Abstract
Aims
The randomized clinical trial RELAX‐AHF demonstrated a positive effect of vasodilator therapy with serelaxin in the treatment of AHF patients. The aim of our study was to compare clinical characteristics and outcomes of patients from the AHEAD registry who met criteria of the RELAX‐AHF trial (relax‐comparable group) with the same characteristics and outcomes of patients from the AHEAD registry who did not meet those criteria (relax‐non‐comparable group), and finally with characteristics and outcomes of patients from the RELAX‐AHF trial.
Methods and results
A total of 5856 patients from the AHEAD registry (Czech registry of AHF) were divided into two groups according to RELAX‐AHF criteria: relax‐comparable (n = 1361) and relax‐non‐comparable (n = 4495). As compared with the relax‐non‐comparable group, patients in the relax‐comparable group were older, had higher levels of systolic and diastolic blood pressure, lower creatinine clearance, and a higher number of comorbidities. Relax‐comparable patients also had significantly lower short‐term as well as long‐term mortality rates in comparison to relax‐non‐comparable patients, but a significantly higher mortality rate in comparison to the placebo group of patients from the RELAX‐AHF trial. Using AHEAD score, we have identified higher‐risk patients from relax‐comparable group who might potentially benefit from new therapeutic approaches in the future.
Conclusions
Only about one in five of all evaluated patients met criteria for the potential treatment with the new vasodilator serelaxin. AHF patients from the real clinical practice had a higher mortality when compared with patients from the randomized clinical trial.
Keywords: Acute heart failure, Mortality, Blood pressure, Vasodilator therapy, AHEAD registry
Introduction
Acute heart failure is still associated with a high hospital mortality, which ranges between 4.0% and 12.7%.1, 2, 3, 4 Emergency physicians, cardiologists, intensivists, nurses, and other healthcare providers have to cooperate to provide optimal benefit. However, many treatment decisions are opinion‐based, while relatively few are evidence‐based. According to the consensus paper on acute heart failure published in 2015, the recommendation for intravenous vasodilator therapy is that it might be administered as an initial therapy for a symptomatic relief in cases when systolic blood pressure (BP) is normal to high (≥110 mmHg). Alternatively, sublingual nitrates may be considered.5
Intravenous vasodilators are the second most commonly used agents in AHF patients. Their use was shown to be associated with a lower mortality, while a delay in administration was associated with a higher mortality.6 Randomized controlled trial (RCT) evidence comparing the clinical outcomes of intravenous vasodilators (especially nitrates) in AHF patients is rather limited, and its methodological quality is relatively low. However, there is a consensus that intravenous vasodilators should be indicated in AHF with rather normal to high bood presure7 and should not be indicated in patients with SBP <110 mmHg.5
Recent Phases III and IV clinical trials have provided promising results with respect to potential management of acute heart failure. These include trials RELAX‐AHF (serelaxin),8 ATOMIC‐AHF trial (omecantiv mecarbil),9 PRONTO (clevidipine),10 TRUE‐AHF (ularitide)11 and ARTS‐HF (finerenone).12
The aim of the analysis was to compare the mortality rates of real‐world AHF patients from the Czech AHEAD registry1, 13 with the published mortality rates of patients from the RELAX‐AHF trial. For the purpose of analysis, we had to divide the real‐world population of Czech AHF patients into two groups: the relax‐comparable group (i.e. population of AHF patients in the AHEAD registry meeting the RELAX‐AHF criteria8) and the relax‐non‐comparable group (the remaining population of AHF patients in the AHEAD registry, not meeting those criteria). Furthermore, we divided the relax‐comparable group according to a recently published risk scoring system AHEAD14 in order to demonstrate that this simple tool can identify high‐risk AHF patients who might potentially benefit from new therapeutic approaches in the future.
Methods
The study protocol complied with the Declaration of Helsinki and was approved by the Multicentre Ethics Committee of University Hospital Brno (Brno, Czech Republic). Written informed consent was obtained from all subjects to participate in the study.
