Abstract
NS-018 is a Janus-activated kinase 2 (JAK2)-selective inhibitor, targeting the JAK–signal transducer and activator of transcription (STAT) pathway that is deregulated in myelofibrosis. In this phase I, dose-escalation portion of a phase I/II study, patients with myelofibrosis received oral NS-018 in continuous 28-day cycles. The primary study objective was to evaluate safety, tolerability and clinically active dose of NS-018. Forty-eight patients were treated; 23 (48%) had previously received a JAK inhibitor (JAKi). The most common drug-related adverse events were thrombocytopenia (27%)/anemia (15%) for hematologic events, and dizziness (23%)/nausea (19%) for non-hematologic events. Once daily NS-018 at 300 mg was chosen as the phase II study dose based on improved tolerability compared with higher doses. A ⩾50% reduction in palpable spleen size was achieved in 56% of patients (47% of patients with prior JAKi treatment), and improvements were observed in myelofibrosis-associated symptoms. Bone marrow fibrosis grade (local assessment) improved from baseline in 11/30 evaluable patients (37%) after 3 cycles of NS-018. JAK2 allele burden was largely unchanged. Changes in cytokine/protein levels were noted after 4 weeks of treatment. NS-018 reached peak plasma concentration in 1–2 h and did not accumulate with multiple dosing. NS-018 will be assessed in patients with previous JAKi exposure in the phase II portion.
Introduction
Myelofibrosis (MF) is a BCR-ABL1-negative chronic myeloproliferative neoplasm (MPN) that can develop as a primary hematologic malignancy or from the progression of polycythemia vera and essential thrombocythemia.1, 2 MF is clinically characterized by splenomegaly, cytopenias, constitutional symptoms and progressive bone marrow fibrosis (BMF).1, 3 The only potential curative treatment for MF is hematopoietic stem cell transplantation, for which the great majority of patients are ineligible.4
Deregulation of Janus-activated kinase (JAK)/signal transducer and activator of transcription (STAT) signaling is a biological hallmark in patients with MF.5, 6 Hyperactivation of this pathway in MF is frequently associated with the JAK2V617F mutation6 but can also result from other mutations (for example, in JAK2 (exon 12), thrombopoietin receptor gene, or calreticulin gene).7, 8 Inhibition of JAK/STAT signaling, particularly via JAK2, is thus recognized as a rational treatment strategy for MF.9
Ruxolitinib is the only JAK1/2 inhibitor approved for the treatment of MF (US approval (2011) for intermediate- or high-risk MF10). Ruxolitinib demonstrated improvements in splenomegaly and constitutional symptoms,11, 12 presumed to be mediated by its antiproliferative effects, and through normalization of cytokine signaling as abnormal cytokine levels have been associated with MF symptoms.13, 14, 15 However, not all patients respond to ruxolitinib, others lose response while on treatment, and some have to discontinue treatment owing to toxicities.16 Guidelines for the management of patients with ruxolitinib-resistant/-intolerant disease have been suggested,16 yet treatment options for these patients remain very limited.
NS-018 is an orally administered, selective, small-molecule inhibitor of JAK2.17 In preclinical assessments, NS-018 inhibited JAK2 with a median inhibitory concentration in the subnanomolar range in an in vitro kinase assay, and reduced splenomegaly in a JAK2V617F-driven mouse model.18 Improvements in leukocytosis, BMF and survival were also observed in the murine model, without reducing peripheral blood erythrocyte or platelet counts.17, 18
Here we report the safety, efficacy, pharmacokinetics (PKs) and pharmacodynamics (PDs) from the phase I portion of a phase I/II study of NS-018 in patients with MF, including patients previously treated with a different JAK2 inhibitor.
Materials and methods
Study population
Eligible patients were ⩾18 years old with intermediate-1, intermediate-2 or high-risk (International Prognostic Scoring System19) primary MF, post-polycythemia vera MF or post-essential thrombocythemia MF20, 21 that required treatment. For patients with intermediate-1 MF, the requirement for treatment was determined by the investigator based on MF symptomatology (for example, symptomatic splenomegaly or transfusion-dependent anemia). Patients had Eastern Cooperative Oncology Group performance status ⩽2, adequate bone marrow reserve (absolute neutrophil count >1 × 109/l and platelet count >50 × 109/l), adequate organ function (serum creatinine ⩽1.5 × upper limit of normal (ULN) or creatinine clearance ⩾60 ml/min/1.73 m2; aspartate aminotransferase and alanine aminotransferase ⩽3 × ULN; and total bilirubin ⩽1.5 × ULN) and wild-type or mutated JAK2 status. Symptomatic palpable splenomegaly was not a requirement for the phase I portion. Key exclusion criteria were: eligible for hematopoietic stem cell transplantation, radiation therapy for splenomegaly within 6 months of screening, prior splenectomy, investigational or anticancer agent therapy within 2 weeks of study treatment, discontinuation of previous JAK2 inhibitor owing to gastrointestinal toxicity, other active malignancy, active systemic infection, serious cardiac condition within 6 months of screening, concurrent medication that strongly inhibits or induces cytochrome P450 (CYP)3A4 or is metabolized by CYP1A2 or CYP3A4. Inclusion and exclusion criteria were specified prior to enrollment.
Study design
This was a phase I/II open-label, dose-escalation study conducted at 8 centers in the United States of America. Patients received continuous 28-day cycles of NS-018 until disease progression, unacceptable toxicity, patient withdrawal or protocol non-compliance. In the phase I portion, patients received oral NS-018 once daily (QD; 75, 125, 200, 300 or 400 mg) or twice daily (BID; 100, 200, 300 or 400 mg) in sequentially enrolled cohorts. BID dosing was introduced to maintain appropriate NS-018 exposure levels as PK analyses revealed that NS-018 half-life was shorter than expected. An additional cohort was opened at 250 mg BID following completion of recruitment for the 400 mg QD/BID cohorts; preliminary results suggested that increased drug exposure might improve efficacy, with the 250 mg BID dose expected to achieve a higher area under the plasma concentration vs time curve value than 300 mg QD but without the toxicity observed at 300 mg BID or 400 mg QD/BID. Dose escalation was based on a standard 3+3 study design and proceeded until the maximum tolerated dose (highest dose at which ⩽1 of 6 patients experienced a dose-limiting toxicity (DLT)) or the highest planned dose was reached.
A DLT was defined as grade 4 neutropenia, thrombocytopenia, anemia, lymphopenia, any other grade 3/4 hematologic toxicity or any grade 3/4 non-hematologic toxicity (excluding nausea, vomiting or diarrhea responsive to antiemetic/antidiarrheal treatment). In the event of a DLT, NS-018 treatment was interrupted but could resume at a reduced dose if the toxicity resolved within 4 weeks. Escalation to the next dose level declared tolerable was permitted if the patient experienced no significant clinical benefit following ⩾3 months of NS-018 treatment and no grade 3/4 drug-related toxicity.
Study objectives
The primary objective of the phase I portion of the study was to evaluate the safety, tolerability and maximum tolerated dose/recommended phase II dose (RP2D) of NS-018 in patients with MF. Secondary objectives included assessment of the PK, PD and preliminary efficacy of NS-018 in patients with MF.
