Abstract
Objectives
We investigated the potential role of cingulum and uncinate fasciculus integrity in trauma-related neural hypervigilance, indexed by less discrimination between amygdala activation to novel and familiar affective images.
Participants
22 women (mean age 21.7 ± 3.9 years) with a history of trauma, and 20 no-trauma controls (mean age 21.9 ± 4.8 years).
Measures
Trauma exposure and trauma-related symptoms were assessed during structured clinical interview. White matter integrity in the anterior cingulum, parahippocampal cingulum, and uncinate fasciculus was measured using diffusion weighted imaging. Amygdala response to novel and familiar affective scenes was measured with functional magnetic resonance imaging.
Results
Trauma-exposed women showed less discrimination between novel and familiar negative images in the amygdala compared to no-trauma controls. In trauma-exposed women, less amygdala discrimination between novel and familiar affective images was associated with less structural integrity in the anterior cingulum, but was not associated with structural integrity of the parahippocampal cingulum or the uncinate fasciculus.
Conclusions
The anterior cingulum might play an important role in impaired novelty discrimination for affective information in the amygdala. This impairment is potentially driven by inefficient habituation and could contribute to persistent behavioral hypervigilance following trauma exposure.
Keywords: Cingulum, White matter, Amygdala, Hypervigilance, Diffusion imaging, Trauma
Highlights
-
•
Trauma-exposed women showed impaired amygdala novelty discrimination for negative images.
-
•
Less novelty discrimination in the amygdala was associated with less anterior cingulum integrity.
-
•
The anterior cingulum might play a role in trauma-related behavioral hypervigilance.
1. Introduction
Exposure to traumatic events can lead to lasting changes in how people respond to affective information in the environment. Many trauma survivors experience chronic hypervigilance, which behaviorally and physiologically is a state of elevated arousal, increased alertness, and constant visual scanning of the surroundings for potential threat (e.g., Dalgleish et al., 2001, Kimble et al., 2010). Hypervigilance can cause significant distress, impair functioning by reducing the attentional resources to focus on the task at hand, and contribute to the maintenance or onset of other symptoms of posttraumatic stress disorder (PTSD) such as re-experiencing and avoidance (e.g., Chemtob et al., 1988, Constans, 2005). Previous neuroimaging work has suggested that abnormal amygdala activation to salient affective information (e.g., Etkin and Wager, 2007, Yoon and Weierich, 2016) and diminished cognitive control by the medial prefrontal cortex (e.g., Bishop et al., 2004) underlie such a hypervigilant state. However, affective and cognitive processes depend on the organization and functional coordination of interconnected brain regions, rather than isolated neural activity. Although a number of studies have investigated variations in the structural connectivity of affective brain regions in trauma-exposed people (e.g., Daniels et al., 2013), as well as beginning to integrate structural and functional connectivity (e.g., Fani et al., 2016), the potential relation between white matter structure and a neural signature of behavioral hypervigilance is still unknown. Taking a multi-method approach that combines structural and functional neuroimaging, we tested a more comprehensive neural model of trauma-related hypervigilance, or over-alertness for threat in the absence of threat.
In hypervigilant states, people show impaired habituation of the affective response to information encountered in daily life, and they remain in a tonic alert and ready state even in the absence of threat. Behaviorally, this state is characterized by heightened attention to the environment, including visual scanning behavior, and heightened physiological readiness to act. Because novel information is affectively salient, by virtue of constituting potential threat, novel information initially activates the brain regions involved in the affective response and anchored by the amygdala (e.g., Balderston et al., 2011, Weierich et al., 2010). However, with repeated presentation of stimuli, this alerting response quickly habituates in healthy people. For example, fMRI studies show that the amygdala response to affective stimuli decreases quickly – regardless of valence (i.e., unpleasant, neutral, or pleasant) – when stimuli are presented repeatedly (e.g., Breiter et al., 1996, Fischer et al., 2003, Weierich et al., 2010). This normative reduction in amygdala response to familiar affective information is impaired in hypervigilant and other stress-related states (e.g., Andreano et al., 2014, Blackford et al., 2011, van den Bulk et al., 2016). Similarly and relatedly, people with trauma-related symptoms also fail to show discrimination between novel and familiar negative information in the amygdala (e.g., Protopopescu et al., 2005, Shin et al., 2005, Tuescher et al., 2011), as less habituation to familiar stimuli results in what essentially is a persistent novelty response. Further, PTSD is associated with abnormally persistent responses to familiar trauma-related stimuli in the lateral occipital complex, which is implicated in object recognition and is modulated by the amygdala response (Hendler et al., 2001).