The Acute Heart Failure Database (AHEAD) network registry was described previously.1, 13 In short, the registry includes consecutive patients from 10 centres with 24 h catheterization laboratory services and a centralized care for patients with acute coronary syndromes (previously described as ‘AHEAD Main’) and five regional centres without a catheterization laboratory; nevertheless, the capacity of catheterization laboratories is sufficient to cover the entire population of the Czech Republic (over 10 million inhabitants). The following inclusion criteria were in accordance with the European guidelines for AHF issued in 2005: signs and symptoms of AHF, confirmed left‐ventricular dysfunction (systolic or diastolic) and/or positive response to therapy.15 At the time of analysis, the AHEAD registry included 7318 hospitalizations for AHF; out of which, 6242 were first hospitalizations for AHF (for a given patient during the evaluated period of the AHEAD registry); the dataset of first hospitalizations for AHF with available key variables for the analysis of comparability with the RELAX‐AHF trial included n = 5856 patients (Figure 1).
Figure 1.
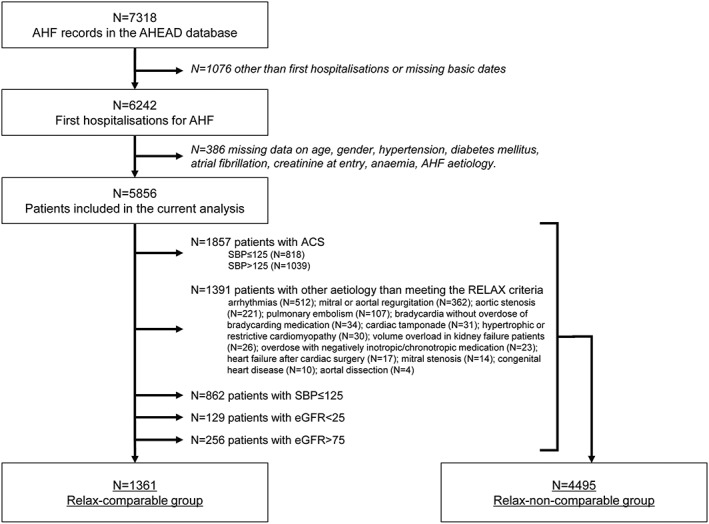
Analysis flowchart.
RELAX‐AHF was an international, double‐blind, placebo‐controlled trial, enrolling patients admitted to hospital for acute heart failure who were randomly assigned to standard care plus 48 h intravenous infusions of placebo or serelaxin. All patients had dyspnoea, congestion on chest radiograph, increased brain natriuretic peptide (BNP) or N‐terminal prohormone of BNP, mild‐to‐moderate renal insufficiency, and systolic BP greater than 125 mmHg. The 6 month mortality was 7.3% in the serelaxin group and 11.3% in the placebo group (P = 0.02).8
According to the RELAX‐AHF criteria, the evaluated cohort of 5856 AHF patients from the AHEAD registry was divided into two groups: the relax‐comparable group and the relax‐non‐comparable group. Exclusion criteria for the relax‐comparable group included acute coronary syndrome, specific aetiology of AHF (arrhythmias, severe form of valvular heart disesase, e.g. mitral or aortal regurgitation or stenosis, with severe bradycardia without overdose of beta‐blockers, cardiac tamponade, hypertrophic, or restrictive cardiomyopathy, volume overload in kidney failure patients, overdose with negatively inotropic/chronotropic medication, heart failure after cardiac surgery and those with aortal dissection), systolic BP ≤ 125 mmHg, eGFR < 25 ml/min/1.73 m2, and eGFR > 75 ml/min/1.73m2. Data were collected prospectively and evaluated continuously between September 2006 and October 2012. Mortality data were obtained from a centralized database of the Ministry of Health of the Czech Republic: the 30‐day mortality was 13.7%, and the 1‐year mortality 32.3%.
