‐value of 0.1.
Table 1.
Mean values and change between day of activity and the day before the activity for the different measurements performed
| Activity | Measurement | Day before activity | Activity day | Change [95% IC] | P‐value |
|---|---|---|---|---|---|
| Mean value | Mean value | ||||
| After salty food | SBP | 110.5 | 108.8 | −1.74 [−4.05; 0.57] | 0.14 |
| DBP | 67.7 | 66.1 | −1.55 [−3.12; 0.01] | 0.05 | |
| HR | 66.5 | 67.2 | 0.74 [−0.66; 2.14] | 0.30 | |
| Weight | 84.0 | 84.3 | 0.28 [0.14; 0.43] | <0.001 | |
| After skipping a dose of diuretic | SBP | 111.1 | 112.1 | 1.05 [−2.13; 4.23] | 0.51 |
| DBP | 66.6 | 66.6 | −0.08 [−1.62; 1.47] | 0.92 | |
| HR | 66.0 | 67.1 | 1.10 [−0.16; 2.36] | 0.09 | |
| Weight | 84.2 | 84.3 | 0.13 [−0.03;0.29] | 0.10 | |
| While listening to loud and irritating noise | SBP | 110.7 | 111.4 | 0.76 [−2.05; 3.58] | 0.59 |
| DBP | 67.0 | 68.0 | 1.04 [−0.74; 2.82] | 0.25 | |
| HR | 66.0 | 67.2 | 1.26 [−0.52; 3.03] | 0.16 | |
| Weight | 83.9 | 84.0 | 0.13 [−0.03; 0.29] | 0.10 | |
| After tea or coffee | SBP | 112.6 | 113.1 | 0.44 [−3.14; 4.02] | 0.81 |
| DBP | 68.7 | 68.0 | −0.71 [−2.51; 1.10] | 0.44 | |
| HR | 66.9 | 66.6 | −0.28 [−1.98; 1.42] | 0.75 | |
| Weight | 84.5 | 84.5 | 0.06 [−0.08; 0.20] | 0.38 | |
| After a large meal | SBP | 111.9 | 111.0 | −0.93 [−3.65; 1.80] | 0.50 |
| DBP | 67.2 | 66.1 | −1.09 [−2.84; 0.66] | 0.22 | |
| HR | 66.4 | 67.7 | 1.30 [−0.63; 3.22] | 0.18 | |
| Weight | 83.9 | 84.2 | 0.25 [0.07; 0.42] | 0.006 | |
| Just after exercise | SBP | 112.4 | 114.3 | 1.84 [−1.22; 4.90] | 0.24 |
| DBP | 67.8 | 66.7 | −1.10 [−2.66; 0.45] | 0.16 | |
| HR | 66.6 | 69.5 | 2.91 [1.20; 4.61] | 0.001 | |
| Weight | 83.4 | 83.4 | 0.03 [−0.10; 0.16] | 0.65 | |
| When hungry | SBP | 110.4 | 111.7 | 1.29 [−1.50; 4.09] | 0.36 |
| DBP | 66.1 | 67.0 | 0.84 [−0.77; 2.46] | 0.30 | |
| HR | 66.6 | 66.9 | 0.30 [−1.52; 2.13] | 0.74 | |
| Weight | 83.7 | 83.7 | 0.02 [−0.11; 0.14] | 0.82 | |
| After skipping a medication dose other than diuretics | SBP | 111.2 | 112.7 | 1.52 [−0.97; 4.02] | 0.23 |
| DBP | 68.5 | 68.2 | −0.33 [−2.13; 1.48] | 0.72 | |
| HR | 66.5 | 68.2 | 1.78 [−0.34; −3.21] | 0.02 | |
| Weight | 84.2 | 84.4 | 0.12 [−0.01; 0.25] | 0.06 | |
| While listening to relaxing music | SBP | 111.7 | 109.7 | −1.97 [−4.19; 0.25] | 0.08 |
| DBP | 67.7 | 66.3 | −1.41 [−2.95; 0.13] | 0.07 | |
| HR | 67.0 | 66.9 | −0.09 [−1.61; 1.44] | 0.91 | |
| Weight | 84.1 | 84.2 | 0.10 [−0.04; 0.24] | 0.16 | |
| After shower/bath | SBP | 111.0 | 113.7 | 2.76 [−0.12; 5.63] | 0.06 |
| DBP | 67.0 | 66.5 | −0.47 [−2.21; 1.27] | 0.59 | |
| HR | 67.8 | 66.9 | −0.92 [−2.60; 0.77] | 0.28 | |
| Weight | 84.5 | 84.6 | 0.05 [−0.07; 0.16] | 0.41 |
SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); HR, heart rate (bpm); Weight (kg).