Structurally, the amygdala is connected to the major white matter pathways implicated in affective processing, and in particular the cingulum and the uncinate fasciculus (e.g., Catani et al., 2012). The cingulum is a medial association pathway that connects the frontal, parietal, and temporal lobes (e.g., Beevor, 1891, Schmahmann and Pandya, 2006). Due to its many short fibers, the cingulum is composed of distinct sub-regions that are associated with different neural functions (Heilbronner and Haber, 2014, Jones et al., 2013a, Schmahmann and Pandya, 2006). Heterogeneity within the tract is further shown by minimal correlation between indices of structural integrity (e.g., fractional anisotropy) and cellular composition in distinct cingulum sub-regions (Jones et al., 2013a, Vogt et al., 2001).
The cingulum bundle can be divided into the cingulate part of the cingulum (CGC; also “anterior cingulum”) and the parahippocampal part of the cingulum (PHC; also “posterior cingulum”, although note that some studies parcellate the PHC and the posterior cingulum separately). The CGC fibers extend through the dorsal and ventral prefrontal cortices, the subgenual anterior cingulate cortex (sgACC), and the dorsal anterior cingulate cortex (dACC). Only a small portion of the fibers from the amygdala and other temporal regions terminate in the CGC. On the other hand, the majority of the PHC fibers contain axons projecting to and from the amygdala, parahippocampal gyrus, and other regions in the medial temporal lobe, with fewer fibers connecting to the prefrontal cortex or the sgACC (e.g., Heilbronner and Haber, 2014). Additionally, the uncinate fasciculus (UF) association fiber bundle carries information to and from the limbic affective regions by connecting the temporal lobe with the medial orbital frontal cortex (e.g., von Der Heide et al., 2013). The CGC and UF both are involved in affect regulation, including top-down modulation of affective responses, whereas the PHC is involved in memory creation and recall of visual scenes (e.g., Keedwell et al., 2016, Suzuki, 1996).
Basic structural studies using diffusion weighted imaging (DWI) in trauma-exposed people have been inconsistent. Some show lower structural integrity in the CGC (e.g., Daniels et al., 2013, Hu et al., 2016, Kim et al., 2006, Sanjuan et al., 2013, Schuff et al., 2011), although increased CGC integrity also has been reported (e.g., Abe et al., 2006, Kennis et al., 2015). In addition, several studies have reported that trauma exposure is associated with decreased (Choi et al., 2009, Fani et al., 2014) or increased (Zhang et al., 2012) structural integrity in the PHC. There also have been mixed findings regarding UF integrity, with some evidence for decreased UF integrity in people with trauma-related symptoms (e.g., Costanzo et al., 2016, Eluvathingal et al., 2006) and some evidence for no association (e.g., Fani et al., 2012). These inconsistencies might be attributed to the wide range of post-trauma symptom profiles, the developmental stage of the brain at the time of first trauma exposure, self-report response biases in symptom assessments, and variation among trauma types (e.g., Naifeh et al., 2008). More recently researchers have begun to test the associations between structure (i.e., white matter integrity) and function (i.e., neural activation patterns) in the affective circuitry of trauma-exposed people. For example, people with PTSD were shown to have less structural integrity of the cingulum, and a genetically-differentiated sample subset also showed poorer hippocampus – anterior cingulate functional connectivity at rest (i.e., Fani et al., 2016). Existing DWI studies have not yet tested the relation between structural integrity in the cingulum and task-based function that is consistent with over-alertness or hypervigilance in the brain.
Given that the CGC and the UF are extensively connected to prefrontal cortices and the sgACC, which are implicated in top-down cognitive control (e.g., Shin et al., 2004, Williams et al., 2006), lower structural integrity in the CGC and the UF might be associated with less habituation to affective information as the amygdala response persists for familiar information rather than habituating to repeated stimulus presentation (e.g., Wright et al., 2001). On the other hand, increased structural integrity in the PHC might reflect greater functional connectivity between the amygdala and the adjacent limbic areas (e.g., parahippocampal gyrus, hippocampus), which has been linked to increased threat sensitivity (e.g., Hahn et al., 2010).