In order to identify the subgroup of higher‐risk patients in the relax‐comparable group, who might potentially benefit from new therapeutic approaches in future, we divided this group of patients according to AHEAD, a recently published scoring14 system. This score is based on the presence of comorbidities that worsen the patient's prognosis: the patient obtains one point for the presence of each comorbidity, and the value of AHEAD score can thus range between 0 and 5. These monitored comorbidities are as follows: A—atrial fibrillation, anaemia (H—haemoglobin level lower than 130 g/L for men and 120 g/L for women), E—elderly (age ≥70 years), A—abnormal renal parameters (creatinine ≥130 µmol/L) and D—diabetes mellitus. Standard descriptive statistics were applied in the analysis: absolute and relative frequencies were used for categorical variables, median values supplemented with 5th–95th percentile range (or mean and standard deviation) were used for continuous variables. The statistical significance of differences between data from the AHEAD registry and published data from the RELAX‐AHF trial was computed using t‐test for continuous variables and chi‐square test for categorical variables. The survival of patients was described and visualized using the Kaplan–Meier methodology. The analysis was performed with the use of SPSS 22.0.0.1 (IBM Corporation, 2014).
Results
The evaluated cohort (n = 5856) of AHF patients was divided according to the RELAX‐AHF criteria into two groups: the relax‐comparable group and the relax‐non‐comparable group. The baseline characteristics of both groups of patients recorded in the AHEAD registry compared with the published characteristics of the placebo group of patients from the RELAX‐AHF trial are shown in Table 1.
Table 1.
Baseline characteristics of two AHEAD groups (relax‐comparable and relax‐non‐comparable) compared to the RELAX‐AHF placebo group
| Characteristics | AHEAD Relax‐non‐comparable (n = 4495) | AHEAD Relax‐comparable (n = 1361) | P | RELAX—placebo (n = 580) | P (AHEAD Relax‐non‐comparable) | P (AHEAD Relax‐comparable) |
|---|---|---|---|---|---|---|
| Age (years) | 71.6 (12.3) | 74.6 (10.8) | <0.001 | 72.5 (10.8) | 0.093 | <0.001 |
| Men | 2616 (58.2%) | 702 (51.6%) | <0.001 | 357 (62.0%) | 0.123 | <0.001 |
| BMI | 28.4 (5.3) | 29.4 (5.8) | <0.001 | 29.5 (6.1) | <0.001 | 0.732 |
| Systolic BP (mmHg) | 132.2 (32.0) | 162.1 (28.5) | <0.001 | 142.1 (17.0) | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 77.9 (17.3) | 91.1 (16.4) | <0.001 | 81.7 (13.2) | <0.001 | <0.001 |
| Heart rate (min−1) | 93.5 (27.5) | 92.0 (24.7) | 0.064 | 80.4 (14.9) | <0.001 | <0.001 |
| Ejection fraction (%) | 38.9 (14.8) | 38.7 (14.6) | 0.717 | 38.6 (14.3) | 0.645 | 0.890 |
| Ejection fraction ≤40% | 2462 (58.7%) | 739 (59.9%) | 0.457 | 295 (55.0%) | 0.105 | 0.057 |
| Creatinine clearance ml/min/1.73 m2 | 54.4 (24.7) | 50.6 (12.8) | <0.001 | 53.3 (12.9) | 0.292 | <0.001 |
| Medical history | ||||||
| Hypertension | 3267 (72.7%) | 1148 (84.3%) | <0.001 | 510 (88.0%) | <0.001 | 0.038 |
| Stroke | 771 (17.2%) | 321 (23.6%) | <0.001 | 84 (14.0%) | 0.049 | <0.001 |
| Smoking | 879 (41.9%) | 165 (34.7%) | 0.004 | 81 (14.0%) | <0.001 | <0.001 |
| PAD | 647 (14.4%) | 235 (17.3%) | 0.010 | 82 (14.0%) | 0.792 | 0.070 |
| Ischaemic heart disease | 2489 (55.4%) | 762 (56.0%) | 0.689 | 307 (53.0%) | 0.280 | 0.230 |
| Atrial fibrillation | 1359 (30.2%) | 439 (32.3%) | 0.157 | 305 (53.0%) | <0.001 | <0.001 |
| COPD | 859 (19.1%) | 377 (27.7%) | <0.001 | 88 (15.0%) | 0.016 | <0.001 |
| Diabetes mellitus | 1939 (43.1%) | 664 (48.8%) | <0.001 | 272 (47.0%) | 0.076 | 0.465 |
| Medication at admission | ||||||
| ACE inhibitors | 2000 (46.7%) | 736 (55.0%) | <0.001 | 320 (55.0%) | <0.001 | 0.992 |
| ARBs | 500 (11.7%) | 193 (14.4%) | 0.007 | 97 (17.0%) | <0.001 | 0.149 |
| Beta‐blockers | 2132 (49.8%) | 804 (60.1%) | <0.001 | 407 (70.0%) | <0.001 | <0.001 |
| Spironolactone | 966 (22.6%) | 314 (23.5%) | 0.481 | 173 (30.0%) | <0.001 | 0.003 |
| Digoxin | 717 (16.8%) | 270 (20.2%) | 0.004 | 108 (19.0%) | 0.187 | 0.555 |
ACE, angiotension converting enzyme; ARBs, angiotensin‐2 receptor blockers; BP, blood pressure; COPD, chronic obstructive pulmonary disease; PAD, Peripheral arterial disease.
