Abstract
Aims
The role of donor/recipient gender matching on the long‐term rejection process and clinical outcomes following heart transplantation (HT) outcomes is still controversial. We aim to investigate the impact of gender matching on early and long‐term outcome HT.
Methods and results
The study population comprised 166 patients who underwent HT between 1991 and 2013 and were prospectively followed up in a tertiary referral centre. Early and late outcomes were assessed by the type of donor–recipient gender match (primary analysis: female donor–male recipient [FD–MR, n = 36] vs. male donor–male recipient [MD–MR, n = 109]). Early mortality, need for inotropic support, length of hospital stay, and major perioperative adverse events did not differ between the FD–MR and MD–MR groups. However, the FD–MR group experienced significantly higher rates of early major rejections per patient as compared with the MD–MR group (1.2 ± 1.6 vs. 0.4 ± 0.8; P = 0.001), higher rates of overall major rejections (16 vs. 5.5 per 100 person years; P < 0.05), and higher rate of cardiac allograft vasculopathy (43% vs. 20%; P = 0.01). Kaplan–Meier survival analysis showed that the cumulative probabilities of survival free of rejections and major adverse events were significantly higher in MD–MR group (P = 0.002 and 0.001, respectively). Multivariate analysis showed that FD–MR status was associated with >2.5‐fold (P = 0.03) increase in the risk for rejections and with a >3‐fold (P = 0.01) increase in the risk for major adverse events during follow‐up.
Conclusions
Donor–recipient gender mismatch is a powerful independent predictor of early and late rejections and long‐term major adverse events following HT.
Keywords: Heart transplantation, Endomyocardial biopsies, Cardiac allograft vasculopathy, Donor, Recipient
Introduction
Heart transplantation (HT) is the gold standard curative therapy for selected patients with end stage heart failure. Over the last decades survival and outcomes of heart transplant recipients have markedly improved with an estimated median survival of 11 years, and 14 years for those who survive to the first year following transplantation.1 Factors contributing to the improved outcomes include improvement in surgical techniques, new immunosuppressive therapies, empowerment in the post‐transplant care, and improved surveillance. Yet, lack of donors is a major limiting factor rendering HT as a curative treatment. According to the 2014 SRTR and OPTN annual data report, of candidates listed in 2011, 55.9% underwent transplant during the first year on the waiting list, 30.2% were still waiting, and 7.2% had died. At 3 years, 9.2% had died, 12.6% had been removed from the list, 68.6% had undergone transplant, and 9.5% were still waiting.2 Thus, all efforts should be made in order to maximize the success rate; among these a proper match between the recipient and the donor is of major importance.
The impact of gender matching on the rejection process is still controversial. Studies which examined the impact of gender matching on the rejection process are scarce mostly referring to rejections only in the first year after transplantation. Some have shown gender mismatching to be associated with an increase in the rejection rate during the first year3, 4, 5 while others have not shown a significant effect.6, 7, 8 Similarly, data regarding the effect of gender matching on survival are conflicting, possibly because of inconsistent assessment of the risk for early and late mortality in previous studies.1, 3, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14
Accordingly, in the present study we aimed to comprehensively investigate the impact of gender matching on early and late outcomes following HT including a comprehensive analysis of the long‐term rejection process among patients enrolled in the Sheba Medical Center (SMC) HT Registry.
Methods
Study population and registry design
Our registry includes 215 patients who underwent HT between 1991 and 2013, and are being followed at the tertiary Heart Transplantation Clinic at SMC. Excluded from further analysis were nine children under the age of 10 and 10 additional patients who underwent HT in China between 2005 and 2007 and whose donors might have been executed prisoners, in accordance with current ethical guidelines of transplantation societies and journals.15, 16, 17, 18 Thirty additional patients transplanted abroad whose donors' gender is unknown were also excluded. Thus, the present study sample comprised 166 patients who were followed up at the SMC Heart Transplantation Clinic, of whom 91% were also transplanted at SMC.
Each patient's data were systematically recorded at enrollment and at each visit or medical contact. Clinical data were recorded on prospectively designed forms and included comprehensive information regarding the transplantation procedure, immunosuppression, rejections, and the occurrence of major cardiac events during long‐term follow‐up. Average follow‐up period of study patients was 9.6 ± 5.2 years (range 0–21). The study was approved by the Institutional Review Board at SMC.
Definition and endpoints
Immunosuppression
All patients were treated with triple‐drug immunosuppression regimen (cyclosporine or tacrolimus, azathioprine or mycophenolated mofetil, and corticosteroids). All patients also received induction therapy consisting of anti‐thymocyte globulin.
