Abstract
Background
Over 50% of patients with symptomatic heart failure (HF) experience HF with preserved ejection fraction (HFpEF). Exercise training (ET) is effective in improving cardiorespiratory fitness and dimensions of quality of life in patients with HFpEF. A systemic pro‐inflammatory state induced by comorbidities as the cause of myocardial structural and functional alterations has been proposed in HFpEF. ET modifies myocardial structure and has been related to inflammatory state. We investigated Ghrelin, related adipokines, markers of inflammation, and neuro‐hormonal activation in patients undergoing a structured ET vs. usual care are with HFpEF.
Methods and results
Ex‐DHF‐P was a prospective, controlled, randomized multi‐centre trial on structured and supervised ET in patients with HFpEF. We performed a post hoc analysis in 62 patients from Ex‐DHF‐P. Ghrelin, adiponectin, leptin, IL‐1ß, IL‐6, IL‐10, tumour necrosis factor‐alpha, MR‐proANP, MR‐proADM, CT‐proET1, and CT‐proAVP were assessed to seize the impact of ET on these markers in patients with HFpEF. Thirty‐six (58%) patients were female, mean age was 64 years, and median ghrelin was 928 pg/mL (interquartile range 755–1156). When stratified for high versus low ghrelin, groups significantly differed at baseline in presence obesity, waist circumference, and adiponectin levels (P < 0.05, respectively). Overall, ghrelin levels rose significantly to 1013 pg/mL (interquartile range 813–1182) (P < 0.001). Analysis of covariance modelling for change in ghrelin identified ET (P = 0.013) and higher baseline adiponectin levels (P = 0.035) as influencing factors.
Conclusions
Exercise training tended to increase ghrelin levels in Ex‐DHF‐P. This increase was especially pronounced in patients with higher baseline adiponectin levels. Future trials are needed to investigate the effect of ET on endogenous ghrelin levels in regard to interactions with cardiac structure and clinically meaningful surrogate parameters.
Keywords: Heart failure, Exercise, Training, Ghrelin, Biomarkers
Introduction
Over 50% of patients with symptomatic heart failure (HF) experience HF with preserved ejection fraction (HFpEF).1 The advent of pharmacologic management promises improved left ventricular diastolic function2 and a reduced rate of hospitalization in HFpEF patients.3 Regular physical activity and structured exercise training (ET) are a cornerstone of non‐pharmacological management in HF patients.4, 5 ET was found to be effective in improving cardiorespiratory fitness6, 7 and dimensions of quality of life in patients with HFpEF.8 Strikingly, ET as a mean of cardiovascular rehabilitation has also been associated to improved left ventricular diastolic function in patients with reduced6 and preserved7 ejection fraction.
Recently, a new paradigm for HFpEF has been proposed, which identifies a systemic pro‐inflammatory state induced by comorbidities as the cause of myocardial structural and functional alterations.9 The pleiotropic stimulus of ET leaves room for mechanistic considerations regarding the beneficial effect of ET in HFpEF, including anti‐inflammation10 and reduced neuro‐hormonal activation.11 Hormonal alterations may play a pivotal role in mediating this process. Inflammatory cytokines are associated with the progression of cardiac hypertrophy,12 and low cytokine levels may be protective for myocytes, whereas persistently high levels are detrimental.13, 14 The growth hormone‐releasing peptide ghrelin has been mainly attributed to the metabolic system and changes in body composition. Noteworthy, ghrelin was found to inhibit apoptosis of cardiomyocytes and endothelial cells in vitro,15 decreases blood pressure, and increases cardiac output.16 It has been suggested that repeated administration of ghrelin may improve left ventricular function, exercise capacity, and muscle wasting and significantly decrease plasma norepinephrine in patients with HF.17 In older adults, ET was not only related to inflammatory state but also modified ghrelin.18 Thus, the encouraging mechanistic effects of ET on myocardial structural and functional alterations may be attributable to endogenously altered ghrelin and hormonal levels.
The purpose of the present post hoc analysis of the Ex‐DHF‐P trial7 was to investigate the impact of structured and supervised ET on ghrelin, related adipokines, markers of inflammation, and neuro‐hormonal activation in the setting of a prospective, controlled, randomized multi‐centre trial in patients with HFpEF. ET improved exercise capacity and physical dimensions of quality of life in HFpEF. This benefit was associated with atrial reverse remodelling and improved left ventricular diastolic function.7
Methods
Study design, sample size
This is a secondary analysis of Ex‐DHF‐P. To date, the trial is the largest, prospective, multicentre, randomized‐controlled trial on ET in patients with HFpEF. Of the originally 71 patients screened for the trial, 67 were included, and 64 were analysed in the primary study.7 Supervised structured endurance/resistance ET on top of usual care (UC) was tested against UC alone. ET improved exercise capacity and physical dimensions of quality of life in HFpEF. This benefit was associated with atrial reverse remodelling and improved left ventricular diastolic function.