As compared with the relax‐non‐comparable group, patients in the relax‐comparable group were older, had higher systolic, and diastolic blood pressure at admission and lower calculated creatinine clearance. They had a higher number of comorbidities in their history, such as hypertension, stroke, or TIA, peripheral artery disease, COPD, and diabetes mellitus. At admission, they were more frequently treated by angiotensin‐converting enzyme inhibitors, angiotensin‐2 receptor blockers, beta‐blockers, and digoxin.
As compared with the placebo group of patients from the RELAX‐AHF trial (which was comparable with the seralaxin‐treated group8), patients in the relax‐comparable group were older, had higher systolic, and diastolic blood pressure, and higher heart rate; in terms of medical history, they had less frequently hypertension and atrial fibrillation but more frequently stroke or TIA, smoking, and COPD. At admission, patients from the relax‐comparable group were less frequently treated with beta‐blockers and spironolactone.
The Kaplan–Meier survival estimation (as shown in Figure 2) demonstrates a significantly higher mortality of the relax‐non‐comparable group as compared with the relax‐comparable group. However, the 180‐day mortality of the relax‐comparable group is significantly higher in comparison with both RELAX‐AHF groups (patients treated with seralaxin and placebo).
Figure 2.
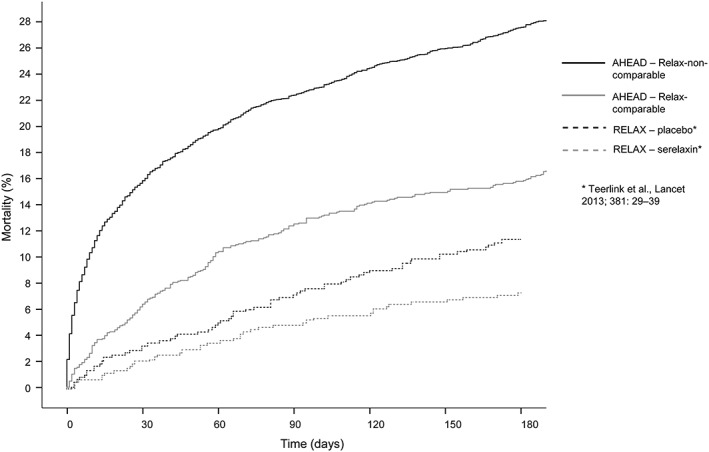
Mortality of two groups of patients from Acute Heart Failure Database (AHEAD) registry (relax‐comparable and relax‐non‐comparable) compared to mortality in the RELAX‐AHF study.
According to the systolic blood pressure at admission, patients from both groups of the AHEAD registry were divided into six groups. In the relax‐non‐comparable group, the highest mortality was associated with BP < 110 mmHg. Mortality in the relax‐non‐comparable group was consistently higher across all systolic blood pressure categories in comparison with the relax‐comparable group (Figure 3).
Figure 3.