Safety assessments
Adverse events (AEs) were assessed throughout the study and graded according to Common Toxicity Criteria for Adverse Events (version 4.0), with the causal relationship of AEs described. Safety assessments also included laboratory tests, electrocardiograms, vital signs and physical examinations.
Efficacy assessments
Spleen size was assessed by palpation in the phase I portion. Treatment responses (splenic and hematologic) were evaluated according to the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria22 on Day 1 of Cycles 2, 3 and 4 and every 3 cycles thereafter. MF-associated symptoms were evaluated using the Myelofibrosis Symptom Assessment Form (MF-SAF23), which was completed at baseline and on Day 1 of Cycles 2 and 4, and on Day 1 of every 3 cycles thereafter. Bone marrow samples were taken during screening (28 days before first dose of study drug), on Day 1 of Cycle 4 and on Day 1 of every 3 cycles thereafter. BMF was assessed in biopsy specimens according to standard practice at the local study site and was graded (0–3) using the European Consensus Grading Criteria.24 Changes in BMF grade were categorized as improvement of ⩾1 grade, stabilization or worsening of ⩾1 grade compared with baseline.
PK assessments
Plasma samples for PK analysis were obtained on Days 1, 8 and 29. Samples were taken predose and approximately 0.5, 1, 2, 3, 4, 6, 8 and 24 h (Days 1 and 29 only) after NS-018 administration. NS-018 concentration was determined using liquid chromatography-tandem mass spectrometry (Sumika Chemical Analysis Service Ltd, Tokyo, Japan), with a lower limit of quantification of 1 ng/ml. PK parameters were calculated using WinNonlin (version 6.4; Certara USA, Inc., Princeton, NJ, USA).
PD assessments
Plasma samples for biomarker assessment were taken predose on Day 1 of Cycles 1–4. The concentrations of 88 cytokines were determined using the RBM HumanMAP assay (version 2.0; Myriad RBM, Austin, TX, USA). Allele burden was assessed on Day 1 of Cycles 1, 2 and 4 and every 3 cycles thereafter, with on-treatment data compared with predose baseline.
Statistical assessments
Continuous variables were summarized using descriptive statistics, including mean, s.d., median and range. The safety analysis population consisted of all patients who received ⩾1 dose of study drug. The splenic response population included all patients who had received ⩾1 cycle of treatment and who had baseline splenomegaly ⩾5 cm. For each symptom, the analysis population included patients who were evaluable at the given time point, had received ⩾1 cycle of treatment and had a baseline score >2 for that symptom. The PD population included all patients who received ⩾1 dose of study drug and had baseline and ⩾1 evaluable postbaseline PD assessment. The PK population included patients who received ⩾1 dose of study drug and had ⩾1 evaluable PK profile.
Study oversight
This study was conducted according to the guidelines of the International Conference on Harmonisation and Good Clinical Practice, and written informed consent was obtained from all participants. The study protocol was approved by the local Institutional Review Board and Ethics Committee, in accordance with local requirements. All subsequent protocol amendments underwent the same approval procedure. This trial was registered at www.clinicaltrials.gov (NCT01423851). The Safety Review Committee (SRC) consisted of actively recruiting investigators, a medical monitor and a representative from the sponsor; SRC evaluated the safety of each dosing cohort and advised on changes in dosing schedule and the RP2D.
Results
Patient demographics and disposition
From June 2011 through August 2014, 48 patients were enrolled across 10 NS-018 dose cohorts in the phase I portion of this study. Three patients were initially dosed at 75 mg QD, 125 mg QD, 100 mg BID, 200 mg QD/BID and 400 mg QD, 6 patients at 300 mg QD, and 8 patients at 250 mg BID, 300 mg BID and 400 mg BID. Subsequent dose escalations and reductions are shown in Supplementary Table S1.
The data cutoff for this analysis was 21 July 2015 (median follow-up time of 279.5 (range 5–1461) days from screening), at which time 16 patients remained on treatment. The median number of treatment cycles was 4 (1–45). Reasons for treatment discontinuation were disease progression (n=13), AEs (n=8), physician consideration (n=6), patient request (n=4) and protocol non-compliance (n=1). Overall, median patient age was 69.0 (38–83) years and 35 (73%) patients had received prior MF treatment; 23 (48%) patients had previously been treated with a JAK2 inhibitor. No patient received erythropoietin or danazol during treatment. A total of 14 patients had intermediate-1 MF at baseline, of whom 12 had symptomatic splenomegaly; 1 had anorexia/weight loss and 1 had red blood cell transfusion dependency (without constitutional symptoms). Baseline demographics and disease characteristics of the entire population are shown in Table 1.
Table 1. Baseline demographics of patient population.
| Characteristics | All patients (n=48) |
|---|---|
| Median age, years (range) | 69.0 (38–83) |
| Male sex, n (%) | 29 (60) |
| Median time from diagnosis, years (range) | 2.2 (0–26.0) |
| MF classification, n (%) | |
| PMF | 37 (77) |
| Post-PV MF | 6 (13) |
| Post-ET MF | 5 (10) |
| ECOG performance status, n (%) | |
| 0 | 12 (25) |
| 1 | 34 (71) |
| 2 | 2 (4) |
| JAK2 mutation, n (%)a | |
| JAK2V617F-positive | 35 (73) |
| JAK2V617F-negative | 13 (27) |
| Palpable splenomegaly, n (%) | 43 (90) |
| Median palpable spleen size, cm (range)b | 13 (2–24) |
| RBC transfusion dependent, n (%)c | 10 (21)d |
| Median platelet count, × 109/l (range) | 141 (52–859) |
| Median hemoglobin, g/l (range) | 100 (67–139) |
| BMF grade, n (%)e | |
| 0 | 0 |
| 1 | 2 (4) |
| 2 | 15 (31) |
| 3 | 31 (65) |
| Previous treatment, n (%) | 35 (73)f |
| JAK2 inhibitor | 23 (48)g |
| Ruxolitinib | 17 |
| Pacritinib | 4 |
| Fedratinib | 4 |
| AZD1480 | 1 |
| LY-2784544 | 1 |
| Hydroxyurea | 14 (29) |
| Darbepoetin alfa | 4 (8) |
| Pegylated interferon-α2b | 3 (6) |
| Danazol | 3 (6) |
Abbreviations: BMF, bone marrow fibrosis; ECOG, Eastern Cooperative Oncology Group; ET, essential thrombocythemia; JAK, Janus-activated kinase; MF, myelofibrosis; PMF, primary myelofibrosis; PV, polycythemia vera; RBC, red blood cell.
Information regarding other mutations (for example, CALR (calreticulin gene) or MPL (myeloproliferative leukemia virus oncogene)) was not available in this study.
In patients with palpable splenomegaly (n=43).
RBC transfusion dependency defined as transfusions of ⩾6 units of packed RBCs in the 12 weeks before study enrollment for a hemoglobin level of <85 g/l, in the absence of bleeding or treatment-induced anemia. Further, the most recent transfusion episode must have occurred in the 28 days before study enrollment.25
Four patients had hemoglobin level of 85–93 g/l at baseline.