Taken together, prior research shows that that normatively the amygdala responds to novelty in much the same way as to other affective properties (e.g., Balderston et al., 2011, Weierich et al., 2010), and also that trauma exposure can be associated with an overly alert salience response. This overactive salience response is anchored in large part by abnormally persistent amygdala activation in the absence of threat, such as when viewing familiar neutral information (Yoon and Weierich, 2016). Our primary objective was to test the relation between novelty discrimination in the amygdala, as one potential neural index of behavioral hypervigilance, and the structural integrity of relevant white matter tracts. We thus integrated diffusion weighted imaging (DWI) to measure cingulum and UF integrity, and task-based functional magnetic resonance imaging (fMRI) to measure of trauma-related “neural hypervigilance”, indexed by less discrimination between novel and familiar affective images in the amygdala. We tested two primary structure-function hypotheses. First, given the need for prefrontal cognitive control in the process of habituation we hypothesized that novelty discrimination for affective information in the amygdala would be associated with less structural integrity in the CGC, and greater integrity in the PHC. Second, we hypothesized that less amygdala habituation to affective information would be associated with less structural integrity in the UF.
2. Method
2.1. Participants
We recruited 22 trauma-exposed (TE) women and 20 women with no trauma exposure (see Table 1 for participant characteristics) from a large urban university in the northeast US. Given known sex differences in affective processing, we restricted our sample to one sex. The presence or absence of trauma exposure was assessed using the trauma exposure criterion (Criterion A) of the posttraumatic stress disorder (PTSD) module of the Diagnostic and Statistical Manual of Mental Disorders IV. All 42 participants were right-handed and eligible for an MRI scan when assessed with a standard MRI safety screen (e.g., no metal in the body, no history of claustrophobia).
Table 1.
Participant characteristics (N = 42).
| Variable | Trauma-exposed (n = 22) | Control (n = 20) |
|---|---|---|
| Age in years, M (SD), range | 21.7 (3.9), 18–34 | 21.9 (4.8), 18–37 |
| Race/ethnicity, n (%) | ||
| White, non-Hispanic | 4 (18.2) | 9 (45.0) |
| Black, non-Hispanic | 3 (13.6) | 4 (20.0) |
| Asian/Pacific Islander | 8 (36.4) | 4 (20.0) |
| Hispanic | 1 (4.5) | 2 (10.0) |
| Multiple | 2 (9.1) | 0 (0.0) |
| Other | 4 (18.2) | 1 (5.0) |
| PSS, M (SD) | 22.3 (6.6)⁎ | 17.4 (7.0) |
| STAI-S, M (SD) | ||
| Session 1 | 46.5 (13.0)⁎ | 36.5 (10.9) |
| Session 2 | 41.9 (9.5)⁎ | 35.5 (9.9) |
| BDI II, M (SD) | ||
| Session 1 | 15.5 (7.1)⁎⁎ | 9.6 (6.4) |
| Session 2 | 11.8 (7.3)⁎⁎ | 5.7 (5.5) |
| Number of trauma types, M (SD) | 2.4 (1.0) | |
| Trauma type, n (%) | ||
| Natural disaster | 1 (4.5) | |
| Fire/explosion | 3 (13.6) | |
| Motor vehicle accident | 6 (27.3) | |
| Other serious accident | 5 (22.7) | |
| Physical assault | 10 (45.5) | |
| Sexual assault | 8 (36.4) | |
| Other unwanted sexual experience | 1 (4.5) | |
| Life-threatening injury/illness | 3 (13.6) | |
| Witness violent death | 2 (9.1) | |
| Sudden, unexpected death of loved one | 6 (27.3) | |
| Caused serious injury/death of another | 1 (4.5) | |
| Other very stressful event | 6 (27.3) | |
| Total number of PTSD symptoms, M (SD), range | 6.7 (5.0), 0–15 | |
| Re-experiencing symptoms | 2.5 (1.7), 0–5 | |
| Avoidance symptoms | 2.5 (1.8), 0–6 | |
| Hyperarousal symptoms | 1.7 (1.9), 0–5 | |
| Total n meeting DSM-IV PTSD criteria | 5 |
Group differences.
p < 0.05.
p < 0.01.
2.2. Procedure
Two study sessions were conducted on two separate days. The first study session included the Structured Clinical Interview for DSM-IV (SCID) and a brief set of questionnaires. The second session was scheduled within a week of the first session and included the MRI scan. The MRI scan sequence consisted of T1-weighted structural scans, BOLD T2*-weighted task fMRI scans, and a diffusion-weighted structural scan. All procedures were approved by the Institutional Review Board and were conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.2.1. Structured clinical interview
We conducted all modules of the SCID for all DSM-IV Axis I disorders to exclude participants who met criteria for major disorders with the exception of PTSD. No participant met criteria for other major diagnoses, so none were excluded. The TE participants represented the range of trauma-related symptoms. Five of the 22 TE participants met DSM-IV criteria for current PTSD. Of the remaining 17 TE participants, 4 endorsed subclinical levels of current symptoms (in this case met re-experiencing and hyperarousal criteria but did not meet avoidance criteria), 9 endorsed at least some symptoms, and 4 endorsed zero current symptoms. Three participants reported current use of prescription medications: one trauma-exposed participants reported prescription medication (Prozac), as did 2 control participant (Wellbutrin & Lexapro; 1 unspecified non-psychoactive medication). The structural and functional imaging data from these participants did not differ from the data of the other participants in each group when we conducted independent sample t-tests within groups. For the TE group ts ranged from − 0.925–0.003, corresponding p-values from 0.366–0.998, and all 95% CIs of the mean difference included zero. For the no trauma controls, ts ranged from − 1.751–0.124, corresponding p-values from 0.097–0.903, and all 95% CIs of the mean difference included zero. Given the absence of differences on the critical data, we retained the participants on medication in the analyses.