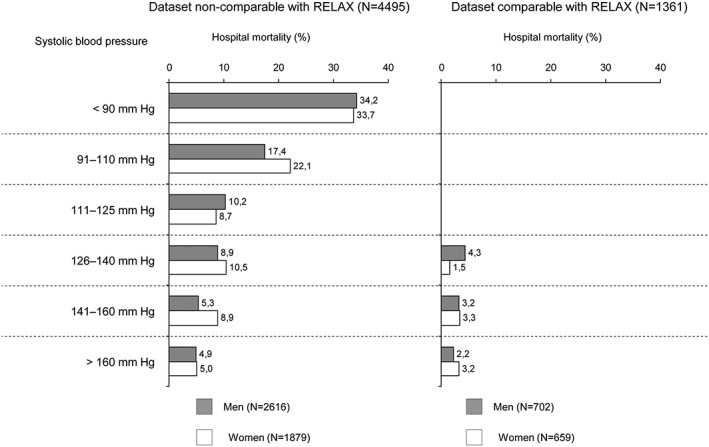
Systolic blood pressure as a risk predictor for hospital mortality in Acute Heart Failure Database (AHEAD) registry.
Overall, 33.8% of patients in the relax‐comparable group were treated with intravenous vasodilators (VD), namely nitrates. The hospital mortality of patients without VD therapy and with VD therapy was 3.1% and 2.9%, respectively, the 1‐month mortality was 7.1% and 6.1%, respectively, and the 1‐year mortality was 23.9% and 26.1%, respectively (neither of these differences is statistically significant).
According to the recently published risk scoring system AHEAD, patients from the relax‐comparable group were divided into three groups: low‐risk (AHEAD score 0–1), intermediate‐risk (score 2–3), and high‐risk (score 4–5). Basic characteristics of patients are given in Table 2. Figure 4 demonstrates that this simple stratification tool clearly differentiates low‐risk patients (AHEAD score 0–1) from patients with a higher risk of death (AHEAD score 2–3 and 4–5), even within the first year after the patient's discharge from hospital.
Table 2.
Baseline characteristics of relax‐comparable group stratified by AHEAD score
| Characteristics | AHEAD score | ||
|---|---|---|---|
| 0–1 (n = 404) | 2–3 (n = 806) | 4–5 (n = 151) | |
| Age (years) | 68.0 (12.3) | 76.9 (8.9) | 79.9 (6.4) |
| Men | 211 (52.2%) | 390 (48.4%) | 101 (66.9%) |
| BMI | 29.6 (6.1) | 29.3 (5.7) | 29.7 (5.8) |
| Systolic BP (mmHg) | 164.5 (30.6) | 162.2 (28.1) | 155.7 (24.3) |
| Diastolic BP (mmHg) | 93.9 (17.6) | 91.0 (15.9) | 84.4 (13.1) |
| Heart rate (min−1) | 93.8 (24.5) | 92.3 (25.0) | 85.5 (22.4) |
| Ejection fraction (%) | 35.3 (15.1) | 39.7 (14.2) | 42.8 (13.4) |
| Ejection fraction ≤40% | 256 (68.3%) | 418 (57.9%) | 65 (47.4%) |
| Creatinine clearance ml/min/1.73m2 | 57.4 (11.0) | 49.2 (12.3) | 40.2 (10.6) |
| Medical history | |||
| Hypertension | 315 (78.0%) | 696 (86.4%) | 137 (90.7%) |
| Stroke | 71 (17.6%) | 206 (25.6%) | 44 (29.1%) |
| Smoking | 45 (36.9%) | 100 (34.4%) | 20 (32.3%) |
| PAD | 53 (13.1%) | 147 (18.2%) | 35 (23.2%) |
| Ischaemic heart disease | 178 (44.1%) | 480 (59.6%) | 104 (68.9%) |
| Atrial fibrillation | 32 (7.9%) | 306 (38.0%) | 101 (66.9%) |
| COPD | 113 (28.0%) | 226 (28.0%) | 38 (25.2%) |
| Diabetes mellitus | 73 (18.1%) | 455 (56.5%) | 136 (90.1%) |
| Medication at admission | |||
| ACE inhibitors | 204 (51.8%) | 444 (55.8%) | 88 (59.5%) |
| ARBs | 37 (9.4%) | 131 (16.5%) | 25 (16.9%) |
| Beta‐blockers | 207 (52.5%) | 500 (62.9%) | 97 (65.5%) |
| Spironolactone | 76 (19.3%) | 194 (24.4%) | 44 (29.7%) |
| Digoxin | 54 (13.7%) | 179 (22.5%) | 37 (25.0%) |
ACE, angiotension converting enzyme; ARBs, angiotensin‐2 receptor blockers; BP, blood pressure; COPD, chronic obstructive pulmonary disease; PAD, Peripheral arterial disease.