Laboratory biomarker testing
Blood sampling in Ex‐DHF‐P was performed according to protocol under standardized conditions. Sampling in one respective patient was performed at the same time of day, preferably fasting in the morning, to eliminate the influence of circadian variation and after a 20 min supine resting period. All samples were immediately centrifuged and stored at −80 °C. Laboratory marker analysis was performed centrally and post hoc. For assessment of ghrelin, a commercially available radio‐immuno‐assay (ghrelin RIA‐Kit, R90, Mediagnost GmbH, Reutlingen, Germany) was used. Solid‐phase ELISAs were used for assessment of adiponectin (human adiponectin, DRP 300, R&D Systems Inc., Minneapolis, MN, USA), Leptin (human leptin, DLP 00, R&D), interleukin‐1‐beta (human IL‐1ß, HSLB 00C, R&D), interleukin‐6 (human IL‐6, HS 600B R&D), interleukin‐10 (human IL‐10, HS 100B, R&D), and tumour necrosis factor alpha (human TNF alpha, HSTA 00D, R&D). Aforementioned assessments were performed according to the respective manual by an experienced and certified laboratory technician at Ulm University Medical Centre (Department for Internal Medicine II, Cardiology, Albert‐Einstein‐Allee 23, 89081 Ulm, Germany). MR‐proANP, MR‐proADM, CT‐proET1, and CT‐proAVP (BRAHMS Kryptor Assays, Thermo Fisher Scientific Clinical Diagnostics B · R · A · H · M · S GmbH, Hennigsdorf, Germany) were measured by time‐resolved amplified cryptate emission technology by a certified reference laboratory (through Thermo Fisher Scientific Clinical Diagnostics BRAHMS).
Ethics
The German Health Authorities and the ethics committees at each centre approved the study. Written informed consent was obtained from all patients before any study‐related procedure was performed.
Statistics
Changes of the level of the investigated biomarkers were assessed by linear model with follow‐up measurement as dependent variable and treatment group as fixed factor. The model was adjusted by the baseline value. To specify the additional impact of ghrelin, we computed several multivariate models for the change of ghrelin—computed as follow‐up value minus baseline value. We transformed ghrelin for all analysis to the logarithmic scale. Metric data were shown as mean ± SD. For skewed distributed laboratory data, presented at baseline and follow‐up, data were shown by median and interquartile range (IQR). Nominal or categorical data are presented with n and %. Analyses were performed according to the intent‐to‐treat principle. The software used for statistical analyses was R 3.0.3 (cran.r‐project.org).
Results
The primary results of Ex‐DHF‐P have been reported elsewhere.7 Of the 64 patients available for analysis, two patients were excluded for quality concerns with blood serum samples. Twenty‐six (42%) of the 62 analysed patients were male. Table 1 shows baseline characteristics of the study population stratified by high vs. low ghrelin serum levels according to a median split (median ghrelin 928 pg/mL) of data. Age did not differ between groups, whereas significantly more women showed higher ghrelin levels. Waist circumferences were higher and obesity more prevalent in the group with lower ghrelin levels. In regard to demographics, risk factors, echocardiographic structure, cardiopulmonary function, quality of life, and treatment subjects were comparable in both groups.
Table 1.
Baseline characteristics of study population stratified by a ghrelin median split
| Ghrelin at baseline no. of subjects | Total (n = 62) | Low ghrelin (n = 31) | High ghrelin (n = 31) | P‐value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 64.4 ± 7.2 | 63.2 ± 7.5 | 65.6 ± 6.7 | 0.17 |
| Sex (female) | 36 (58) | 11 (35) | 25 (81) | 0.001 |
| Heart rate (bpm) | 64 ± 10 | 66 ± 11 | 62 ± 9 | 0.20 |
| Systolic BP (mmHg) | 150 ± 21 | 149 ± 21 | 150 ± 21 | 0.91 |
| Diastolic BP (mmHg) | 85 ± 11 | 84 ± 12 | 87 ± 10 | 0.22 |