Rejection, surveillance, and treatment
Rejections were diagnosed by routine, or as clinically indicated, endomyocardial biopsies (EMB), classified according to the International Society for Heart and Lung Transplantation (ISHLT) classification system for rejection.19 Routine EMB were performed every week for 4 weeks after transplantation, twice per month in the second and third months, then once a month for the next 3 months, and every 3 months until the end of the first year. After the first year post‐transplant annual protocol biopsies were carried out.
Major rejection was defined as cellular grade 2R, 3R, and humoral AMR1 according to the 2004 revised classification, with appropriate matching of earlier biopsies to the 1990 ISHLT grading system.19 Also, any symptomatic acute cellular rejection irrespective of the ISHLT EMB grade was defined as major rejection episode.
Symptomatic acute cellular rejections were treated by steroid pulse therapy. Additional cytolytic immunosuppressive therapy with antithymocyte antibodies was administered if hemodynamic compromise was present or when no clinical improvement within 12 to 24 hours of pulse therapy administration was observed.
Asymptomatic major acute cellular rejection was also treated by high dose corticosteroids. Humoral rejection episodes were rare (3 in all) treated by plasmapheresis and intravenous immunoglobulin.
Cardiac allograft vasculopathy
Cardiac allograft vasculopathy (CAV) was diagnosed by coronary angiography performed annually (or earlier if clinically indicated). Significant graft coronary artery disease was defined angiographically as >50% stenosis in a major epicardial coronary artery.
Gender matching
Patients were categorized into four groups based on donor and recipient gender: male donor–male recipient (MD–MR, n = 109); female donor–male recipient (FD–MR, n = 36); male donor–female recipient (MD–FR, n = 14); and female donor–female recipient (FD–FR, n = 7). Because of the small number of women who underwent HT, the primary analysis was carried out among male recipients.
Outcome measures
The primary outcome measures of the present study included (i) the first and repeated occurrence of major rejections; (ii) the first occurrence of a major adverse event (see definition below); and (iii) all‐cause mortality.
Major adverse events included development of CAV, acute myocardial infarction or need for percutaneous coronary intervention, congestive heart failure, implantable pacemaker, stroke, new‐onset peripheral vascular disease, malignancy, and end stage renal disease.
Early outcomes were also assessed as a secondary outcome measures and included need for prolonged inotropic support, length of hospital stay, and major perioperative complications (sepsis, coagulopathy, acute renal failure, cerebrovascular insult, prolonged mechanical ventilation, early graft failure/dysfunction).
Statistical analysis
The clinical characteristics of study patients were compared by gender matching using the chi‐square test for categorical variables and the t‐test for continuous variables. Comparisons were carried out separately within the male and female recipient groups. The Kaplan–Meier estimator was used to assess the time to the first occurrence of each endpoint by the donor–recipient groups, and groups were compared using the log‐rank test.
Multivariable Cox proportional hazard regression analysis was used to evaluate the association between donor–recipient status and the first occurrence of endpoints during follow‐up. Covariates included in the multivariate models were identified using a best subset procedure among variables that were predictive of the endpoint and were unbalanced among the two groups (candidate covariates are listed in Tables 1 and 2).
Table 1.
Baseline characteristics of male recipients and comparison of donors and recipients
| Recipients from male donors n = 109 | Recipients from female donors n = 36 | P value† | |
|---|---|---|---|
| Age recipient (y) | 52 ± 11 | 48 ± 12 | 0.15 |
| Age donor (y) | 34 ± 14 | 36 ± 11 | 0.37 |
| P value* | <0.001 | <0.001 | |
| Weight recipient (kg) | 79 ± 14 | 70 ± 12 | 0.004 |
| Weight donor (kg) | 79 ± 12 | 69 ± 12 | <0.001 |
| P value* | 0.95 | 0.44 | |
| Height recipient (cm) | 174 ± 7 | 173 ± 8 | 0.22 |
| Height donor (cm) | 178 ± 7 | 167 ± 9 | <0.001 |
| P value* | <0.001 | 0.007 | |
| BMI recipient | 26 ± 4 | 24 ± 3 | 0.006 |
| BMI donor | 25 ± 3 | 25 ± 3 | 0.50 |
| P value* | 0.05 | 0.06 |
y, years; BMI, body mass index.
Values are mean ± SD.
For comparison of recipient and donor.
For comparison according to donor's gender.
Table 2.