Figure 4.
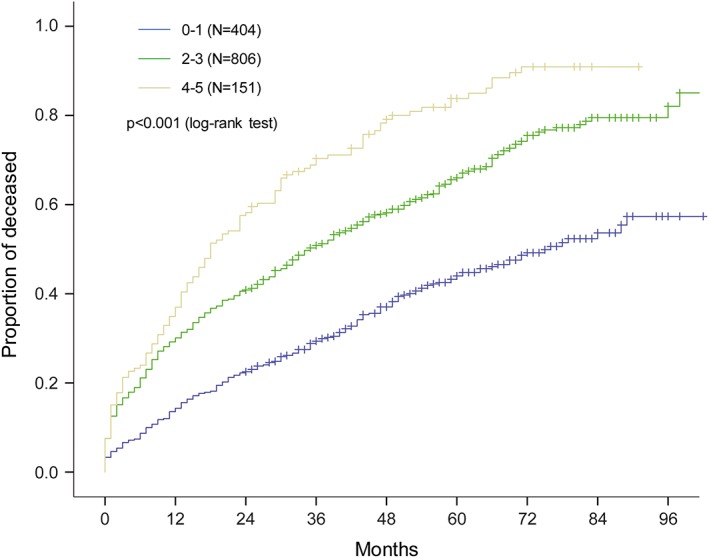
Mortality of patients in relax‐comparable group stratified by Acute Heart Failure Database (AHEAD) score.
Discussion
Our results highlight several very important points: (i) only 23% of all consecutive patients hospitalized with AHF met criteria of the large RELAX‐AHF trial; (ii) mortality of patients who met criteria of the RELAX‐AHF trial (relax‐comparable group) is significantly lower in comparison to the rest of patients recorded in the AHEAD registry (relax‐AHF‐non‐comparable group); (iii) mortality of relax‐comparable group of patients from registry of consecutive AHF patients is significantly higher than mortality of patients in RCT regardless to study treatment; (iv) systolic BP at hospital admission significantly affects the prognosis of AHF patients.
Based on our data, we were not able to confirm a reduction of mortality associated with the treatment by vasodilators, as it was previously demonstrated by results from the ALARM registry, where the treatment by combination of diuretics and vasodilators led to a lower mortality in comparison to diuretics alone (7.6% and 14.2%, respectively, P < 0.001).16 On the other hand, the ASCEND HF trial also did not confirm any statistically significant effect of nesiritide‐based vasodilator therapy on AHF‐related mortality or rehospitalization.17
Furthermore, our results demonstrated two to three times higher unadjusted mortality of patients with systolic BP ≤ 110 mmHg as compared with those with systolic BP > 110 mmHg at admission. Similar results have been shown by the published data from the OPTIMIZE HF registry, where a systolic BP lower than 100 mmHg was associated with three times higher hospital mortality rate in patients with normal (2.6% and 7.5%, respectively) or with decreased renal functions (5.5% and 16.3%, respectively).18
Finally, mortality analysis in the subgroup relax‐comparable showed that the AHEAD score is a simple tool that can easily identify higher risk patients who might potentially benefit from new therapeutic approaches in the future.