| Waist (cm) | 99 ± 13 | 104 ± 12 | 94 ± 13 | 0.004 |
| New York Heart Association (NYHA) functional class | 0.73 | |||
| II | 52 (84) | 25 (81) | 27 (87) | |
| III | 10 (16) | 6 (19) | 4 (13) | |
| Risk factors | ||||
| Arterial hypertension | 54 (87) | 26 (84) | 28 (90) | 0.71 |
| Hyperlipidaemia | 29 (47) | 15 (48) | 14 (45) | 1.00 |
| Diabetes mellitus | 9 (15) | 4 (13) | 5 (16) | 1.00 |
| Obesity (BMI ≥ 30 kg/m2) | 32 (52) | 21 (68) | 11 (35) | 0.021 |
| (BMI > 25 kg/m2) | 55 (89) | 28 (90) | 27 (87) | 1.00 |
| Abnormal waist (>102 cm (male); >88 cm (female)) | 38 (61) | 19 (61) | 19 (61) | 1.00 |
| Smoker | 6 (10) | 4 (13) | 2 (6) | 0.67 |
| Cardiac structure and function | ||||
| Left ventricular ejection fraction (LVEF) (%) | 67 ± 7 | 67 ± 7 | 68 ± 6 | 0.40 |
| Left atrial volumen index (LAVI) (mL/m2) | 27.7 ± 8.0 | 29.1 ± 8.8 | 26.2 ± 7.1 | 0.16 |
| Left ventricular mass index (LVMI) (g/m2) | 134.1 ± 34.2 | 132.8 ± 33.7 | 135.4 ± 35.2 | 0.77 |
| E/e′ lateral | 9.7 ± 2.8 | 9.8 ± 2.9 | 9.5 ± 2.8 | 0.70 |
| E/e′ medial | 12.9 ± 3.7 | 13.1 ± 4.1 | 12.7 ± 3.2 | 0.69 |
| e′ lateral (mm) | 7.5 ± 2.0 | 7.5 ± 2.1 | 7.5 ± 1.9 | 1.00 |
| e′ medial (mm) | 5.6 ± 1.3 | 5.6 ± 1.2 | 5.5 ± 1.5 | 0.92 |
| VO2 max | 16.2 ± 4.8 | 16.3 ± 5.2 | 16.0 ± 4.5 | 0.84 |
| Watt max | 114 ± 37 | 123 ± 41 | 105 ± 31 | 0.07 |
| 6MWD (m) | 544 ± 88 | 546 ± 101 | 543 ± 74 | 0.89 |
| Laboratory | ||||
| Haemoglobin (mmol/L) | 8.9 (8.3–9.5) | 9.2 (8.8–9.6) | 8.6 (8.3–9.2) | 0.11 |
| NT‐proBNP (pg/mL) | 124 (78–183) | 118 (62–157) | 128 (80–210) | 0.11 |
| Sens. CRP (mg/L) | 1.83 (0.93–3.63) | 1.85 (0.78–3.71) | 1.82 (0.98–3.04) | 0.56 |
| Treatment | ||||
| Angiotensin converting enzyme inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) | 41 (67) | 19 (63) | 22 (71) | 0.59 |
| β‐Blocker | 31 (51) | 17 (57) | 14 (45) | 0.45 |
| Mineralcorticoid Receptor Antagonist (MRB) | 2 (3) | 1 (3) | 1 (3) | 1.00 |
| Diuretic | 26 (43) | 13 (43) | 13 (42) | 1.00 |
| Lipid lowering drug | 16 (26) | 10 (33) | 6 (19) | 0.25 |
| Uric acid lowering drug | 6 (10) | 3 (10) | 3 (10) | 1.00 |
| Quality of life | ||||
| SF‐36 physical sum score | 42.8 ± 9.7 | 44.2 ± 9.3 | 41.3 ± 10.0 | 0.26 |
| SF‐36 mental sum score | 49.5 ± 10.6 | 48.8 ± 10.7 | 50.3 ± 10.6 | 0.61 |
| SF‐36 physical functioning scale | 66.8 ± 21.0 | 68.9 ± 19.7 | 64.6 ± 22.4 | 0.43 |
| Patient Health Questionnaire (PHQ) sum score | 6.2 ± 5.6 | 6.6 ± 6.1 | 5.8 ± 5.1 | 0.62 |
BMI, body mass index; BP, blood pressure.
Values are n (%), mean ± SD, or median (interquartile range) tests are χ 2 for index or nominal variables tauB for ordinal and analysis of variance for metric measurements.
Adiponectin levels tended to be higher in patients with higher ghrelin levels [median adiponectin levels 8828 ng/mL (IQR 6270–13637) in high ghrelin and 6417 ng/mL (IQR 4068–9167) in low ghrelin, P = 0.012]. Levels of TNF‐α, IL‐1ß, IL‐6, IL‐10, MR‐proANP, MR‐proADM, CT‐proET1, and CT‐proAVP were comparable in the setting of high vs. low ghrelin (P > 0.05).
Aside from significantly higher values for MR‐proANP in the UC group at baseline (P = 0.007), no significant (P > 0.05) differences between the respective treatment groups was observed at the respective time‐point (Table 2). Overall changes and changes over the course of biomarkers in the respective treatments groups are displayed in Table 3. In summary, we observed a significant change in ghrelin. Ghrelin increased significantly under ET. This shift in ghrelin was especially pronounced in the setting of low ghrelin levels at baseline in the ET intervention group (Figure 1). With regard to the increase in ghrelin, analysis of covariance models for change in ghrelin and ratio of geometric means are shown in Table 4. Among baseline ghrelin values, ET and higher adiponectin levels (Figure 2) in the ET group significantly contributed to the model.
Table 2.