2.2.2. Questionnaires
Following the SCID, participants completed a set of questionnaires, which included the Perceived Stress Scale (PSS, Cohen et al., 1983), the State-Trait Anxiety Inventory – State Version (STAI-S, Spielberger et al., 1983), and the Beck Depression Inventory II (BDI-II, Beck et al., 1996). During the second study session, participants completed STAI-S and BDI-II before the MRI scan.
2.2.3. FMRI task
The fMRI task consisted of 4 event-related functional runs. These runs began approximately 20 min into the scan session. This timing minimized the potential for a confounding influence of scanner-related stress on the BOLD response, as the runs began after the 15-min window during which normative scanner-related stress has been shown to occur and then subside (Muehlhan et al., 2011). During each run, participants viewed 60 full-color images of randomly presented complex scenes that were positive, negative, or neutral in valence.
We selected task stimuli from a stimulus set currently being normed in our lab. The set is designed to depict scenes (rather than discrete objects or single people/animals), and allows us to balance relevant affective elements such as social versus non-social content. Importantly, because this stimulus set is designed to help assess how people respond to information in typical daily life, the scenes have been selected to approximate the affective value of visual information typically encountered in daily life. This criterion means that the unpleasant scenes do not include extreme or explicitly traumatic content such as mutilation or interpersonal violence and the pleasant scenes do not include, for example, highly erotic content. It follows that the ranges of arousal and valence for this set are not as broad as those of, for example, the International Affective Picture System (IAPS; Lang et al., 2008) set, which was specifically designed to capture more of the affective range. We selected scenes for this task based on valence and arousal ratings collected from an initial sample of 748 healthy adults. Valence was rated from 1 to 9, with 1 as most unpleasant and 9 as most pleasant. For the images in this study, valence ratings were: unpleasant (M = 2.61, SD = 1.02), neutral (M = 5.59, SD = 0.84), and pleasant (M = 6.85, SD = 0.86). Arousal also was rated from 1 to 9, with 1 for low arousal and 9 for high arousal. For the images in this study, arousal ratings were: unpleasant (M = 5.60, SD = 1.02), neutral (M = 3.88, SD = 0.65), pleasant (M = 4.58, SD = 0.69). Although the arousal ratings for the unpleasant images were slightly higher than arousal ratings for pleasant images, they were not significantly different.
Runs 1 and 2 were novel; participants viewed each of the images in each run for the first time. Runs 3 and 4 were familiar; the images from Runs 1 and 2 were repeated in Runs 3 and 4. We used the Optseq2 sequence optimization tool (https://surfer.nmr.mgh.harvard.edu/optseq/) to optimize trials within the rapid event-related runs. Inter-trial jitter ranged from 1500 ms to 6000 ms and each run was 332 s long. During each trial, participants viewed a fixation cross for 500 ms, followed by an image for 3500 ms. Participants were asked to press a button on a button box to indicate whether the scene was indoors or outdoors (trauma-exposed n = 14; control n = 17) or to rate the arousal level for each image (trauma-exposed n = 8; control n = 3).1 The task was designed and presented using E-prime experimental software (Psychology Software Tools, Pittsburgh, PA) on a PC. Images were rear-projected to a screen in the magnet bore, and participants viewed images via a mirror mounted on the head coil.
2.2.4. MR image acquisition
We used a Siemens Magnetom Trio Tim 3 T fMRI scanner with a 32-channel gradient head coil. We conducted a localizer scan, followed by a whole brain magnetization prepared rapid gradient echo (MPRAGE) sequence to acquire high-resolution T1-weighted images (TR/TE/flip angle = 2.17 s/4.33 ms/7°, field of view (FOV) = 256 × 256 mm2, matrix = 256 × 256, slice thickness = 1.2 mm, voxel size = 1 × 1 × 1.2 mm3).