BMF grading was not available for 1 patient.
Ten patients had received more than one of the listed categories of prior MF treatment (JAK2 inhibitor, hydroxyurea (n=6); darbepoetin alfa, JAK2 inhibitor (n=2); hydroxyurea, darbepoetin alfa, JAK2 inhibitor (n=1); danazol, darbepoetin alfa, JAK2 inhibitor (n=1)).
Two patients received treatment with 2 different JAK2 inhibitors (one patient received pacritinib, then LY-2784544; the other patient received fedratinib, then ruxolitinib).
Safety
DLTs were observed in 2 patients: grade 3 QT prolongation in 1 patient in the 250 mg BID cohort and grade 3 dizziness in 1 patient in the 400 mg BID cohort. The patient with grade 3 QT prolongation recovered following NS-018 discontinuation. This was the only case of QT prolongation observed in the study. The patient with grade 3 dizziness recovered after the NS-018 dose was reduced. An additional 7 patients discontinued NS-018 owing to AEs (3 patients at 300 mg BID (involuntary muscle contraction, dizziness and insomnia; anemia; neutropenia); 2 patients at 400 mg BID (dizziness, dysphagia and nervous system disorder) and one each at 250 mg BID (thrombocytopenia, hemolysis and hemolytic anemia) and at 300 mg QD (transient ischemic attack)). Overall, 11 patients underwent dose reductions owing to AEs, with these events most frequent at NS-018 doses ⩾250 mg BID (Supplementary Table S1). The most common reason for dose reduction was dizziness (n=4).
The most common drug-related hematologic events were thrombocytopenia (all grades, 27% grade 3/4, 10%) and anemia (all grades, 15% grade 3/4, 6%) (Table 2). No cases of grade 4 anemia were reported. Thrombocytopenia was more frequent in patients with baseline platelet count <100 × 109/l, compared with those with platelet count ⩾100 × 109/l (all grades, 44% vs 19% grade 3/4, 38% vs 6%, respectively (Table 2)). Drug-related non-hematologic events were typically grade 1/2 neurological and gastrointestinal disorders, with dizziness (23%) and nausea (19%) the most common toxicities in these classes, respectively (Table 3).
Table 2. Drug-related hematological AEs observed in >2 patients in any cohort.
| AE, n (%) |
NS-018 dose cohort |
Baseline platelet count | All dose cohorts (n=48) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
QD |
BID |
|
|||||||||||
| 75 mg (n=3) | 125 mg (n=4) | 200 mg (n=9) | 300 mg (n=16) | 400 mg (n=4) | 100 mg (n=5) | 200 mg (n=12) | 250 mg (n=8) | 300 mg (n=12) | 400 mg (n=8) | <100 × 109/l (n=16) | ⩾100 × 109/l (n=32) | ||
| Thrombocytopenia | 1 (33) | 0 | 0 | 3 (19) | 0 | 1 (20) | 1 (8) | 3 (38) | 4 (33) | 1 (13) | 7 (44) | 6 (19) | 13 (27) |
| Grade 1/2 | 0 | — | — | 0 | — | 0 | 1 (8) | 2 (25) | 3 (25) | 0 | 1 (6) | 4 (13) | 5 (10) |
| Grade 3 | 0 | — | — | 2 (13) | — | 1 (20) | — | 0 | 1 (8) | 1 (13) | 3 (19) | 2 (6) | 5 (10) |
| Grade 4 | 1 (33) | — | — | 1 (6) | — | 0 | — | 1 (13) | 0 | 0 | 3 (19) | 0 | 3 (6) |
| Anemia | 0 | 0 | 1 (11) | 1 (6) | 1 (25) | 0 | 0 | 1 (13) | 3 (25) | 2 (25) | NC | NC | 7 (15) |
| Grade 1/2 | — | — | 1 (11) | 1 (6) | 1 (25) | — | — | 1 (13) | 1 (8) | 1 (13) | NC | NC | 4 (8) |
| Grade 3 | — | — | — | — | — | — | — | 0 | 2 (17) | 1 (13) | NC | NC | 3 (6) |
| Grade 4 | — | — | — | — | — | — | — | 0 | 0 | 0 | NC | NC | 0 |
Abbreviations: AE, adverse event; BID, twice-daily dosing; NC, not calculated; QD, once-daily dosing. n includes patients in the original dose cohort and those who had their dose escalated or reduced to the dose level of that cohort. Patients may be included more than once if they experienced events in more than one dose cohort.
Table 3. Drug-related non-hematological AEs observed in >2 patients in any cohort.
| All grade/grade 3 or 4, n (%) |
NS-018 dose cohort |
All dose cohorts (n=48) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
QD |
BID |
||||||||||
| 75 mg (n=3) | 125 mg (n=4) | 200 mg (n=9) | 300 mg (n=16) | 400 mg (n=4) | 100 mg (n=5) | 200 mg (n=12) | 250 mg (n=8) | 300 mg (n=12) | 400 mg (n=8) | ||
| Dizziness | 0 | 0 | 0 | 3 (19)/1 (6) | 1 (25)/0 | 0 | 1 (8)/0 | 1 (13)/1 (13) | 3 (25)/0 | 2 (25)/1 (13) | 11 (23)/3 (6) |
| Nausea | 0 | 0 | 1 (11)/0 | 0 | 1 (25)/0 | 0 | 1 (8)/0 | 2 (25)/1 (13) | 4 (33)/0 | 0 | 9 (19)/1 (2) |
| Diarrhea | 0 | 0 | 2 (22)/0 | 2 (13)/0 | 1 (25)/0 | 0 | 2 (17)/0 | 0 | 1 (8)/0 | 1 (13)/0 | 7 (15)/0 |
| Paresthesia | 0 | 0 | 0 | 1 (6)/0 | 1 (25)/0 | 0 | 0 | 0 | 2 (17)/1 (8) | 0 | 4 (8)/1 (2) |
| Weight increased | 0 | 0 | 2 (22)/0 | 0 | 1 (25)/0 | 0 | 0 | 0 | 1 (8)/0 | 0 | 4 (8)/0 |
| Headache | 0 | 0 | 0 | 2 (13)/0 | 0 | 0 | 0 | 0 | 0 | 1 (13)/0 | 3 (6)/0 |
| Vomiting | 0 | 0 | 2 (22)/0 | 1 (6)/0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (6)/0 |
| Esophageal reflux | 0 | 0 | 0 | 1 (6)/0 | 0 | 0 | 0 | 1 (13)/0 | 1 (8)/0 | 0 | 3 (6)/0 |
Abbreviations: AE, adverse event; BID, twice-daily dosing; QD, once-daily dosing. n includes patients in the original dose cohort and those who had their dose escalated or reduced to the dose level of that cohort. Patients may be included more than once if they experienced events in more than one cohort.
NS-018 400 mg BID/QD was considered intolerable by the SRC based on the proportion of drug-related neurological AEs. Dizziness, peripheral neuropathy, headache, disturbance in attention, vertigo and dysesthesia were observed at 400 mg BID; dizziness, paresthesia, disturbance in attention and dysphasia were observed at 400 mg QD. At the time of the SRC decision, any patient receiving 400 mg BID/QD underwent dose reduction (400 mg BID cohort, n=1; 400 mg QD cohort, n=3).