Laboratory values at baseline and follow‐up
| Time point | Baseline | Follow‐up | ||||
|---|---|---|---|---|---|---|
| Arm | Overall | Usual care | Excercise training | Overall | Usual care | Excercise training |
| No. of subjects | (N = 62) | (N = 19) | (N = 43) | (N = 62) | (N = 19) | (N = 43) |
| Ghrelin [pg/mL] | 928 (755–1156) | 833 (755–1114) | 988 (758–1166) | 1013 (813–1182) | 913 (796–1027) | 1092 (852–1203) |
| Leptin [pg/mL] | 22 579 (7106–40 487) | 28 379 (7768–50 159) | 20 326 (6726–36 376) | 22 487 (8979–43 155) | 33 774 (9536–53 063) | 18 320 (9106–34 361) |
| Adiponectin [ng/mL] | 7596 (4328–11 148) | 8777 (4162–11 704) | 7590 (4427–10 782) | 7028 (4868–11 345) | 7987 (5146–11 246) | 6647 (4211–11 002) |
| TNF‐α [pg/mL] | 1.66 (1.11–2.45) | 1.93 (1.40–2.33) | 1.54 (1.07–2.55) | 2.03 (1.40–3.07) | 2.30 (1.69–3.59) | 1.95 (1.37–2.57) |
| IL‐1β [pg/mL] | ||||||
| Below detection cut‐off | 36 (58.06) | 10 (52.63) | 26 (60.47) | 54 (87.10) | 17 (89.47) | 37 (86.05) |
| Above detection cut‐off | 26 (41.94) | 9 (47.37) | 17 (39.53) | 8 (12.90) | 2 (10.53) | 6 (13.95) |
| IL‐1β [pg/mL] (if measurable) | 0.20 (0.11–0.52) | 0.18 (0.14–0.38) | 0.22 (0.11–0.59) | 0.09 (0.06–0.56) | 0.31 (0.19–0.43) | 0.08 (0.06–0.46) |
| IL‐6 [pg/mL] | 1.57 (1.13–2.48) | 1.61 (0.96–2.15) | 1.56 (1.26–2.63) | 1.44 (1.00–2.60) | 1.42 (1.23–2.02) | 1.45 (0.91–2.84) |
| IL‐10 [pg/mL] | ||||||
| Below detection cut‐off | 31 (50.00) | 13 (68.42) | 18 (41.86) | 42 (67.74) | 12 (63.16) | 30 (69.77) |
| Above detection cut‐off | 31 (50.00) | 6 (31.58) | 25 (58.14) | 20 (32.26) | 7 (36.84) | 13 (30.23) |
| IL‐10 [pg/mL] (if measurable) | 8.42 (4.26–16.70) | 9.10 (5.92–15.49) | 8.16 (4.13–16.28) | 1.80 (0.96–4.12) | 0.61 (0.44–1.48) | 2.22 (1.64–6.02) |
| MR‐proANP [pmol/L] | 96 (78–129) | 118 (98–148) | 91 (68–114) | 91 (71–124) | 97 (75–136) | 86 (61–108) |
| MR‐proADM [nmol/L] | 0.52 (0.48–0.66) | 0.52 (0.46–0.64) | 0.52 (0.49–0.66) | 0.57 (0.50–0.72) | 0.57 (0.52–0.68) | 0.58 (0.49–0.73) |
| CT‐proET1 [nmol/L] | 55.0 (51.0–63.8) | 56.0 (51.0–63.5) | 55.0 (50.5–64.0) | 58.0 (51.0–66.8) | 57.0 (50.5–70.0) | 59.0 (52.0–66.5) |
| CT‐proAVP [pmol/L] | 4.10 (2.45–7.20) | 3.40 (2.60–6.45) | 4.10 (2.45–7.95) | 3.98 (2.48–6.17) | 3.73 (2.21–5.89) | 4.15 (2.60–6.38) |
TNF, tumour necrosis factor.
Values median (interquartile range) resp. N (%).
Usual care with significantly (P < 0.05) higher values for MR‐proANP at baseline. A selection bias is assumed.
Table 3.