Our results based on the Czech registry AHEAD are fully consistent with the recently published results. Miro et al. demonstrated similar results from the Spanish registry EAHFE,19 and Wang et al. showed that approximately 2 out of 10 patients hospitalized because of AHF in the USA, Latin America, or Asia‐Pacific are potentially eligible for the RELAX‐AHF criteria. Even after multivariate adjustement, RELAX‐AHF type of patients had lower mortality rates as compared with non‐RELAX‐AHF type of patients.20
Two large RCTs with VD therapy—RELAX II and TRUE HF are currently ongoing. The RELAX II trial is a multicentre, randomized, double‐blind, placebo‐controlled Phase III study to evaluate the efficacy, safety, and tolerability of serelaxin when added to standard therapy in AHF patients. The TRUE AHF clinical trial has finished enrolment in spring 2015, while the RELAX II trial is supposed to finish enrolment in winter 2015/2016. Results of both trials are expected to be available in 2016, expectantly bringing the definitive answer on the role of vasodilators in acute heart failure treatment.
Our work has several limitations. The AHEAD registry was originally not designed to assess the treatment with vasodilators. An increased value of the natriuretic peptide level was one of the important criteria for the enrolment in the RELAX‐AHF trial, as well as for the subsequent RELAX II and True HF trials. We could not use the natriuretic peptide levels because of a limited number of patients in which this parameter was known at the time of their admission. However, we can assume that a low value of the natriuretic peptide would exclude low‐risk patients from the relax‐comparable group and that it could lead to an even higher mortality in the relax‐comparable group in comparison to RELAX‐AHF patients than we demonstrated in this study. Moreover, we were not able to exclude patients with acute infections that could lead to an acute decompensated heart failure.
Conclusions
According to results from the AHEAD registry, we can expect that about only 20–25% of patients hospitalized because of AHF meet the criteria for a potential treatment with new vasodilator serelaxin. Significantly, lower mortality rates can be expected in participants of ongoing randomized clinical trials in comparison with real‐world AHF patients. AHEAD score is a simple tool that can easily identify higher risk patients who might potentially benefit from new therapeutic approaches in the future. The new treatment of AHF patients with contraindication for vasodilator therapy remains the challenge for future research.
Conflict of interest
None declared.
Funding
The work was supported by a project of conceptual development of research organization 65269705 (University Hospital Brno) funded by the Ministry of Health of the Czech Republic, by the Grant MUNI/A/1362/2015.
Acknowledgements
We would like to thank to all investigators of the AHEAD registry, especially to H.A., M.F., R.F., A.K., T.M, S.B, L.P, F.R., D.V., K.V. and M.B.
Spinar, J. , Jarkovsky, J. , Spinarova, L. , Vitovec, J. , Linhart, A. , Widimsky, P. , Miklik, R. , Zeman, K. , Belohlavek, J. , Malek, F. , Cihalik, C. , Spac, J. , Felsoci, M. , Ostadal, P. , Dusek, L. , Kettner, J. , Vaclavik, J. , Littnerova, S. , Monhart, Z. , Malek, J. , Parenica, J. , and on behalf of the AHEAD registry investigators (2017) Worse prognosis of real‐world patients with acute heart failure from the Czech AHEAD registry in comparison to patients from the RELAX‐AHF trial. ESC Heart Failure, 4: 8–15. doi: 10.1002/ehf2.12105.