At doses <400 mg, 300 mg QD was considered better tolerated than 250 or 300 mg BID, with a lower rate of discontinuations owing to AEs (13%, 50% and 38%, respectively); NS-018 300 mg QD was the RP2D. Drug-related grade 3/4 AEs in the 300 mg QD cohort included 2 cases (13%) of grade 3 thrombocytopenia and 1 case (6%) each of grade 4 thrombocytopenia, grade 3 dizziness and grade 3 dyspnea. Only 1 case of anemia (grade 2) was observed at 300 mg QD. Other laboratory abnormalities related to NS-018 were rarely observed and were generally of grade 1 severity. Furthermore, no laboratory abnormalities other than thrombocytopenia and anemia were observed in the RP2D 300 mg QD cohort.
Clinical response: spleen
Among 36 patients evaluable for splenic response, 20 (56%) achieved a ⩾50% reduction in palpable spleen size (Table 4; evaluable patients had received ⩾1 treatment cycle and had baseline splenomegaly ⩾5 cm). These 20 patients all met IWG-MRT criteria for spleen response25 (15 patients with baseline spleen >10 cm achieved a ⩾50% reduction in spleen size and 5 patients with baseline spleen ⩽10 cm attained a 100% reduction). Overall, splenic reduction reached 100% in 11 patients (31%), including all 5 patients who responded at 300 mg QD. For the patients who attained a ⩾50% reduction in spleen size, 13/20 maintained the reduction for ⩾8 weeks, thus satisfying the IWG-MRT criteria for splenic clinical improvement (Table 4). The median duration of splenic response was 5.5 (1–24) cycles. The time to ⩾50% splenic reduction ranged from 1 to 12 cycles of treatment, with a median of 2 cycles for the RP2D 300 mg QD cohort.
Table 4. Clinical responses.
| Patient group | Splenic reduction ⩾50%, n/N (%)a | Median (range) cycles to splenic reduction ⩾50%a | Splenic reduction of 100%, n/N (%)a | Splenic clinical improvement, n/N (%)a,b | Hb/Plt clinical improvement, n/N (%)b,c |
|---|---|---|---|---|---|
| NS-018 dose cohort | |||||
| 75 mg QD | 0/2 (0) | — | — | — | 0/3 (0) |
| 125 mg QD | 0/4 (0) | — | — | — | Plt: 1/4 (25) |
| 200 mg QD | 3/5 (60) | 3 | 1/5 (20) | 1/5 (20) | 0/6 (0) |
| 300 mg QD | 5/9 (56) | 2 (1–8) | 5/9 (56) | 4/9 (44) | Hb: 2/9 (22) |
| 400 mg QD | 3/3 (100) | 1 | 0/3 (0) | 1/3 (33) | 0/3 (0) |
| 100 mg BID | 2/3 (66) | 7.5 (3–12) | 0/3 (0) | 1/3 (33) | Hb: 1/3 (33) |
| 200 mg BID | 1/3 (33) | 1 | 1/3 (33) | 1/3 (33) | 0/4 (0) |
| 250 mg BID | 1/4 (25) | 3 | 1/4 (25) | 0/4 (0) | 0/4 (0) |
| 300 mg BID | 3/6 (50) | 1 | 3/6 (50) | 2/6 (33) | Hb: 1/7 (14) |
| 400 mg BID | 4/6 (67) | 1.5 (1–2) | 1/6 (17) | 4/6 (67) | 0/6 (0) |
| Prior JAK2 inhibitor treatment | 9/19 (47) | 2 (1–12) | 5/19 (26) | 6/19 (32) | Hb: 3/18 (17) Plt: 1/18 (6) |
| All dose cohorts | 20/36 (56) | 1 (1–12) | 11/36 (31) | 13/36 (36) | Hb: 4/40 (10) Plt: 1/40 (3) |
Abbreviations: BID, twice-daily dosing; Hb, hemoglobin; JAK, Janus-activated kinase; N, number of evaluable patients; Plt, platelet; QD, once-daily dosing.
Splenic response was assessed in patients who had received ⩾1 cycle of treatment and with baseline splenomegaly ⩾5 cm.
All measures of clinical improvement had to be confirmed for ⩾8 weeks.22
Hb/Plt response was assessed in patients who had received ⩾1 cycle of treatment.
Of the evaluable patients with the JAK2V617F mutation, 13/26 (50%) achieved a ⩾50% reduction in palpable spleen size, of whom 8 had 100% splenic reduction. By comparison, 7/10 (70%) JAK2V617F-negative patients achieved a ⩾50% reduction in spleen size, with 100% reduction in 3 of these patients. Among evaluable patients who had received prior JAK2 inhibitor treatment, 9/19 (47%) achieved a ⩾50% reduction in spleen size, with splenic clinical improvement recorded in 6 of these patients (Table 4).
Clinical response: blood counts
Hemoglobin improvement (⩾20 g/l increase) was observed in 11 patients, with 4 attaining IWG-MRT clinical improvement (Table 4). Platelet improvement (100% increase) was recorded in 2 patients, of whom 1 met the criteria for clinical improvement according to IWG-MRT criteria (Table 4).
Of the 10 patients who were transfusion dependent at baseline, 3 achieved transfusion independence (absence of any packed red blood cell transfusions during any consecutive 'rolling' 12-week interval during the treatment phase25); each of these patients has since discontinued treatment, with 2 requiring transfusions at or subsequent to discontinuation. For the other 7 patients who were transfusion dependent at baseline, the frequency of transfusions remained similar throughout treatment. A total of 29 patients were treated who had not received a packed red blood cell tranfusion in the 12 weeks prior to study entry, of whom 4 became transfusion dependent while on treatment (⩾6 units of packed red blood cells during any consecutive ‘rolling' 12-week interval); 2 of these 4 patients subsequently achieved transfusion independence spontaneously while still on treatment.