Functional MRI images were acquired using a blood oxygen level dependent (BOLD) echoplanar (EPI) T2*-weighted sequence (TR/TE/flip angle = 2.0 s/30 ms/90°, FOV = 220 × 220 mm2, matrix = 64 × 64, slice thickness = 4 mm, voxel size = 3.44 × 3.44 × 4 mm3). The T1- and T2*-weighted images were collected in the same plane (30 axial slices angled perpendicular to the AC/PC line) with an interleaved excitation order and foot to head phase encoding.
We acquired whole brain diffusion-weighted images using a spin-echo echo-planar sequence along 30 diffusion gradient directions and with a b value of 1000s/mm2 (TR/TE/flip angle = 9.5 s/91 ms/90°, b value = 1000s/mm2, FOV = 240 × 240 mm2, matrix = 96 × 96, slice thickness = 2.5 mm, voxel size = 2.5 × 2.5 × 2.5 mm3). Two normalization images with no diffusion encoding (b value = 0) were acquired in the beginning of the sequence.
2.3. Data preparation
2.3.1. fMRI
Functional MRI data were analyzed using Freesurfer FS-FAST software (version 5.3; http://surfer.nmr.mgh.harvard.edu). Functional imaging data were motion corrected to the middle time point of each BOLD run, and inspected for gross motion. Slices were excluded if motion was > 1 mm. In addition, BOLD data were intensity normalized and spatially smoothed (full-width half-maximum = 4 mm) using a 3D Gaussian filter. The first three volumes in each run were discarded to allow for T2* equilibration. Following preprocessing, functional images for each participant were registered to that participant's 3D MPRAGE image.
We conducted a first-level analysis using a general linear model, in which the blood oxygen level-dependent (BOLD) response to each event was modeled using a SPM canonical hemodynamic response function. Bilateral amygdalae were defined a priori based on the Desikan-Killiany atlas (Desikan et al., 2006) using an automated segmentation tool in Freesurfer. BOLD percent signal change in the amygdala (threshold p < 0.05) was modeled for the following 3 contrasts: novel negative versus familiar negative, novel neutral versus familiar neutral, and novel positive versus familiar positive.
2.3.2. DWI
Diffusion weighted images were preprocessed using FMRIB's Software Library (FSL; version 5.0.8; http://fsl.fmrib.ox.ac.uk). Images were first skull-stripped using the brain extraction tool. We then corrected for Eddy current-induced distortions and head motion using an automated affine registration algorithm. Gradient directions (bvecs) were adjusted according to image rotation done during the previous motion correction step. Diffusion tensor maps and scalar maps including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) maps were generated for each participant. FA is a summary measure of structural integrity and is highly sensitive to microstructural changes in the white matter tract. FA varies between 0 (isotropic diffusion) and 1 (anisotropic diffusion), thus higher FA indicates greater structural integrity. MD represents the average magnitude of diffusion in all directions, and RD reflects perpendicular diffusivity. Higher MD and RD indicate decreased integrity (e.g., Alexander et al., 2011, Jones et al., 2013b).
Probabilistic fiber tractography was performed using the FSL plugin AutoPtx (De Groot et al., 2013; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/AutoPtx). The AutoPtx uses the Bayesian Estimation of Diffusion Parameter Obtained using Sampling Techniques (BEDPOSTx) to fit fiber orientation for each voxel (Behrens et al., 2007). Next, using the nonlinear image registration algorithm in FSL (FNIRT), each participant's FA maps were aligned to the FMRIB-58 template FA image. The inverse of this nonlinear warp matrix was applied to predefine seed, target, exclusion, and termination masks for CGC, PHC, and UF provided by AutoPtx. These masks were then warped to native diffusion space for each participant, and probabilistic tractography was conducted using PROBTRACKX in FSL. For each tract, we applied tract-specific thresholds derived by de Groot et al. (2015) from a subsample of 30 participants who each were scanned twice to test reproducibility (thresholds at maximum reproducibility were CGC = 0.01; PHC = 0.02; UF = 0.01) to filter voxels that could be incorrectly classified as part of a tract (Fig. 1). We then computed average FA, MD, and RD.
Fig. 1.