References
- 1. Spinar J, Parenica J, Vitovec J, Widimsky P, Linhart A, Fedorco M, Malek F, Cihalik C, Spinarová L, Miklik R, Felsoci M, Bambuch M, Dusek L, Jarkovsky J. Baseline characteristics and hospital mortality in the Acute Heart Failure Database (AHEAD) Main registry. Crit Care Lond Engl 2011; 15: R291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rossi JS, Flaherty JD, Fonarow GC, Nunez E, Gattis Stough W, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, Yancy CW, Young JB, Davidson CJ, Gheorghiade M. Influence of coronary artery disease and coronary revascularization status on outcomes in patients with acute heart failure syndromes: a report from OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure)*. Eur J Heart Fail 2008; 10: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 3. Siirilä‐Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola V‐P. Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure. Eur Heart J 2006; 27: 3011–3017. [DOI] [PubMed] [Google Scholar]
- 4. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V‐P, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006; 27: 2725–2736. [DOI] [PubMed] [Google Scholar]
- 5. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola V‐P, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray JJV, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emerg…. Eur Heart J 2015; 36: 1958–1966. [DOI] [PubMed] [Google Scholar]
- 6. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009; 15: 256–264. [DOI] [PubMed] [Google Scholar]
- 7. Sharon A, Shpirer I, Kaluski E, Moshkovitz Y, Milovanov O, Polak R, Blatt A, Simovitz A, Shaham O, Faigenberg Z, Metzger M, Stav D, Yogev R, Golik A, Krakover R, Vered Z, Cotter G. High‐dose intravenous isosorbide‐dinitrate is safer and better than Bi‐PAP ventilation combined with conventional treatment for severe pulmonary edema. J Am Coll Cardiol 2000; 36: 832–837. [DOI] [PubMed] [Google Scholar]
- 8. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin‐2, for treatment of acute heart failure (RELAX‐AHF): a randomised, placebo‐controlled trial. Lancet 5; 381: 29–39. [DOI] [PubMed] [Google Scholar]
- 9. Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double‐blind, placebo‐controlled, crossover, dose‐ranging phase 2 trial. Lancet 2011; 378: 676–683. [DOI] [PubMed] [Google Scholar]
- 10. Peacock WF, Chandra A, Char D, Collins S, Der Sahakian G, Ding L, Dunbar L, Fermann G, Fonarow GC, Garrison N, Hu M, Jourdain P, Laribi S, Levy P, Möckel M, Mueller C, Ray P, Singer A, Ventura H, Weiss M, Mebazaa A. Clevidipine in acute heart failure: results of the A Study of Blood Pressure Control in Acute Heart Failure—A Pilot Study (PRONTO). Am Heart J 2014; 167: 529–536. [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J 2015; 36: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitt B, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Nowack C, Kim S‐Y, Pieper A, Kimmeskamp‐Kirschbaum N, Filippatos G. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study‐Heart Failure (ARTS‐HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail 2015; 17: 224–232. [DOI] [PubMed] [Google Scholar]
- 13. Parenica J, Spinar J, Vitovec J, Widimsky P, Linhart A, Fedorco M, Vaclavik J, Miklik R, Felsoci M, Horakova K, Cihalik C, Malek F, Spinarova L, Belohlavek J, Kettner J, Zeman K, Dušek L, Jarkovsky J. Long‐term survival following acute heart failure: the Acute Heart Failure Database Main registry (AHEAD Main). Eur J Intern Med 2013; 24: 151–160. [DOI] [PubMed] [Google Scholar]
- 14. Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, Linhart A, Widimsky P, Miklik R, Zeman K, Belohlavek J, Malek F, Felsoci M, Kettner J, Ostadal P, Cihalik C, Vaclavik J, Taborsky M, Dusek L, Littnerova S, Parenica J. AHEAD score–Long‐term risk classification in acute heart failure. Int J Cardiol 2016; 202: 21–26. [DOI] [PubMed] [Google Scholar]
- 15. Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez‐Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Dickstein K, Albuquerque A, Conthe P, Crespo‐Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Moreno R, Singer M, Singh S, Tendera M, Thygesen K; ESC Committe for Practice Guideline (CPG) . Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 384–416. [DOI] [PubMed] [Google Scholar]
- 16. Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas F, Delgado JF, Follath F. Short‐term survival by treatment among patients hospitalized with acute heart failure: the global ALARM‐HF registry using propensity scoring methods. Intensive Care Med 2011; 37: 290–301. [DOI] [PubMed] [Google Scholar]
- 17. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in‐hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol 2008; 52: 347–356. DOI:10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 19. Miró Ò, Gil V, Müller C, Mebazaa A, Bueno H, Martín‐Sánchez F, Herrero P, Jacob J, Llorens P. How does a clinical trial fit into the real world? The RELAX‐AHF study population into the EAHFE registry. Clin Res Cardiol 2015; 104: 850–860. [DOI] [PubMed] [Google Scholar]
- 20. Wang TS, Hellkamp AS, Patel CB, Ezekowitz JA, Fonarow GC, Hernandez AF. Representativeness of RELAX‐AHF Clinical Trial Population in Acute Heart Failure. Circ Cardiovasc Qual Outcomes 2014; 7: 259–268. [DOI] [PubMed] [Google Scholar]