Clinical response: symptoms
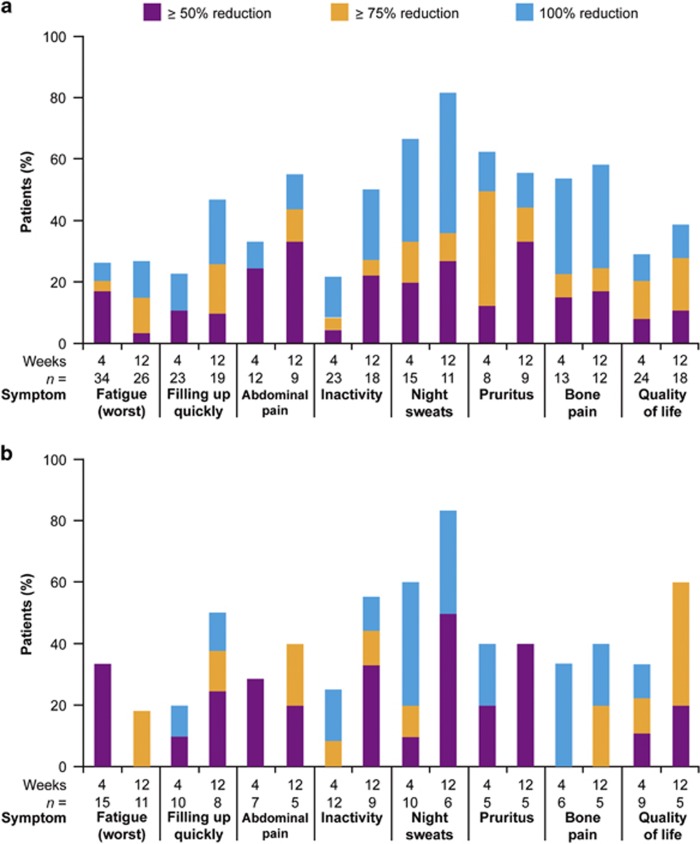
Across the total evaluable population, reductions in mean MF-SAF scores were observed by Week 4 for all the 8 assessed symptoms. The proportion of patients with ⩾50% reduction in symptom scores was similar or increased from Week 4 to Week 12 for each symptom, based on the number of patients evaluable at each time point (Figure 1a). The degree of symptom improvement varied between patients, but reductions in symptom scores of up to 100% were achieved in at least some patients across all symptoms measured. MF-SAF scores were available for 5 patients in the 300 mg cohort; for each symptom, at least 1 patient achieved a ⩾50% improvement at Week 4 and/or Week 12.
Figure 1.
Patients with ⩾50% reduction in individual symptoms for (a) all evaluable patients and (b) evaluable patients who had previously received a JAK2 inhibitor. n, number of evaluable patients (⩾1 cycle treated and score >2 at baseline).
A reduction in mean score for all symptoms was also observed in those patients who had received previous JAK2 inhibitor treatment, with the pattern of symptom improvement broadly similar to the total evaluable population (Figure 1b).
Bone marrow fibrosis
Changes in BMF were assessed in 30 patients (BMF population) who had a grading after 3 cycles of treatment. BMF grading was according to the local study site, based on the assessment of ⩾2 hematopathologists per site. At baseline, grade 3 BMF was observed in 18/30 patients (60%), grade 2 in 10/30 patients (33%) and grade 1 in 2/30 patients (7%). After 3 cycles of NS-018, 11/30 patients (37%) attained an improvement of ⩾1 BMF grade, which was maintained for >12 weeks in 5 of these patients (Table 5).
Table 5. Summary of changes in BMF grade and relationship to clinical symptoms in patients with BMF grade evaluable after completion of 3 cycles of treatment.
| Patient group | BMF grade change to Cycle 4 Day 1 (duration) | Spleen responsea | Increase in Hb of ⩾20 g/l | 100% increase in Plt count |
|---|---|---|---|---|
| NS-018 dose cohort | ||||
| 75 mg QD | 3→2 (3 cycles) | × | ✓ (not CI) | × |
| 125 mg QD | 3→2 (12 cycles) | ✓ | ✓ (not CI) | ✓ (CI) |
| 300 mg QD | 3→2 (3 cycles) | × | × | × |
| 300 mg QD | 3→2 (39 cycles) | ✓ | ✓ (CI) | ✓ (not CI) |
| 300 mg QD | 3→1 (9 cycles) | ✓ | × | × |
| 400 mg QD | 3→2 (6 cycles) | ✓ | × | × |
| 250 mg BID | 3→2 (2 cycles) | × | × | × |
| 300 mg BID | 2→0 (2 cycles) | NP | ✓ (CI) | × |
| 300 mg BID | 3→2 (1 cycles) | × | × | × |
| 400 mg BID | 3→2 (1 cycles) | ✓ | × | |
| 400 mg BID | 2→1 (12 cycles) | ✓ | ✓ (not CI) | × |
| BMF change, n/N (%) | ||||
| BMF improvement after 3 cycles (n=11 evaluable) | — | 6/10 (60)b | 5/11 (45) | 2/11 (18) |
| BMF improvement at any time | — | 11/15 (73)c | 5/17 (29) | 2/17 (12) |
| BMF stabilization or worsening (n=14 evaluable) | — | 7/14 (50) | 2/14 (14) | 0/14 (0) |
Abbreviations: BID, twice-daily dosing; BMF, bone marrow fibrosis; CI, International Working Group for Myelofibrosis Research and Treatment clinical improvement; Hb, hemoglobin; NP, spleen non-palpable (0 cm at baseline); Plt, platelet; QD, once-daily dosing. ✓, achieved specified criterion; ×, did not achieve specified criterion.
Spleen response=reduction in spleen size of ⩾50%.
Excludes 1 patient with NP.
Excludes 2 patients with NP.
Sixteen (53%) patients had unchanged BMF grade after Cycle 3, of whom 6 achieved a BMF improvement from baseline later in the study. Three (10%) patients had BMF worsening at completion of Cycle 3, yet BMF grade returned to baseline at the last reading for 2 of these patients; the other patient was the only case in the BMF population in which the grade remained worsened compared with baseline at the last reading.
Of the patients with improvement in BMF after 3 cycles, 5/11 (45%) achieved a hemoglobin improvement and 2/11 (18%) achieved a platelet improvement (Table 5); whereas in patients without an improvement after 3 cycles (n=19), only 2 hemoglobin responses and no platelet responses were observed. Overall, the rates of spleen response, hemoglobin improvement and platelet improvement were higher in patients who had achieved improvement in BMF at any time during treatment compared with those patients who had stable or worsening BMF grade (Table 5), although there were only a small number of patients in each group.
PD: JAK2V617F allele burden
A total of 35 patients (73%) were positive for the JAK2V617F mutation (Table 1). Allele burden fluctuated only slightly during treatment, and it remained similar to the baseline value in almost all patients. A decrease in allele burden of ⩾25% was observed in only 2 patients: at Cycle 7 in one and at Cycle 16 in the other.
PD: biomarker analysis
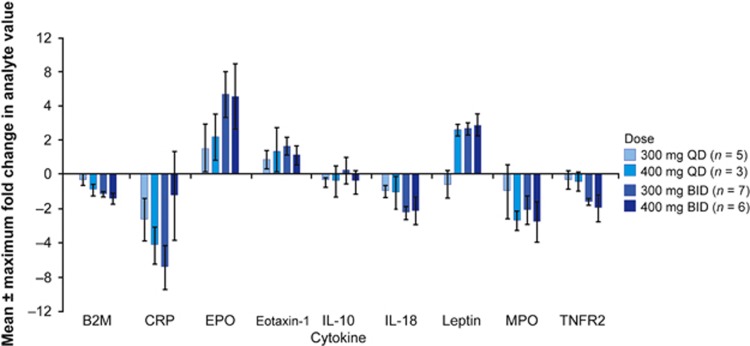
Biomarker concentrations were assessed in 21 patients following 4 cycles of NS-018 doses ⩾300 mg QD (Figure 2). Following NS-018 treatment, a dose-dependent upregulation of erythropoietin, leptin and eotaxin-1 was observed; these biomarkers are associated with improved outcomes in MPNs.26, 27, 28 Dose-dependent downregulation was observed in β-2 macroglobulin (B2M), C-reactive protein (CRP), interleukin (IL)-18, myeloperoxidase and tumor necrosis factor receptor 2 (TNFR2), demonstrating inhibition of the JAK2 pathway. Levels of cytokines tumor necrosis factor-α and IL-6, previously associated with outcomes in MF,27, 29 were unchanged after 4 weeks of treatment.