Male recipients—pre heart transplantation baseline disease characteristics
| Recipients from male donors n = 109 | Recipients from female donors n = 36 | P value | |
|---|---|---|---|
| Aetiology of end stage heart failure | |||
| Ischemic heart disease (%) | 71 | 64 | 0.42 |
| Dilated cardiomyopathy (%) | 21 | 19 | 0.83 |
| Status of heart transplant waiting list candidates: | |||
| Status 1A (%) | 20 | 25 | 0.84 |
| Status 1B (%) | 42 | 39 | |
| Status 2 (%) | 38 | 36 | |
| ICD (%) | 23 | 22 | 0.96 |
| Assist device (%) | 20 | 14 | 0.39 |
| Creatinine (mg/dL) | 1.9 ± 4.9 | 1.5 ± 0.6 | 0.63 |
| Bilirubin (mg/dL) | 2.0 ± 6.5 | 1.3 ± 1.1 | 0.57 |
| Systolic pulmonary artery pressure (mmHg) | 55 ± 20 | 50 ± 20 | 0.36 |
| Diastolic pulmonary artery pressure (mmHg) | 27 ± 10 | 26 ± 12 | 0.55 |
| Mean pulmonary artery pressure (mmHg) | 38 ± 13 | 36 ± 14 | 0.41 |
| Pulmonary wedge pressure (mmHg) | 27 ± 10 | 25 ± 13 | 0.43 |
| Cardiac output (L/min) | 3.7 ± 1.1 | 3.2 ± 0.8 | 0.06 |
| Transpulmonary pressure gradient (mmHg) | 11.4 ± 7.5 | 11.1 ± 6.6 | 0.85 |
| Duration of hospitalization: from admission to transplantation (days) | 76 ± 134 | 39 ± 60 | 0.03 |
| PRA (mean, %) | 0.79 ± 4.9 | 1.08 ± 3.9 | 0.78 |
| Blood type | |||
| A (%) | 50 | 44 | 0.81 |
| AB (%) | 14 | 17 | |
| B (%) | 18 | 17 | |
| O (%) | 18 | 22 |
Values are mean ± SD, or percentage.
Data were analysed with SPSS software version 19.0. (SPSS Inc., Chicago, IL, USA).
A two‐sided 0.05 significance level was used for hypothesis testing.
Results
Clinical and perioperative characteristics of study patients by donor–recipient gender match
The 166 HT patients were categorized into four groups by type of donor–recipient match as follows: male donor–male recipient (MD–MR, n = 109), female donor–male recipient (FD–MR, n = 36), male donor–female recipient (MD–FR, n = 14), and female donor–female recipient (FD–FR, n = 7). Because of the small number of transplanted women (n = 21), this subgroup was excluded from the primary analysis. The clinical characteristics and outcomes of transplanted women by donor–recipient gender match are reported in Tables S1–S4 , Supporting Information.
The baseline characteristics of male recipients by gender match status are summarized in Table 1. The mean age of study patients was 51 ± 11 years and was not significantly different between male recipients of female or male hearts.
Donors were significantly younger than recipients in both groups, and mean age of donors did not differ between groups. Male recipients of male hearts were significantly shorter than donors, whereas the opposite trend was observed among male recipients of female hearts. Similar trends were shown for female recipients (shown in Table S1 ).
Table 2 summarizes baseline disease characteristics of male recipients prior to HT by donor sex. Aetiology for end stage heart disease was mostly ischemic in both groups. Creatinine prior to implantation did not differ between the two groups as well as mean serum bilirubin values. Furthermore, hemodynamic parameters, the need for inotropic support pre‐transplantation, and the use of assist device as bridge for transplantation were comparable between groups. Status of heart transplant waiting list candidates was equally dispersed between groups; however, duration of hospitalization prior to transplantation was shorter among patients who received heart from female—as compared with male—donors (39 ± 60 days vs. 76 ± 134 days, respectively, P = 0.028).
Operative and postoperative data are summarized in Table 3. The clinical characteristics of the two groups were also similar during operative and immediate post‐transplantation periods, including ischemic time, early post‐operative complications, or the need for prolonged inotropic support.
Table 3.