Change of endogenous biomarkers over 12 weeks
| Coefficient | Ratio of geometric mean [95%CI] | P‐value |
|---|---|---|
| Ghrelin | ||
| (Intercept) | 4.23 [2.10–8.54] | <0.001 |
| Baseline value | 1.73 [1.61–1.86] | <0.001 |
| Treatment group (ET) | 1.09 [1.02–1.17] | 0.013 |
| Leptin | ||
| (Intercept) | 6.54 [2.59–16.55] | <0.001 |
| Baseline value | 1.78 [1.67–1.90] | <0.001 |
| Treatment group (ET) | 0.85 [0.68–1.06] | 0.15 |
| Adiponectin | ||
| (Intercept) | 6.27 [1.76–22.26] | 0.005 |
| Baseline value | 1.74 [1.58–1.92] | <0.001 |
| Treatment group (ET) | 0.89 [0.74–1.07] | 0.20 |
| TNF‐α | ||
| (Intercept) | 2.06 [1.56–2.71] | <0.001 |
| Baseline value | 1.26 [1.10–1.44] | 0.001 |
| Treatment group (ET) | 0.77 [0.57–1.04] | 0.09 |
| IL‐6 | ||
| (Intercept) | 1.13 [0.77–1.66] | 0.51 |
| Baseline value | 1.84 [1.53–2.22] | <0.001 |
| Treatment group (ET) | 0.83 [0.53–1.30] | 0.41 |
| IL‐10 | ||
| (Intercept) | 0.11 [0.01–2.04] | 0.12 |
| Baseline value | 1.41 [0.80–2.49] | 0.20 |
| Treatment group (ET) | 10.02 [0.88–114.09] | 0.06 |
| MR‐proANP | ||
| (Intercept) | 1.06 [0.35–3.16] | 0.92 |
| Baseline value | 1.94 [1.66–2.27] | <0.001 |
| Treatment group (ET) | 1.08 [0.89–1.31] | 0.44 |
| MR‐proADM | ||
| (Intercept) | 1.15 [1.05–1.25] | 0.004 |
| Baseline value | 2.14 [1.97–2.34] | <0.001 |
| Treatment group (ET) | 1.00 [0.94–1.07] | 0.99 |
| CT‐proET1 | ||
| (Intercept) | 1.24 [0.60–2.57] | 0.55 |
| Baseline value | 1.93 [1.71–2.19] | <0.001 |
| Treatment group (ET) | 1.03 [0.96–1.10] | 0.47 |
| CT‐proAVP | ||
| (Intercept) | 1.14 [0.90–1.45] | 0.26 |
| Baseline value | 1.80 [1.64–1.97] | <0.001 |
| Treatment group (ET) | 1.10 [0.91–1.33] | 0.34 |
ET, exercise training; TNF, tumour necrosis factor; UC, usual care.
Tabulated is for each measurement the ratio of geometric mean and P‐values of the group difference between the two arms [UC vs. ET interpretation: the ratio of the geometric mean value of ghrelin (at baseline) for UC vs. ET is 1.73 (95% CI: 1.61–1.86)].
Figure 1.
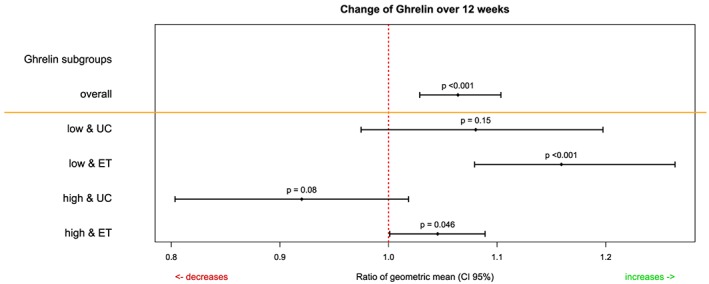
Change of ghrelin over 12 weeks. UC, usual care; ET, exercise training.
Table 4.
ANCOVA models for change in ghrelin over 12 weeks
| Coefficient | Ratio of geometric mean [95%CI] | P‐value |
|---|---|---|
| Change of ghrelin | ||
| Model 1: … by baseline ghrelin | ||
| (Intercept) | 4.31 [2.07–8.96] | <0.001 |
| Ghrelin (baseline) | 0.87 [0.81–0.94] | <0.001 |
| Model 2: … by baseline ghrelin adjusted for treatment group | ||
| (Intercept) | 4.23 [2.10–8.54] | <0.001 |
| Ghrelin (baseline) | 0.86 [0.81–0.93] | <0.001 |
| Exercise training | 1.09 [1.02–1.17] | 0.013 |
| Model 3: … by baseline ghrelin adjusted for treatment group and adiponectin (high) | ||
| (Intercept) | 4.18 [2.02–8.62] | <0.001 |
| Ghrelin (baseline) | 0.87 [0.80–0.93] | <0.001 |
| Exercise training | 1.09 [1.02–1.17] | 0.014 |
| Adiponectin (high) | 1.01 [0.94–1.07] | 0.86 |
| Model 4: … by baseline ghrelin adjusted for treatment group, interaction [exercise training: adiponectin (high)] | ||
| (Intercept) | 4.37 [2.18–8.74] | <0.001 |
| Ghrelin (baseline) | 0.87 [0.81–0.93] | <0.001 |
| Exercise training | 1.01 [0.92–1.11] | 0.79 |
| Adiponectin (high) | 0.90 [0.81–1.01] | 0.06 |
| Exercise training: adiponectin (high) | 1.15 [1.01–1.32] | 0.035 |
ANCOVA, analysis of covariance.
Tabulated is per model the ratio of geometric means and the P‐value of the ANCOVA test.
Figure 2.
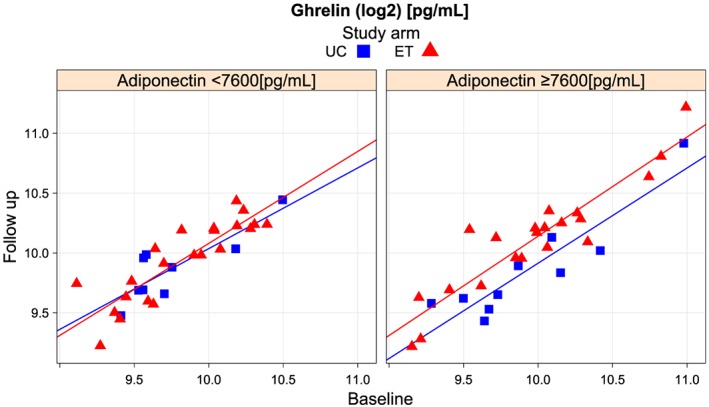
Change of ghrelin over 12 weeks by treatment group and baseline adiponectin. UC, usual care; ET, exercise training.