Figure 2.
Maximum change in biomarker levels following 4 cycles of NS-018 therapy. B2M, β-2 macroglobulin; BID, twice-daily dosing; CRP, C-reactive protein; EPO, erythropoietin α IL, interleukin; MPO, myeloperoxidase; QD, once-daily dosing; TNFR2, tumor necrosis factor receptor 2.
Pharmacokinetics
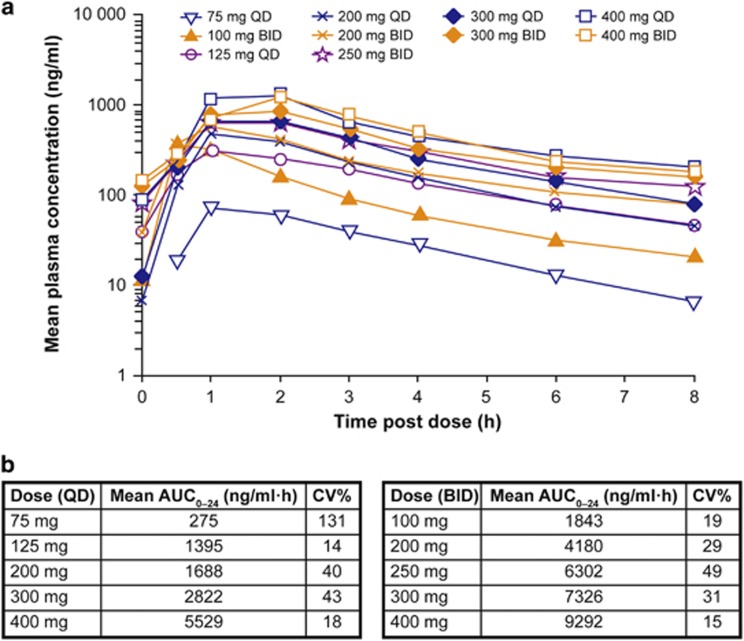
The PK parameters of NS-018 were assessed in 43 patients. On Day 8, maximum plasma concentration (Cmax) was achieved 1–2 h postdosing for all the dose cohorts (Figure 3). Based on Cmax assessments on Day 8, systemic exposure of NS-018 was approximately dose proportional. The half-life of NS-018 was 2–5 h. There was no evidence of drug accumulation as drug exposure was not substantially different between Days 8 and 29.
Figure 3.
Systemic exposure of NS-018. (a) Plot of NS-018 plasma concentration over time on Day 8; (b) AUC0–24 on Day 8. AUC0–24, area under the plasma concentration vs time curve from time 0 to 24 h (the value of AUC0–24 for BID dosing was calculated as twice the value of AUC0–12); BID, twice-daily dosing; CV, coefficient of variation; QD, once-daily dosing.
Discussion
The results of this phase I dose-escalation portion of the phase I/II study indicate that the JAK2 inhibitor NS-018 can improve palpable splenomegaly and constitutional symptoms in patients with MF, even those previously treated with a different JAK2 inhibitor. Among the doses tested, NS-018 300 mg QD was considered to give the best balance between clinical activity and tolerability, and this regimen has been chosen for further assessment in the ongoing phase II portion. This 300 mg QD dose appeared tolerable over long-term dosing, with up to 45 cycles administered to date in 1 patient.
Evaluation of the safety and tolerability of NS-018 (primary objective) revealed that the most common non-hematologic AEs were neurological or gastrointestinal in nature. Of the neurologic AEs, dizziness and paresthesia were the most frequent, which were predominantly grade 1/2 in intensity. No grade 4 neurologic events were observed during the study. The neurologic toxicities had a major influence on the choice of RP2D, with 400 mg QD/BID considered intolerable by the SRC based on the proportion of these events. The study discontinuation rate was lower at 300 mg QD than at 250 mg BID or 300 mg BID, with 2 cases of treatment cessation owing to nervous system disorders in the latter cohort. Three agents in this drug class (fedratinib, XL109 and AZD1480) have been discontinued owing to neurological events30 and a persistent treatment-emergent peripheral neuropathy with momelotinib has been recently reported.31 Although possible mechanisms underlying the neurotoxicity of fedratinib have been proposed,32, 33 there are currently insufficient data from which to generate a hypothesis surrounding neurologic events with NS-018. Irrespective, the need for vigilance around neurologic toxicities in patients treated with JAK inhibitors is clear.
Of the hematologic AEs, thrombocytopenia and anemia were the most common drug-related AEs. These events occurred most frequently with a total daily dose ⩾300 mg, potentially reflecting the greater JAK2-suppressive effects of NS-018 at these dose levels. JAK/STAT signaling is integral in hematopoiesis, so hematologic events are likely a corollary of greater JAK2 inhibition. Indeed, thrombocytopenia and anemia have been frequently observed in clinical trials of other JAK inhibitors.11, 12, 34, 35 However, it should be noted that the effect on hematopoiesis is variable for the different JAK inhibitors, with the JAK2-selective inhibitor pacritinib demonstrating a lack of myelosuppression.36 This favorable effect was ascribed to the lack of JAK1 inhibition and/or suppression of inflammatory signaling through IRAK-1.37 Although comprehensive kinase screening data are not available for NS-018, the specificity of this agent for JAK2 over JAK1 has been reported.18 In the current study of NS-018, drug-related cases of hematologic events at grade 3/4 were not common (thrombocytopenia: 8/48 (17%); anemia: 3/48 (6%)), and anemia (grade 2) was reported in only 1 patient at the 300 mg QD RP2D. Drug-related thrombocytopenia was more frequent in patients with baseline platelet count <100 × 109/l, recorded in 44% (7/16) of this subpopulation compared with 19% of patients with a higher baseline count.
Similar to other JAK2 inhibitors, clinical activity of NS-018 included reduction of splenomegaly and improvement of MF-associated constitutional symptoms. Overall, more than half of the splenic-evaluable patients achieved ⩾50% reduction in spleen size and almost a third attained a 100% reduction by physical examination. Improvement in splenomegaly was observed irrespective of JAK2V617F mutational status, as has been reported for other JAK inhibitors (for example, fedratinib,34 ruxolitinib11). NS-018 was able to elicit durable responses, with clinical improvement (response maintained for at least 8 weeks) in splenomegaly, anemia and thrombocytopenia. A reduction in symptom burden was observed for each of the 8 symptoms measured, with complete resolution (100% reduction) observed in some patients. Symptom improvement (⩾50% reduction in score) was observed after 4 weeks of treatment—the earliest evaluable time—and the proportion of evaluable patients with this level of improvement was maintained or increased at Week 12 for most symptoms, again indicating the durability of response. The efficacy of NS-018 300 mg QD during prolonged follow-up is under investigation in the phase II portion of the study.