Male recipients—operative and postoperative data
| Recipients from male donors n = 109 | Recipients from female donors n = 36 | P value | |
|---|---|---|---|
| A. Early perioperative data | |||
| Ischemic time (min) | 163 ± 46 | 168 ± 57 | 0.81 |
| Early post‐operative complications (%) | 47 | 56 | 0.30 |
| Prolonged ionotropics support (%) | 35 | 46 | 0.16 |
| Days from transplantation to discharge | 21 ± 20 | 26 ± 23 | 0.07 |
| Days from admission to discharge (total length of stay) | 98 ± 138 | 66 ± 64 | 0.23 |
| In‐hospital death (%) | 21 | 22 | 0.53 |
| Cause of in‐hospital death* (%) | |||
| Early graft failure | 26 | 63 | |
| Coagulopathy/Infection | 70 | 37 | |
| Malignancy | 0 | 0 | |
| CVA | 4 | 0 | |
| B. Long‐term data | |||
| Follow‐up adverse events (%) | 32 | 61 | 0.003 |
| Heart failure (%) | 11 | 36 | 0.003 |
| Stroke (%) | 13 | 21 | 0.26 |
| Acute myocardial infarction (%) | 5 | 18 | 0.03 |
| End stage renal failure (%) | 5 | 29 | 0.001 |
| Heart retransplantation (%) | 2 | 4 | 0.75 |
| Cardiac allograft vasculopathy (%) | 20 | 43 | 0.01 |
| Pacemaker (%) | 4 | 11 | 0.16 |
| Malignancy (%) | 18 | 14 | 0.73 |
| New onset peripheral vascular disease (%) | 22 | 39 | 0.06 |
| Death during follow‐up (%) | 21 | 33 | 0.12 |
| Cause of death during follow‐up* (%) | |||
| Late graft failure | 26 | 33 | |
| Coagulopathy/infection | 30 | 25 | |
| Malignancy | 22 | 25 | |
| CVA | 22 | 17 |
Values are mean ± SD, or percentage. CVA; cerebrovascular accident.
Data are shown for descriptive purposes without a statistical comparison because of small numbers.
The corresponding findings regarding perioperative characteristics and outcomes are shown in Tables S2 and S3 .
Risk of rejections and major cardiac events during follow‐up by donor–recipient gender match
Early rejection rates were significantly more frequent in the FD–MR group (Figure 1 A). During the first 3 months after transplantation mean major rejections per patient were 0.7 ± 0.9 for FD–MR vs. 0.3 ± 0.6 for MD–MR (P = 0.001). During the next 9 months (3–12 months after transplantation) major rejections per patient were also more common in the FD–MR group (0.52 ± 0.9 vs. 0.09 ± 0.3 for MD–MR; P = 0.001).
Figure 1.
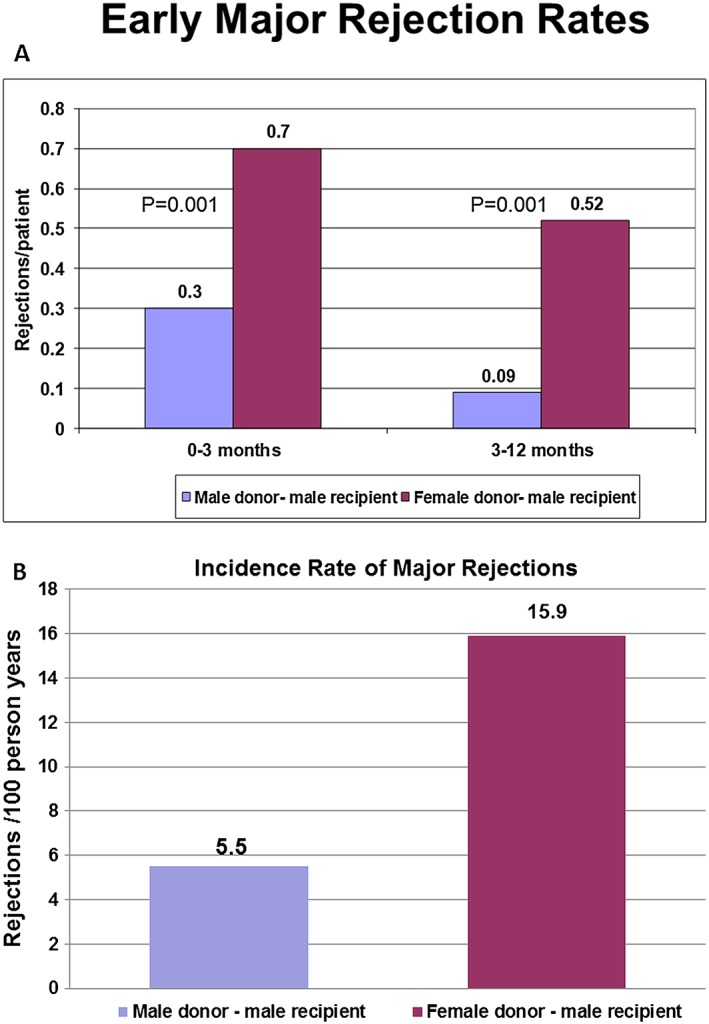
(A) Early major rejection rates. Rate of major rejection episodes at time intervals according to frequency of endomyocardial biopsies sampled. Major rejections were more frequent in the female donor–male recipient group. (B) Incidence rate of major rejections. Incidence rate of major rejection episodes during the follow‐up according to donor's gender. Major rejections were more frequent in the female donor–male recipient group.