Discussion
This study investigates the effect of ET on ghrelin levels and other biomarkers in the setting of HFpEF. We report a significant impact of ET on endogenous ghrelin in this randomized, controlled trial. ET clearly increased ghrelin levels. Patients with lower ghrelin levels at baseline indeed experienced a more pronounced increase in ghrelin under a training intervention. The increase in ghrelin was related to higher adipokine levels at baseline. Of note, beneficial effects in patients with cardiovascular disease, especially HF, have previously been attributed to an altered expression of ghrelin. Further, we investigated the trajectories of adipokines, inflammatory and anti‐inflammatory markers, and surrogates of neuro‐hormonal activation in the EX‐DHF‐P trial. In the current analysis (n = 62), general findings in cardiac structure and surrogate markers of HFpEF (Table 1) are in line with the primary results of Ex‐DHF‐P.7
Ghrelin
In this trial, the median baseline ghrelin level was 928 pg/mL. A clinically described ghrelin reference value for normal weight, control subjects is considered to be between 520 and 700 pg/mL. Makovey et al.19 reported women to have higher ghrelin levels than men and found age to be a significant predictor of ghrelin levels after adjustment for gender, fat mass, and body size. Ghrelin levels are reportedly lower in individuals with a higher body weight.20 Hence, we interpret our observation of differences in percentages of women and obese subjects in subgroups with higher vs. lower ghrelin levels as confirming the literature. Interestingly, Gibas‐Dorna et al.20 reported significantly lower ghrelin levels in patients with hypertension when compared with controls. While we did not observe a difference in regard to blood pressure, this finding is of note when considering the left‐ventricular hypertrophy, diastolic dysfunction, and HFpEF continuum.
In this analysis, we evaluate the effect of ET on endogenous ghrelin levels in the setting of HFpEF. Under standardized conditions of a clinical trial, we observed a significant change of ghrelin over time. This change was seen in the ET group with a trend for change in the group with higher ghrelin levels and a clear change in the group with lower ghrelin levels (Figure 1). Analysis of covariance modelling identified a marked ratio of geometric to be due to ET (Table 4). Adiponectin was found to play an additional role in this setting (Table 4). We interpret the currently observed increase in endogenous ghrelin levels under ET to be consistent with the alterations observed in studies in older adults18 but consider it to may have additional clinical consequences in the setting of HF. Animal data suggest that endogenous ghrelin plays a crucial role in attenuating pressure overload‐induced cardiac hypertrophy and diastolic dysfunction.21 Mao et al.21 discuss these effects because of the activation of a cholinergic anti‐inflammatory pathway. By simultaneously evoking sympathetic inhibition and parasympathetic activation, ghrelin is considered to be effective against cardiovascular diseases.21 Given that ghrelin was found to inhibit apoptosis of cardiomyocytes and endothelial cells in vitro,15 it decreases blood pressure and increases cardiac output.16 Nagaya et al. have previously suggested that repeated administration of ghrelin may improve left ventricular function, exercise capacity, and reduce muscle wasting in patients with HF. Because the relevance of sarcopenia in the clinical picture of HF22 is growing and approximately 20% of HF patients with an average age of nearly 70 years may meet the criteria for diagnosing sarcopenia,23 ghrelin may be an interesting treatment strategy to tackle the wasting continuum in HF.24
In a meta‐analysis on the effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mouse models of HF, Khatib et al.25 consider the existing data to provide suggestive evidence that ghrelin may lower the risk of mortality and improve cardiovascular outcomes. In this work, we show that non‐pharmacologic HF management with regular physical activity and structured ET has the potential to alter endogenous ghrelin levels.
Due to statistical limitations in the present analysis, we refrained from assessing associations of the change of blood values and change in endpoint variables of the Ex‐DHF‐P study (e.g. E/e′ ratio, VO2max, LAVI, and SF‐36 physical functioning scale). However, future analyses in larger, prospective cohorts like the currently recruiting Exercise Training in Diastolic Heart Failure Trial may close this gap and allow for a more subtle pathophysiological evaluation of ghrelin's role.
Adipokines
Leptin, as an adipocyte‐derived hormone, links nutritional status with neuroendocrine and immune functions, that is, by affecting IL‐1 and TNF‐α. Leptin has the potential to influence basal metabolism and angiogenesis. In the present analysis, we did observe an overall change in ghrelin between baseline and follow‐up, but not in regard to UC or ET.