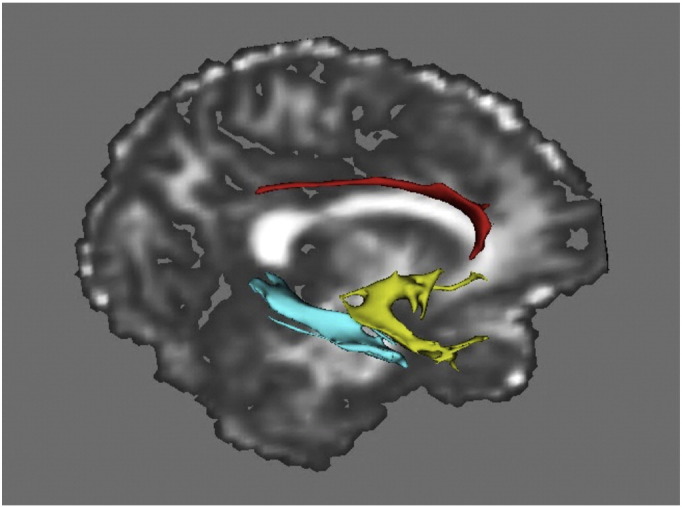
Visualization of the reconstructed cingulate part of cingulum (red), parahippocampal cingulum (blue), and uncinate fasciculus (yellow) from one participant's diffusion data.
2.4. Data analysis
We first tested group differences in amygdala activation for 3 contrasts (novel negative versus familiar negative, novel neutral versus familiar neutral, and novel positive versus familiar positive) with a priori planned comparison t-tests for independent samples. We also tested group differences in white matter integrity between trauma-exposed participants and no-trauma controls in the CGC, PHC, and UF with a priori planned comparison t-tests for independent samples. Next, to test the relation between novelty discrimination in the amygdala and white matter structural integrity, we conducted bivariate correlations between the amygdala response and structural integrity indices (FA, MD, RD) for the three tracts of interest. Correlation analyses were conducted for each group separately. In addition, we tested the relation between white matter tract integrity, novelty discrimination, and PTSD symptoms in the trauma-exposed group.
3. Results
3.1. Descriptive statistics and control variables
Descriptive statistics for functional activation in the bilateral amygdalae and diffusion measures of cingulum and UF integrity are presented in Table 2. The two groups did not differ in age (t(40) = − 0.092, p = 0.927, Cohen's d = − 0.03), and age was not associated with any of the fMRI or DWI measures (ps > 0.05). Trauma-exposed people reported greater perceived stress, state anxiety, and depressed mood, (ps < 0.05; see Table 1 for descriptive statistics). The trauma-exposed participants who met criteria for current PTSD (n = 5) did not differ from the other 17 participants in the TE group (t-values with equal variances not assumed ranged from 1.243 to − 0.161, with corresponding p-values ranging from 0.232 to 0.879).
Table 2.
Amygdala peak magnitude by contrast category and diffusion parameters for white matter tracts.
| Variable | M (SD) | |||
|---|---|---|---|---|
| Trauma-exposed (n = 22) |
Control (n = 20) |
|||
| Right | Left | Right | Left | |
| Amygdala response (% signal change) | ||||
| Novel negative vs Familiar negative | 0.30 (0.58) | 0.11 (0.46)* | 0.25 (0.60) | 0.49 (0.70)* |
| Novel neutral vs Familiar neutral | 0.06 (0.60) | 0.13 (0.27) | 0.28 (0.43) | 0.30 (0.53) |
| Novel positive vs Familiar positive | 0.16 (0.73) | 0.31 (0.72) | 0.12 (0.64) | 0.45 (0.85) |
| Cingulate part of cingulum (CGC) | ||||
| FA | 0.40 (0.02) | 0.43 (0.03) | 0.38 (0.03) | 0.41 (0.04) |
| MD | 79E-05 (3E-05) | 80E-05 (3E-05) | 81E-05 (4E-05) | 83E-05 (5E-05) |
| RD | 63E-05 (3E-05) | 62E-05 (4E-05) | 65E-05(4E-05) | 66E-05 (6E-05) |
| Parahippocampal cingulum (PHC) | ||||
| FA | 0.30 (0.02) | 0.30 (0.02) | 0.29 (0.02) | 0.29 (0.01) |
| MD | 104E-05 (6E-05) | 103E-05 (6E-05) | 108E-05 (6E-05) | 103E-05 (6E-05) |
| RD | 90E-05 (7E-05) | 90E-05 (6E-05) | 96E-05 (6E-05) | 92E-05 (7E-05) |
| Uncinate fasciculus (UC) | ||||
| FA | 0.35 (0.02) | 0.35 (0.02) | 0.35 (0.02) | 0.35 (0.03) |
| MD | 85E-05 (3E-05) | 87E-05 (4E-05) | 86E-05 (3E-05) | 87E-05 (4E-05) |
| RD | 71E-05 (4E-05) | 73E-05 (4E-05) | 73E-05 (5E-05) | 73E-05 (6E-05) |
FA = Fractional anisotropy, MD = mean diffusivity, RD = radial diffusivity.
* denotes significant group difference at p < 0.05.