When the overall follow‐up rate of major rejections was assessed, male recipients who received female hearts were shown to have nearly a three‐fold higher rate of rejections during follow‐up (16 rejections per 100 person‐years) compared with male recipients who received male hearts (5.5 rejections per 100 person‐years; Figure 1 B).
Similarly, the rate of CAV was >2‐fold higher in the female donor group as compared with the male donor group (43% vs. 20% P = 0.01).
Consistent with those findings, the Kaplan–Meier cumulative survival free of major rejections was significantly higher in MD–MR group as compared with the FD–MR group (5 year rates: 72% vs. 50%, respectively; 10 year rates: 69% vs. 29%, respectively; P = 0.002 for the overall difference during follow‐up; Figure 2 A).
Figure 2.
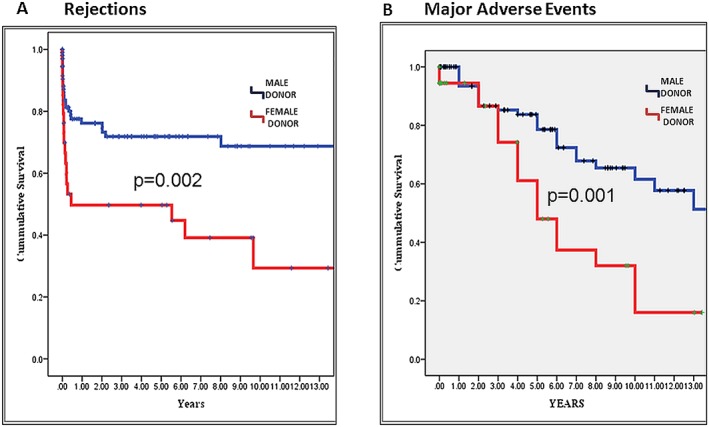
Kaplan–Meier survival curves for rejections and major adverse events. Illustrates (A) probability of cumulative survival free of rejections according to donor's gender (male—blue; female—red) and (B) probability of cumulative survival free of major adverse events according to donor's gender (male—blue; female—red).
Corresponding to the findings relating to rejection rates, significantly higher rates of major adverse events were also observed in the female donor—as compared with the male donor—group (61% vs. 32% P = 0.003 [Table 3]). Thus, the cumulative survival free of major adverse events was significantly higher in MD–MR group compared with the FD–MR groups (5 year rates: 84% vs. 62%, respectively; 10 year rates: 48% vs. 16%, respectively; P = 0.001 for the overall difference during follow‐up, P = 0.001 for the overall difference during follow‐up; Figure 2 B).
Notably, similar trends were shown when specific adverse events during follow‐up were assessed, demonstrating that the development of heart failure was more frequent in men who received female hearts (36% vs. 11%, P = 0.003, respectively) as well as acute myocardial infarction (18% vs. 5% P = 0.031, respectively) and development of end staged renal failure (29% vs. 5%, P = 0.001, respectively).
Multivariate analysis
Results from the multivariate models for the major rejection and adverse events endpoints among male recipients are shown in Table 4. Multivariate analysis showed that female donor was the most powerful predictor of adverse outcomes among study patients, and was associated with >2.5‐fold (P = 0.03) increase in the risk for rejection (Table 4) and a >3‐fold (P = 0.01) increase in the risk for major adverse events during follow‐up (Table 4). Increased transpulmonic gradient (TPG) was also shown to be associated with increased risk for adverse long‐term outcomes (Table 4), whereas the recipient's age, BMI, or length of hospital was not associated with a statistically significant risk increase after adjustment for donor sex and TPG.
Table 4.
Multivariate analysis—risk factors for rejections and major adverse events*
| Rejections | Major adverse events | |||||
|---|---|---|---|---|---|---|
| Risk factor | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value |
| Donor's sex | 2.54 | 1.09–5.93 | 0.03 | 3.27 | 1.29–8.25 | 0.01 |
| TPG (per 1SD = 7 mmHg) | 1.22 | 0.83–1.80 | 0.32 | 1.76 | 1.23–2.53 | 0.002 |
| BMI recipient | 1.07 | 0.97–1.12 | 0.17 | 1.07 | 0.97–1.18 | 0.19 |
Findings are further adjusted for age of recipient, length of hospital stay.
CI: confidential interval; BMI: body mass index; TPG: transpulmonary pressure gradient.
Risk of death by donor–recipient gender match
Risk of death by donor–recipient gender match did not differ between groups as assessed by Kaplan–Meier analysis (10 year survival rates: 56% and 57% among recipients from male and female donors, respectively; P = 0.94). Cause of death was not affected by gender matching (Table 3).