Exercise increased HR, on average +3 bpm, P = 0.001. Skipping a dose of medication other that diuretics also had an effect on HR, but more modest: almost +2 bpm, P = 0.016. Skipping a dose of diuretic showed a tendency on increasing HR (P = 0.087).
There were no statistically significant changes on measured vital signs for beneficial activities such as listening to relaxing music, but this activity showed a tendency on lowering systolic and diastolic blood preassure (P = 0.081 and P = 0.071, respectively). Eating salty food lowered diastolic blood preassure (P = 0.05).
No differences in vital signs were observed the day after any of the activities were performed.
Conclusions
The Motiva telehealth system has previously shown to significantly reduce the number of hospitalizations and days in hospital for HF and other cardiac causes in the CARME‐study.6 It has also shown to satisfy patients that used it and to change patients' behaviours.7 In the present study, we were able to prove that this system is capable of detecting variations of vital signs values (even small ones) because of life routine alterations.
One of the reasons proposed for lack of patient's self‐monitoring is the gradual loss of motivation and the tediousness of routine. Telemonitoring is a promising approach that could empower patients with HF and allow them to take a much more active part in their own management.4 As a visual tool for the patient, it may be useful for their motivation and self care. The MOTIVA system provides an instant, graphic, and visual feedback of self‐measurements that can promote compliance with health and dietary measures as the patient can acknowledge how a deviation from the ‘good path’ alters vital signs. It allows a greater awareness of how variations in daily activities have an impact on parameters such as weight, BP, or HR. This could reinforce the idea of medication and life‐style recommendation benefits in cases of low‐adherence.
Our study has several limitations: on one hand, the inherent to an observational study with no control group; on the other, we have no way to prove that patients did the activities as they were not watched in situ. Some may have lied about their compliance as suspected by some of the clinicians, but according to our results, the compliance seems to be high as the system was able to detect the changes.
In conclusion, our HTM system was able to detect small changes in vital signs related to these activities. Whether individual patient variability in the response to these or other interventions has implications for treatment or prognosis remains to be explored. Further studies should assess if providing such a schedule of activities might be useful for patient education and could improve long‐term adherence to HTM.
Conflict of Interest
None declared.
Funding
This work was supported by the EU 7th Frame Programme under grant number FP7‐216695, Redes Temáticas de Investigación Cooperativa en Salud (RETICS): Red Cardiovascular [RD12/0042/0047] and Red de Terapia Celular–TerCel [RD12/0019/0029], and Ministerio de Economía y Competitividad (Juan de la Cierva, JCI‐2012‐14025).
Acknowledgements
The authors would like to acknowledge the support of Philips Healthcare and Medtronic, especially the technical assistance of Joan Valenzuela, Salvador Dominguez, and Belén Nuñez, and the research nurses of Hull and Heidelberg sites and our hospital.
Gastelurrutia, P. , Lupón, J. , Domingo, M. , Stut, W. , Dovancescu, S. , Cleland, J. , Frankenstein, L. , Bayes‐Genis, A. , and on behalf of the HeartCycle Programme Investigators (2017) Potential role for clinical calibration to increase engagement with and application of home telemonitoring: a report from the HeartCycle programme. ESC Heart Failure, 4: 66–70. doi: 10.1002/ehf2.12104.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JGF, Swedberg K, Follath F, Komajda M, Cohen‐Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme‐‐ a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 2003; 24: 442–463. [DOI] [PubMed] [Google Scholar]
- 3. O'Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT‐HF registry. J Card Fail 2005; 11: 200–205. [DOI] [PubMed] [Google Scholar]
- 4. Dierckx R, Pellicori P, Cleland JGF, Clark AL. Telemonitoring in heart failure: Big brother watching over you. Heart Fail Rev 2015; 20: 107–116. [DOI] [PubMed] [Google Scholar]
- 5. http://www.heartcycle.eu, accessed 21/11/2014.
- 6. Domingo M, Lupón J, González B, Crespo E, López R, Ramos A, Urrutia A, Pera G, Verdú JM, Bayes‐Genis A. Noninvasive remote telemonitoring for ambulatory patients with heart failure: effect on number of hospitalizations, days in hospital, and quality of life. CARME (CAtalan Remote Management Evaluation) study. Rev Esp Cardiol 2011; 64: 277–285. [DOI] [PubMed] [Google Scholar]
- 7. Domingo M, Lupón J, González B, Crespo E, López R, Ramos A, Urrutia A, Pera G, Verdú JM, Bayes‐Genis A. Evaluation of a telemedicine system for heart failure patients: feasibility, acceptance rate, satisfaction and changes in patient behavior: results from the CARME (CAtalan Remote Management Evaluation) study. Eur J Cardiovas Nurs: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 2012; 11: 410–418. [DOI] [PubMed] [Google Scholar]