The activity of NS-018 was also observed in patients who had received prior JAK2 inhibitor treatment. Indeed, almost half of the evaluable, prior JAK2 inhibitor-treated patients achieved a ⩾50% splenic reduction. Furthermore, symptom improvement and clinical improvement in hemoglobin and platelet levels were also observed among this group of patients. Preliminary results from a study of fedratinib in patients resistant to/intolerant of ruxolitinib support re-treatment with another JAK inhibitor,38 with this strategy under investigation in the phase II portion of the current NS-018 study. It should also be noted that re-treatment with the same JAK inhibitor may also be clinically beneficial, as reported in a recent case study of a patient with MF who achieved resensitization to ruxolitinib.39 In vitro, resistance of MPN cells to ruxolitinib was associated with reactivation of JAK-STAT signaling through heterodimerization between JAK2 and JAK1/TYK2, yet this signaling change was reversible on ruxolitinib withdrawal with cells reverting to their previous ruxolitinib-sensitive state.40 However, the potential for resensitization has not been tested in a large clinical population, and different resistant variants may arise during treatment with different JAK inhibitors. Furthermore, specific toxicities associated with one agent, rather than efficacy, may drive the choice of a different JAK inhibitor for re-treatment. The maintenance of clear treatment records that outline the reason(s) for JAK2-inhibitor discontinuation and choice of subsequent treatments is thus of growing importance in the drive to maximize the effectiveness of JAK2 inhibitor therapy in MPNs. A limitation of this phase I study is that the reasons for discontinuation of previous JAK2 inhibitor treatment were not documented; however, these data are being collected in the phase II portion.
Data from the present study suggest that NS-018 is able to elicit an early improvement in BMF by ⩾1 grade in a subset of patients with MF. Reductions in BMF grade were observed in 11/30 evaluable patients after 3 cycles of treatment, and 5 of these patients sustained this improvement for >12 weeks. More advanced BMF grade (grade 2/3 vs grade 0/1) has previously been associated with worse anemia and splenomegaly in patients with MF.41 Our data suggest that an early reduction in BMF grade may be associated with improvements in anemia and thrombocytopenia, as hemoglobin and platelet responses were more frequent in patients with BMF improvement after Cycle 3 compared with those who had stable/worsening BMF grade at this time point. In addition, of splenic-evaluable patients with BMF improvement after Cycle 3, 6/10 (60%) achieved a ⩾50% splenic reduction. However, the small sample size precludes any firm judgment on the effects of early BMF grade reduction on cytopenias or splenomegaly. It should also be noted that there was no central review of BMF specimens, thus introducing the potential for interobserver/intraobserver variability in assessment of bone marrow architecture; this may be considered a limitation of the study.
The JAK-STAT pathway is central to signaling for a number of biomarkers, and growing evidence suggests that modulation of cytokine and protein levels may underpin some of the clinical benefits of JAK inhibitors in MF.15, 27, 42, 43 Downregulation of biomarkers associated with STAT3 activation (B2M, CRP, IL-18, myeloperoxidase, TNFR2)44, 45, 46 was observed in this study, fitting with the JAK-STAT inhibition expected with NS-018. Furthermore, 4 weeks of treatment with NS-018 resulted in upregulation of erythropoietin, leptin and eotaxin-1, notable because elevation of these cytokines has been associated with improved outcomes in patients with MPNs.26, 27, 28 Overall, the pattern of cytokine regulation observed with NS-018 is slightly different to that reported for the approved JAK inhibitor ruxolitinib.27, 47 For example, CRP was downregulated without a concurrent change in IL-6, which may be unexpected based on the close relationship between these molecules (that is, IL-6 induces CRP production48, 49). However, in the proinflammatory state observed in patients with MF, it is possible that other cytokines also regulate CRP production (for example, IL-1750). Of interest, CRP downregulation was reported without significant IL-6 reduction in patients treated with JAK2-selective inhibitor fedratinib, suggesting that the axis around IL-6/CRP merits further investigation. Among other biomarkers measured in this study, B2M is downregulated during NS-018 treatment, yet this has not been reported with ruxolitinib.51 Serum B2M has been shown to increase in hematologic malignancies, and targeting this molecule with monoclonal antibodies induces apoptosis in hematologic tumor cells.52 In contrast, the proinflammatory cytokine TNFα was downregulated following 4 weeks' of ruxolitinib treatment,11 yet no significant changes were observed in this current study. Although these potential differences are intriguing, the relatively small sample size in the current study prevents definitive conclusions from being drawn.
Allele burden was also measured as part of the PD assessments in this study. The vast majority of patients had no reduction of the mutant JAK2V617F clone with NS-018 treatment. These data are generally consistent with those of other JAK2 inhibitors, for example, momelotinib and fedratinib effected no meaningful change in allele burden;34, 35, 53 fedratinib, similar to NS-018, is a JAK2-selective inhibitor. Furthermore, only a modest reduction in allele burden was seen with long-term ruxolitinib treatment.54
The PK data showed that NS-018 is rapidly absorbed, achieving Cmax within 1–2 h. The exposure of NS-018 increased in an approximately dose-proportional manner, with drug clearance unimpaired at higher doses. Indeed, there appeared to be no drug accumulation with multiple dosing—a potential result of the short half-life (2–5 h) of NS-018.
In summary, the aggregate experience of safety and efficacy data across multiple dose levels favored use of the 300 mg daily dose in the phase II portion of the study. This dose was tolerable and led to improvements in palpable splenomegaly and symptom burden in both JAK2 inhibitor-naive and -pretreated patients. The ongoing phase II portion of this study will further evaluate the safety and efficacy of this dose in patients with JAK2 inhibitor-resistant/-intolerant disease.
Acknowledgments
We thank Taishi Yamashita of NS Pharma, Inc. and Thomas J Myers and Linda J Paradiso of Strategia Therapeutics, Inc. for their data analysis, manuscript preparation and critical review. The authors received editorial and writing support from Adelphi Communications Ltd, funded by NS Pharma, Inc.
Author contributions
SV, MT, ER, MW, OO, CJ, BS and RAM were involved in study design, data collection, data interpretation and writing of the manuscript. TO was involved in study design, data analysis, data interpretation and writing of the manuscript. The paper was reviewed and approved by all the authors.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
SV has received research support from NS Pharma, Inc. MT has received research support from Ariad, BMS, Sanofi, Pfizer and Incyte. ER is part of the speakers' bureau for Celgene and Incyte. MW has no conflicts of interest to disclose. OMO has served on the advisory committee for Incyte, CTI/Baxalta, Celgene, Spectrum and Sunesis. CJ has received research funding from J&J and CTI Biopharma. BS has served as part of the advisory committee and speakers' bureau for Incyte and on the advisory committee for Sanofi. TU is an employee of NS Pharma, Inc. RAM has received research support from Incyte, Gilead, Celgene and NS Pharma, Inc.