Discussion
To our knowledge the present study is the first to provide comprehensive data regarding specific early and late outcomes following HT by recipient–donor gender matching, as well as detailed report of the effect on the rejection process, including long‐term serial EMB pathological specimens. Our findings have several important implications that can be used for improved risk assessment among patients undergoing HT. We have shown that (i) male recipients who receive female hearts experience a pronounced and significant increase in the risk for early and late major rejections, with a corresponding increase in CAV; and (ii) donor–recipient gender mismatch was also shown to be a powerful and independent predictor of major adverse events during long‐term follow‐up, including, higher rates of heart failure and end stage renal failure, probably related to the significant increase in rejection rate. Our data suggest that recipient–donor gender mismatching should be avoided whenever possible among patients undergoing HT in order to improve long‐term outcomes.
Recipient–donor gender mismatch and the risk of major rejections
Previous studies which examined the impact of gender matching on the rejections rates after cardiac transplantation are scarce, mostly including rejections only in the first year after transplantation. In a study reviewing data for 57 sex‐mismatched and 179 sex‐matched men who underwent HT between 1990 and 2002,3 median survival was significantly shorter in the sex‐mismatched group (8.1 vs.12.9 years). Recipients of female hearts experienced more often clinically relevant episodes of cellular rejection during the first 3 months post transplantation.
In a study by Prendergast and colleagues4 overall donor–recipient gender mismatching significantly increased the number of rejection episodes and reduced survival in the first year after HT. More specifically, among female recipients, donor–recipient gender mismatching significantly increased the number of rejection episodes in the first year after transplantation.
In contrast to these findings, in the study conducted by Al‐Khaldi et al. 6 freedom from treated rejection was not different between recipients of male or female donors' hearts. Similar results were found in the Collaborative Transplant Study where no significant effect of donor gender on the proportion of patients treated for rejections episodes was noted. Yet, significantly higher frequency was found in females irrespective of donor gender.7
Significantly increased rejection rates might be the key to understanding the poor long‐term outcomes observed in the sex‐mismatched group.
CAV, commonly referred to as chronic rejection, is a complex disease entity and represents the leading cause of mortality after the first year of transplantation. Stoica and colleagues20 have shown that the onset of CAV was significantly increased by cumulative number of moderate/severe rejections. This is supported by the findings of a pioneer study which revealed that male recipients of female allografts have a higher degree of vascular intimal hyperplasia detected by intravascular ultrasound at 1 year after HT.21
Our study further establishes, by multivariate analysis, female donor as a major risk factor for rejections, with >2‐fold higher rates of CAV in the female donor group.
These findings suggest a possible mechanism based on the theory that presumes that the female donor organ has greater antigenicity which leads to more frequent acute and chronic rejections.3 Minor histocompatibility antigens present on the Y chromosome and antibody development during pregnancy may predispose female recipients of male donors to higher incidence of rejection.9 Women may develop blood group antibodies as a consequence of the immunologic reaction to the paternal antigens in the fetus, as such, half of pregnancies result in development of anti‐human leukocyte antigen (HLA) antibodies.22 This possible mechanism is supported by a study by Lietz et al., who showed that female recipients had increased pre‐transplant immunoreactivity (as manifested by higher prevalence of HLA‐B8 and DR3 haplotypes and antinuclear antibodies) and significantly shorter durations to first rejection, further rejection episodes, and earlier production of anti‐HLA antibodies post‐transplant.23
Recipient–donor gender mismatch and the risk for major adverse events
The influence of gender matching on the HT outcomes is still controversial and has been examined by several pioneering studies. According to the ISHLT registry1 female donor is a risk factor for 5 year mortality. On the other hand, male donor is found as a significant risk factor for development of CAV within 8 years. In a study conducted by Solomon and colleagues, who evaluated 137 consecutive heart transplants, female donor was identified as a statistically significant risk factor for early post‐operative low cardiac output and intra aortic balloon pump insertion, but multivariate analysis failed to demonstrate this as a risk factor for early mortality.24 However, in an earlier study, female donor was identified, by multivariate analysis, as a significant risk factor for death after transplantation.25
Other studies have suggested that it may well be the interplay between the gender of donor and recipient (rather than the sex per se) that most influences outcomes.11 According to the ISHLT registry1 MR/FD vs. MR/MD was found as a borderline significant risk factor for 10 year mortality but significant risk factor for 15 year mortality. A study that evaluated the effect of donor gender on early mortality (30 days) did not find that gender mismatch negatively affects early survival.13 Al‐Khaldi and colleagues have reported that male recipients of female hearts had reduced long‐term survival compared with those who received male donors' hearts. Donor gender had no effect on long‐term survival in male recipients < 45 years of age and female recipients. Actuarial freedom from treated rejection was not different between recipients of male or female donor hearts.6
An analysis of data from 25 432 heart transplant (4159 female and 21 273 male) recipients from the Collaborative Transplant Study suggests that cardiac transplants from female donors had significantly inferior actuarial survival in male recipients, whereas no difference according to donor gender was demonstrable in female recipients.7
Weiss and colleagues, using the United Network for Organ Sharing, containing information on more than 18 000 heart‐transplant recipients11 have shown that men who received organs from male donors had the highest cumulative survival at 5 years (74.5%), while men receiving female hearts had a 15% increase in the risk of adjusted cumulative mortality. No significant increase in the relative hazard for death occurred for women receiving opposite sex donor organs. According to this report, female recipients, irrespective of donor sex, had 3.6% lower overall survival at 5 years post‐transplant. The combination that MR/FD carries a higher risk for early mortality is also supported in the study of analysis of 67 855 heart transplants published by Kaczmarek et al. 14
Khush and colleagues, who studied 60 584 adult recipients from the ISHLT registry, have also supported that male recipients of female allografts had a 10% increase in adjusted mortality relative to male recipients of male allografts. However, differences in the effect of donor sex on acute rejection or CAV between male and female recipients were not significant, yet the authors stress that these analyses were limited by the large amount of missing data on these outcomes.8
Hence, our study further supports those studies which have previously shown that male recipients of female hearts have the poorest long‐term outcomes, while providing for the first time comprehensive information on the early and late outcomes, including a detailed report of the effect on the rejection process over a long follow‐up period.