In the trauma-exposed group depressed mood reported during Session 1 was associated with greater left amygdala habituation to positive images (r = 0.423, p = 0.050). In the no-trauma control group, depressed mood (Session 2) was associated with greater left amygdala habituation to negative images (r = 0.593, p = 0.006). Additionally, depressed mood (Session 1) was associated with less left amygdala habituation to positive images (r = − 0.532, p = 0.019). We report results with and without depressed mood as a covariate in all subsequent analyses with amygdala activation.
Unlike controlling for depressed mood, which is associated with trauma exposure but does not overlap phenomenologically with hyperarousal symptoms including hypervigilance, we made the decision that removal of the variance within the state anxiety (STAI) and perceived stress (PSS) measures in the current study actually could remove a considerable portion of the variance of interest. Thus although we report the descriptives for full characterization of our sample, we do not control for STAI or PSS scores in our analyses.
3.2. Group differences between trauma-exposed women and controls
3.2.1. Novelty discrimination in the amygdala
Trauma-exposed women compared with no-trauma controls showed a non-significant trend toward less novelty discrimination (novel versus familiar) across all three valence categories in the left amygdala, t(39) = − 1.88, p = 0.067, d = 0.59 (medium effect). When testing novelty discrimination for only negative and neutral (ambiguous and therefore potentially threatening) scenes consistent with prior studies (e.g., Yoon and Weierich, 2016), trauma-exposed women compared with controls showed less novelty discrimination across the two categories in the left amygdala, t(39) = − 2.60, p = 0.013, d = 0.82 (large effect). Testing valence individually, trauma-exposed women compared with controls showed less novelty discrimination in the left amygdala for novel negative versus familiar negative images, t(39) = − 2.04, p = 0.048, d = 0.65 (see also Table 2). The groups did not differ in novelty discrimination for negative images in the right amygdala, t(39) = − 1.37, p = 0.178, d = 0.43. In addition the two groups did not differ in amygdala novelty discrimination for neutral (novel neutral versus familiar neutral) or positive images (novel positive versus familiar positive; all ps > 0.05). This specific finding of less novelty discrimination for negative scenes held when we conducted ANCOVAs controlling for depressed mood scores; trauma-exposed women compared with no-trauma controls showed less novelty discrimination in the left amygdala for novel negative versus familiar negative images, F(2,38) = 8.24, p = 0.007, d = 0.91. The groups did not differ in novelty discrimination for negative images in the right amygdala, F(2,38) = 0.014, p = 0.905, d = 0.30), nor for the other functional contrasts (all ps > 0.05).
3.2.2. White matter integrity
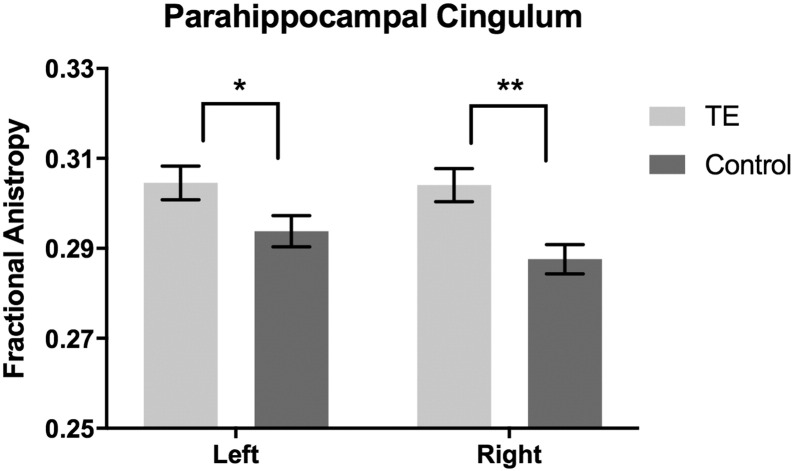
Trauma-exposed women had higher fractional anisotropy (FA) in the PHC bilaterally (right: t(40) = 3.32, p = 0.002, d = 1.04; left: t(40) = 2.08, p = 0.044, d = 0.66) (Fig. 2). In addition, trauma-exposed women had lower mean diffusivity (MD) (t(40) = − 2.28, p = 0.028, d = 0.72) and lower radial diffusivity (RD (t(40) = − 2.51, p = 0.016, d = 0.79) in the right PHC compared to controls. The groups did not differ in the left PHC MD or RD (all ps > 0.05).
Fig. 2.
Trauma-exposed women had higher structural integrity measured via fractional anisotropy (FA) in bilateral PHC compared to no-trauma controls. *p < 0.05, **p < 0.001.