The hormone adiponectin is secreted by adipose tissue. Adiponectin levels tended to be higher in patients with higher ghrelin levels. In this analysis, we report the effect of ET on change in ghrelin in patients with higher baseline adpionectin levels (P = 0.035). While body mass index did not change in either treatment group in the Ex‐DHF‐P trial,7 fat mass and detailed body composition assessments were unfortunately not available in our patients. Levels of the hormone are previously shown to be inversely correlated with body fat percentage in adults.26 Adiponectin is known to suppress TNF‐α production and to possess direct anti‐inflammatory effects. Hence, one may hypothesize the observed ET ghrelin–adipokine interaction to document physiologic pathways that may bear additional effects in diastolic HF.
Inflammatory and anti‐inflammatory markers
Higher levels of TNF‐α have been reported and debated in severe chronic HF.27 In this study, we did observe a significant overall change in regard to TNF‐α and IL‐6 (P < 0.05), but not in regard to treatment group. In fact, TNF‐α may play a role in a systemic pro‐inflammatory state, which has been suggested as a mechanism in HFpEF.9 In their systematic review, Smart et al. 28 suggested that physical exercise employing ≥5 sessions per week may be most likely to reduce serum levels of TNF‐α in HF patients. In this analysis, we did not observe an analogue finding. Further, in regard to IL‐1ß and IL‐10, no relevant trajectory was observed. This may be explained by a marked number of samples with measured values below cut‐off thresholds, indicating a generally low inflammatory state of the present HF population.
Neuro‐hormonal activation
NT‐proBNP levels were reported to be similar at baseline in the respective treatment groups and did not change throughout Ex‐DHF‐P trial.7 In regard to novel markers of neuro‐hormonal activation, we documented overall changes in MR‐proANP, MR‐proADM, CT‐proET1, and CT‐proAVP in this analysis. We observed significantly higher MR‐proANP levels in the UC group as compared with ET at baseline and assume a selection bias to explain this finding. Aside from this, MR‐proADM, CT‐proET1, and CT‐proAVP were comparable in the respective treatment groups at baseline and follow‐up. We did not observe a significant treatment interaction in the markers of neuro‐hormonal activation.
Exercise training confers benefit in terms of enhancements in exercise capacity and health‐related quality of life29 in HFpEF patients. The processes behind better exercise capacity are complex and tend to reduce the level of muscle atrophy, inflammation, and catabolic/anabolic imbalance through molecular mechanisms.30 The present analysis allows for a glance at the hormonal alterations that may play a pivotal role in mediating the underlying process.
Limitations
This analysis is limited in its exploratory, secondary, post hoc character. Ex‐DHF‐P was a multicentre trial with a relatively small number of mildly affected, middle‐aged patients in short‐term follow‐up. The study was not primarily designed to address ghrelin, other endogenous biomarkers, and their interactions and not adequately powered to allow for definite conclusions. The reported results could hence be a statistical phenomenon, not only because the control group is considerably smaller than the ET group and therefore an identical effect in the control group had a lower chance to reach statistical significance than in the ET group. Therefore, future studies with an adequately powered sample size, a broader population with a wider range of stages of HFpEF, and long‐term follow‐up are necessary. The ongoing, larger Ex‐DHF study (recruitment completed) will allow for such analysis.
Conclusions
This study investigates the effect of ET on endogenous ghrelin levels, and other biomarkers in the setting of HFpEF. ET tended to increase ghrelin levels in Ex‐DHF‐P. This increase was especially pronounced in patients with higher baseline adiponectin levels. We documented multiple, not‐treatment‐related changes in other biomarker trajectories in the setting of HFpEF. While suggestive data on the effects in patients with HF have been previously attributed to an altered expression of ghrelin, future trials are urgently needed to address the potential role of ghrelin, interactions, and clinically meaningful surrogate parameters in this setting.
Acknowledgements
We like to thank all subjects for participation in the trial and all investigators, trainers, and study personnel for their time and commitment in the study. Additionally, we like to thank the colleagues at University Medical Centre Ulm and Thermo Fisher Scientific Clinical Diagnostics B · R · A · H · M · S GmbH for their help with biomarker measurements.
Conflict of interest
All authors declare no conflict of interest.
Funding
This work was partially funded by the German Cardiac Society Hans Bloemer Young Investigator Award for Clinical Cardiovascular Research. The assessment of MR‐proANP, MR‐proADM, CT‐proET1, and CT‐proAVP was provided by Thermo Fisher Scientific Clinical Diagnostics B · R · A · H · M · S GmbH (Hennigsdorf, Germany) free of charge, as commercial owner of these tests Thermo Fisher Scientific Clinical Diagnostics B · R · A · H · M · S GmbH was not involved in data‐interpretation, drafting, or writing of this manuscript. Other than disclosed, no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors was involved.