Limitations
Retrospective analysis of single centre registry data despite comprehensive data collection; unable to assess outcomes by donor sex among female recipients because of sample size limitations.
Conclusions
Our data suggest that gender per se and gender matching impact major outcomes following HT. Because of shortage of donors, and life‐threatening situation of recipients, complete avoidance of gender mismatch is unrealistic. Yet, knowing the limitation of gender mismatching and predicting clinical implications can help in future patients' care management.
Conflict of interest
None declared.
Supporting information
Table S1. Baseline characteristics of female recipients and comparison of donors and recipients.
Table S2. Female recipients—pre heart transplantation baseline disease characteristics.
Table S3. Operative and postoperative data.
Table S4. Rejections.
Supporting Information: Because of the small number of transplanted women (n = 21), this subgroup was excluded from the primary analysis. The clinical characteristics and outcomes of transplanted women by donor–recipient gender match are reported in the Supplementary Appendix Tables 1–4.
Peled, Y. , Lavee, J. , Arad, M. , Shemesh, Y. , Katz, M. , Kassif, Y. , Asher, E. , Elian, D. , Har‐Zahav, Y. , Goldenberg, I. , and Freimark, D. (2017) The impact of gender mismatching on early and late outcomes following heart transplantation. ESC Heart Failure, 4: 31–39. doi: 10.1002/ehf2.12107.
The copyright line for this article was changed on 14 November 2016 after original online publication
References
- 1. Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty‐eighth Adult Heart Transplant Report—2011. J Heart Lung Transplant 2011; 10: 1078–1094. [DOI] [PubMed] [Google Scholar]
- 2. Colvin M, Smith JM, Skeans MA, Edwards LB, Callahan ER, Snyder JJ, Israni AK, Kasiske BL. Heart. Am J Transplant 2016; 16(Suppl 2): 115–140. [DOI] [PubMed] [Google Scholar]
- 3. Welp H, Spieker T, Erren M, Scheld HH, Baba HA, Stypmann J. Sex mismatch in heart transplantation is associated with increased number of severe rejection episodes and shorter long‐term survival. Transplant Proc 2009; 41: 2579–2584. [DOI] [PubMed] [Google Scholar]
- 4. Prendergast TW, Furukawa S, Beyer AJ III, Browne BJ, Eisen HJ, Jeevanandam V. The role of gender in heart transplantation. Ann Thorac Surg 1998; 65: 88–94. [DOI] [PubMed] [Google Scholar]
- 5. Reed RM, Netzer G, Hunsicker L, Mitchell BD, Rajagopal K, Scharf S, Eberlein M. Cardiac size and sex‐matching in heart transplantation: size matters in matters of sex and the heart. JACC Heart Fail 2014; 2: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Khaldi A, Oyer PE, Robbins RC. Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant 2006; 25: 461–468. [DOI] [PubMed] [Google Scholar]
- 7. Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol 2002; 13: 2570–2576. [DOI] [PubMed] [Google Scholar]
- 8. Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant 2012; 31: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kittleson MM, Shemin R, Patel JK, Ardehali A, Kawano M, Davis S, Moriguchi JD, Kobashigawa JA. Donor–recipient sex mismatch portends poor 10‐year outcomes in a single‐center experience. J Heart Lung Transplant 2011; 30: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 10. Tsai FC, Marelli D, Bresson J, Gjertson D, Kermani R, Ardehali A, Esmailian F, Hamilton M, Fonarow GC, Moriguchi J, Plunkett M, Hage A, Tran J, Kobashigawa JA, Laks H. Recent trends in early outcome of adult patients after heart transplantation: a single‐institution review of 251 transplants using standard donor organs. Am J Transplant 2002; 2: 539–545. [DOI] [PubMed] [Google Scholar]