Supplementary Material
References
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med 2000; 342: 1255–1265. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007; 31: 737–740. [DOI] [PubMed] [Google Scholar]
- Stein BL, Cervantes F, Giles F, Harrison CN, Verstovsek S. Novel therapies for myelofibrosis. Leuk Lymphoma 2015; 56: 2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F. How I treat myelofibrosis. Blood 2014; 124: 2635–2642. [DOI] [PubMed] [Google Scholar]
- Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 2014; 123: e123–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 2007; 7: 673–683. [DOI] [PubMed] [Google Scholar]
- Tabarroki A, Tiu RV. Molecular genetics of myelofibrosis and its associated disease phenotypes. Transl Med UniSa 2014; 8: 53–64. [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014; 123: 3714–3719. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JO, Cross NC, Mesa RA. The future of JAK inhibition in myelofibrosis and beyond. Blood Rev 2014; 28: 189–196. [DOI] [PubMed] [Google Scholar]
- Incyte Corporation. Highlights of prescribing information: JAKAFITM (ruxolitinib) tablets, for oral use. Approval: 2011 (cited 31 March 2016). Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf.
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, Dipersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–798. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Kantarjian HM, Pardanani AD, Mesa RA, Newton RC, Scherle PA et al. The clinical phenotype of myelofibrosis encompasses a chronic inflammatory state that is favorably altered by INCB018424, a selective inhibitor of JAK1/2. Blood 2008; 122, Abstract 2804. [Google Scholar]
- Fridman J, Nussenzveig R, Liu P, Rodgers J, Burn T, Haley P et al. Discovery and preclinical characterization of INCB018424, a selective JAK2 inhibitor for the treatment of myeloproliferative disorders. Blood 2007; 110: Abstract 3538. [Google Scholar]
- Squires M, Harrison CN, Barosi G, Vannucchi AM, Barbui T, Gisslinger H et al. The relationship between cytokine levels and symptoms in patients (pts) with myelofibrosis from COMFORT-II, a phase 3 study of ruxolitinib (RUX) vs best available therapy (BAT). Blood 2013; 122, Abstract 4070. [Google Scholar]
- Pardanani A, Tefferi A. Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J 2014; 4: e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Shide K, Naito H, Niwa T, Horio T, Miyake J et al. Effect of NS-018, a selective JAK2V617F inhibitor, in a murine model of myelofibrosis. Blood Cancer J 2014; 4: e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Shide K, Niwa T, Homan J, Sugahara S, Horio T et al. Efficacy of NS-018, a potent and selective JAK2/Src inhibitor, in primary cells and mouse models of myeloproliferative neoplasms. Blood Cancer J 2011; 1: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008; 22: 437–438. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006; 108: 1497–1503. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res 2009; 33: 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005; 90: 1128–1132. [PubMed] [Google Scholar]
- Tefferi A, Cervantes F, Mesa R, Passamonti F, Verstovsek S, Vannucchi AM et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013; 122: 1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvasnicka HM, Thiele J, Bueso-Ramos CE, Sun W, Cortes JE, Kantarjian HM et al. Ruxolitinib-induced modulation of bone marrow microenvironment in patients with myelofibrosis is associated with inflammatory cytokine levels. Blood 2014; 124, Abstract 3182. [Google Scholar]
- Harrison CN, Kiladjian JJ, Gisslinger H, Passamonti F, Sirulnik LA, Wang L et al. Association of cytokine levels and reductions in spleen size in COMFORT-II, a phase III study comparing ruxolitinib to best available therapy (BAT). J Clin Oncol 2012; 30(Suppl)abstract 6625. [Google Scholar]
- Cervantes F, Alvarez-Larrán A, Hernández-Boluda JC, Sureda A, Torrebadell M, Montserrat E. Erythropoietin treatment of the anaemia of myelofibrosis with myeloid metaplasia: results in 20 patients and review of the literature. Br J Haematol 2004; 127: 399–403. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira BV, Harrison C. How many JAK inhibitors in myelofibrosis? Best Pract Res Clin Haematol 2014; 27: 187–195. [DOI] [PubMed] [Google Scholar]
- Abdelrahman RA, Begna KH, Al-Kali A, Hogan WJ, Litzow MR, Pardanani A et al. Momelotinib treatment-emergent neuropathy: prevalence, risk factors and outcome in 100 patients with myelofibrosis. Br J Haematol 2015; 169: 77–80. [DOI] [PubMed] [Google Scholar]
- Scott BL, Becker PS. JAK/STAT pathway inhibitors and neurologic toxicity: above all else do no harm? JAMA Oncol 2015; 1: 651–652. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang Y, Diamond S, Boer J, Harris JJ, Li Y et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke's encephalopathy. Drug Metab Dispos 2014; 42: 1656–1662. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 2015; 1: 643–651. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Tefferi A, Jamieson C, Gabrail NY, Lebedinsky C, Gao G et al. A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 2015; 5: e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Komrokji RS. A comprehensive review of pacritinib in myelofibrosis. Future Oncol 2015; 11: 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a non-myelosuppressive JAK2 kinase inhibitor in Phase 3 development in primary and post ET/PV myelofibrosis. Blood 2014; 124, Abstract 1874. [Google Scholar]
- Harrison CN, Schaap NPM, Zweegman S, Jourdan E, Kiladjian J-J, Cervantes F et al. Efficacy and Safety of Fedratinib (SAR302503/TG101348) in Patients with Intermediate- or High-Risk Myelofibrosis (MF), Post-Polycythemia Vera (PV) MF, of Post-Essential Thrombocythemia (ET) MF Previously Treated with Ruxolitinib: Interim Results from a Phase II Study (JAKARTA-2). Blood 2013; 122, Abstract 661. [Google Scholar]
- Gisslinger H, Skrabs C, Gisslinger B, Schoder R, Mullauer L, Kralovics R.. A Case Study of Resensitization to Ruxolitinib, a JAK1/JAK2 Inhibitor, in a Patient with Myelofibrosis (MF). Haematologica 2013; 98(Suppl 1)Abstract P827. [Google Scholar]
- Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 2012; 489: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM. Grade of bone marrow fibrosis is associated with relevant hematological findings-a clinicopathological study on 865 patients with chronic idiopathic myelofibrosis. Ann Hematol 2006; 85: 226–232. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM. How do JAK2-inhibitors work in myelofibrosis: an alternative hypothesis. Leuk Res 2009; 33: 1581–1583. [DOI] [PubMed] [Google Scholar]
- Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev 2013; 24: 133–145. [DOI] [PubMed] [Google Scholar]
- Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem 1996; 271: 9503–9509. [DOI] [PubMed] [Google Scholar]
- Hamilton KE, Simmons JG, Ding S, Van Landeghem L, Lund PK. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol Cancer Res 2011; 9: 1718–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S et al. IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-gamma production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol 2000; 165: 1307–1313. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 2012; 18: 3008–3014. [DOI] [PubMed] [Google Scholar]
- Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica 2011; 96: 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res 2015; 21: 1248–1257. [DOI] [PubMed] [Google Scholar]
- Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem 2007; 282: 27229–27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CN, Kiladjian J-J, Gisslinger H, Passamonti F, Sirulnik A, Wang L et al. Association of Cytokine Levels and Reductions in Spleen Size in COMFORT-II: A Phase 3 Study Comparing Ruxolitinib with Best Available Therapy (BAT). Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, USA, 1–5 June 2012 Poster of abstract 6625. [Google Scholar]
- Yang J, Qian J, Wezeman M, Wang S, Lin P, Wang M et al. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell 2006; 10: 295–307. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013; 27: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013; 122: 4047–4053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.