Trippel, T. D. , Holzendorf, V. , Halle, M. , Gelbrich, G. , Nolte, K. , Duvinage, A. , Schwarz, S. , Rutscher, T. , Wiora, J. , Wachter, R. , Herrmann‐Lingen, C. , Duengen, H.‐D. , Hasenfuß, G. , Pieske, B. , and Edelmann, F. (2017) Ghrelin and hormonal markers under exercise training in patients with heart failure with preserved ejection fraction: results from the Ex‐DHF pilot study. ESC Heart Failure, 4: 56–65. doi: 10.1002/ehf2.12109.
The legal statement for this article was changed on 5 October 2016 after original online publication.
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobson SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 2. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo‐DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA 2013; 309: 781–91. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, for the TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines . Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 5. Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011; 13: 347–357. [DOI] [PubMed] [Google Scholar]
- 6. Sandri M, Kozarez I, Adams V, Mangner N, Höllriegel R, Erbs S, Linke A, Möbius‐Winkler S, Thiery J, Kratzsch J, Teupser D, Mende M, Hambrecht R, Schuler G, Gielen S. Age‐related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: the Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur Heart J 2012; 33: 1758–1768. [DOI] [PubMed] [Google Scholar]
- 7. Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann‐Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex‐DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol 2011; 58: 1780–1791. [DOI] [PubMed] [Google Scholar]
- 8. Nolte K, Herrmann‐Lingen C, Wachter R, Gelbrich G, Düngen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex‐DHF‐P trial. Eur J Prev Cardiol 2015; 22: 582–593. [DOI] [PubMed] [Google Scholar]
- 9. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 10. Gielen S, Adams V, Möbius‐Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti‐inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 2003; 42: 861–868. [DOI] [PubMed] [Google Scholar]
- 11. Conraads VM, Beckers P, Vaes J, Martin M, Van Hoof V, De Maeyer C, Possemiers N, Wuyts FL, Vrints CJ. Combined endurance/resistance training reduces NT‐proBNP levels in patients with chronic heart failure. Eur Heart J 2004; 25: 1797–1805. [DOI] [PubMed] [Google Scholar]
- 12. Prabhu SD. Cytokine‐induced modulation of cardiac function. Circ Res 2004; 95: 1140–1153. [DOI] [PubMed] [Google Scholar]
- 13. Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science 1988; 242: 941–944. [DOI] [PubMed] [Google Scholar]
- 14. Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor‐alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation 1998; 97: 1392–1400. [DOI] [PubMed] [Google Scholar]
- 15. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des‐acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3‐kinase/AKT. J Cell Biol 2002; 159: 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 2001; 280: R1483–R1487. [DOI] [PubMed] [Google Scholar]
- 17. Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 2004; 110: 3674–3679. [DOI] [PubMed] [Google Scholar]
- 18. Markosfi MM, Carillo AE, Timmerman KL, Jennings K, Coen PM, Pence DB, Flynn MG. Exercise training modifies ghrelin and adiponectin concentrations and is related to inflammation in older adults. J Gerontol A Biol Sci Med Sci 2014; 69: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite‐sex twin study. Clin Endocrinol 2007; 66: 530–537. [DOI] [PubMed] [Google Scholar]
- 20. Gibas‐Dorna M, Nowak D, Piatek J, Pupek‐Musialik D, Krauss H, Kopczynski P. Plasma ghrelin and interleukin‐6 levels correlate with body mass index and arterial blood pressure in males with essential hypertension. J Physiol Pharmacol 2015; 66: 367–372. [PubMed] [Google Scholar]
- 21. Mao Y, Tokudome T, Kishimoto I, Otani K, Nishimura H, Yamaguchi O, Otsu K, Miyazato M, Kangawa K. Endogenous ghrelin attenuates pressure overload‐induced cardiac hypertrophy via a cholinergic anti‐inflammatory pathway. Hypertension 2015; 65: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 22. Von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol 2013; 45: 2257–2265. [DOI] [PubMed] [Google Scholar]
- 23. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 24. von Haehling S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 2015; 74: 367–377. [DOI] [PubMed] [Google Scholar]
- 25. Khatib MN, Shankar A, Kirubakaran R, Agho K, Simkhada P, Gaidhane S, Saxena D, B U, Gode D, Gaidhane A, Zahiruddin SQ. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: a systematic review and meta‐analysis. PLoS One 2015; 10: e0126697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med 2002; 80: 696–702. [DOI] [PubMed] [Google Scholar]
- 27. Ceconi C, Curello S, Bachetti T, Corti A, Ferrari R. Tumor necrosis factor in congestive heart failure: a mechanism of disease for the new millennium? Prog Cardiovasc Dis 1998; 41: 25–30. [DOI] [PubMed] [Google Scholar]
- 28. Smart NA, Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest Heart Fail 2011; 17: 110–114. [DOI] [PubMed] [Google Scholar]
- 29. Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta‐analysis of comparative studies. Int J Cardiol 2012; 162: 6–13. [DOI] [PubMed] [Google Scholar]
- 30. Lainscak M, Anker SD, von Haehling S. No train, no gain: does this apply to heart failure with preserved ejection fraction? Int J Cardiol 2013; 162: 69–70. [DOI] [PubMed] [Google Scholar]