- 11. Weiss ES, Allen JG, Patel ND, Russell SD, Baumgartner WA, Shah AS, Conte JV. The impact of donor–recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail 2009; 2: 401–408. [DOI] [PubMed] [Google Scholar]
- 12. Patel ND, Weiss ES, Nwakanma LU, Russell SD, Baumgartner WA, Shah AS, Conte JV. Impact of donor‐to recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation 2008; 118(14 Suppl): S83–S88. [DOI] [PubMed] [Google Scholar]
- 13. Izquierdo MT, Almenar L, Martínez‐Dolz L, Moro J, Agüero J, Sánchez‐Lázaro I, Cano O, Ortiz V, Sánchez R, Salvador A. Analysis of the impact of donor gender on early mortality. Transplant Proc 2007; 39: 2375–2376. [DOI] [PubMed] [Google Scholar]
- 14. Kaczmarek I, Meiser B, Beiras‐Fernandez A, Guethoff S, Überfuhr P, Angele M, Seeland U, Hagl C, Reichart B, Eifert S. Gender does matter: gender‐specific outcome analysis of 67,855 heart transplants. Thorac Cardiovasc Surg 2013; 61: 29–36. [DOI] [PubMed] [Google Scholar]
- 15. Caplan AL, Rockman HA, Turka LA. Editorial position on publishing articles on human organ transplantation. J Clin Invest 2012; 122: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO . Draft guiding principles on human organ transplantation. WHO web site. http://www.who.int/ethics/topics/transplantation_guiding_principles/en/index1.html (August 7 2011).
- 17. WMA . WMA council resolution on organ donation in China. World Medical Association web site. http://www.wma.net/en/30publications/10policies/30council/cr_5/ (August 7 2011).
- 18. Lavee J, West LJ. A call for a policy change regarding publications based on transplantation of organs from executed prisoners. J Heart Lung Transplant 2012; 31: 555–562. [DOI] [PubMed] [Google Scholar]
- 19. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu‐Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24: 1710–1720. [DOI] [PubMed] [Google Scholar]
- 20. Stoica SC, Cafferty F, Pauriah M, Pauriah M, Taylor CJ, Sharples LD, Wallwork J, Large SR, Parameshwar J. The cumulative effect of acute rejection on development of cardiac allograft vasculopathy. J Heart Lung Transplant 2006; 25: 420–425. [DOI] [PubMed] [Google Scholar]
- 21. Mehra MR, Stapleton DD, Ventura HO, Escobar A, Cassidy CA, Smart FW, Collins TJ, Ramee SR, White CJ. Influence of donor and recipient gender on cardiac allograft vasculopathy. An intravascular ultrasound study. Circulation 1994; 90: II78–82. [PubMed] [Google Scholar]
- 22. Csete M. Gender issues in transplantation. Anesth Analg 2008; 107: 232–238. [DOI] [PubMed] [Google Scholar]
- 23. Lietz K, John R, Kocher A, Schuster M, Mancini DM, Edwards NM, Itescu S. Increased prevalence of autoimmune phenomena and greater risk for alloreactivity in female heart transplant recipients. Circulation 2001; 104(suppl I:I‐177–I‐183). [DOI] [PubMed] [Google Scholar]
- 24. Solomon NA, McGiven JR, Alison PM, Ruygrok PN, Haydock DA, Coverdale HA, West TM. Changing donor and recipient demographics in a heart transplantation program: influence on early outcome. Ann Thorac Surg 2004; 77: 2096–2102. [DOI] [PubMed] [Google Scholar]
- 25. McCarthy JF, McCarthy PM, Massad MG, Cook DJ, Smedira NG, Kasirajan V, Goormastic M, Hoercher K, Young JB. Risk factors for death after heart transplantation: does a single‐center experience correlate with multicenter registries? Ann Thorac Surg 1998; 65: 1574–1578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of female recipients and comparison of donors and recipients.
Table S2. Female recipients—pre heart transplantation baseline disease characteristics.
Table S3. Operative and postoperative data.
Table S4. Rejections.
Supporting Information: Because of the small number of transplanted women (n = 21), this subgroup was excluded from the primary analysis. The clinical characteristics and outcomes of transplanted women by donor–recipient gender match are reported in the Supplementary Appendix Tables 1–4.