There were no group differences in FA, MD, or RD between two groups in the CGC or the UF (all ps > 0.05), although there was a trend toward greater left CGC FA (t(40) = 1.87, p = 0.069, d = 0.59) and RD (t(40) = − 1.70, p = 0.055, d = 0.54) in the trauma-exposed group compared to controls.
3.3. Associations between white matter integrity and novelty discrimination in the amygdala
We tested the bivariate correlations between white matter tract integrity and amygdala activation, as well as the partial correlations controlling for depressed mood.
3.3.1. Trauma-exposed group
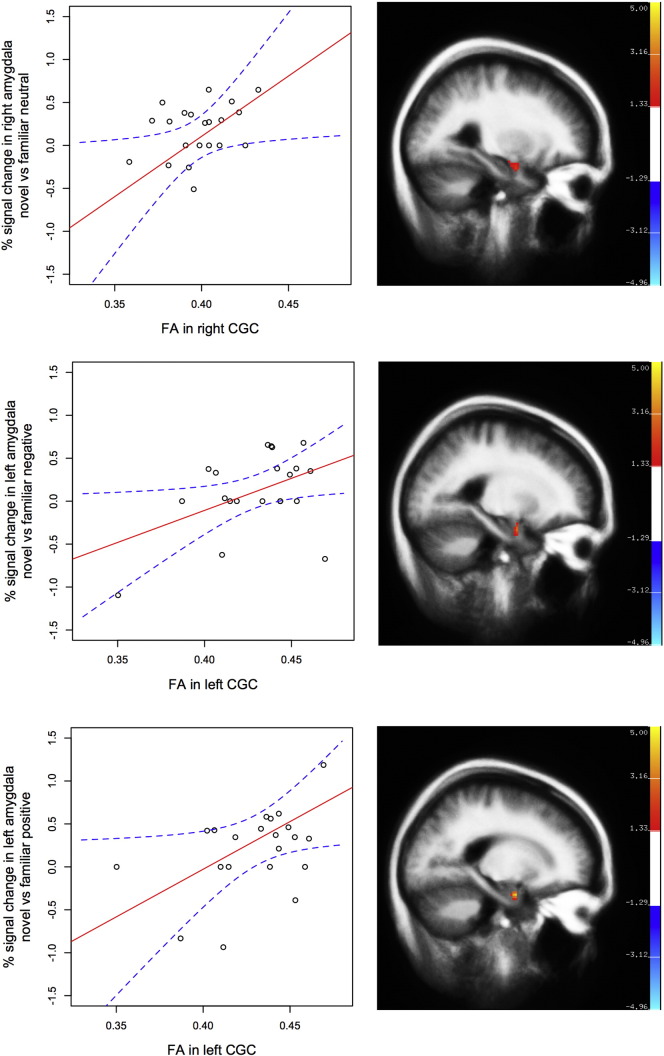
In the trauma-exposed women (n = 22), decreased structural integrity in the CGC was associated with less novelty discrimination in the amygdala for affective images. Lower FA in the right CGC was associated with less discrimination between novel versus familiar neutral images in the right amygdala, r = 0.447, p = 0.037 (Fig. 3). In addition, lower FA in the left CGC was associated with less left amygdala novelty discrimination for negative images (r = 0.459, p = 0.036) and positive images (r = 0.436, p = 0.043; Fig. 3). Higher RD in the left CGC was also associated with less left amygdala novelty discrimination for positive images (r = − 0.475, p = 0.025).
Fig. 3.
In trauma-exposed women (n = 22), fractional anisotropy (FA) in the cingulate part of cingulum (CGC) was inversely associated with less novelty discrimination in the amygdala for affective information. Dotted lines (blue) indicate 95% CI of best-fit line (solid; red).
Novelty discrimination in the amygdala for affective images was not associated with structural integrity in the PHC or the UF (all ps > 0.05). The pattern of results did not differ when we conducted partial correlations with depressed mood scores as covariates.
3.3.2. No-trauma control group
In the no-trauma controls (n = 20), decreased structural integrity in the right UF was associated with less right amygdala novelty discrimination for negative images, indexed by lower FA (r = 0.487, p = 0.029) and higher MD (r = − 0.460, p = 0.042) in the right UF. However, when controlling for depressed mood, although these associations were of a medium effect size (FA partial r = 0.313; MD partial r = − 0.313), they were no longer statistically significant (ps > 0.05).
Novelty discrimination in the amygdala for affective images was not associated with structural integrity in the CGC or the PHC in the control group (all ps > 0.05).
3.4. Relation between white matter integrity and novelty discrimination and trauma-related hyperarousal symptoms.
We conducted these analyses within the trauma-exposed group, as the no-trauma control group did not endorse trauma-related symptoms. A greater number of hyperarousal symptoms (i.e., SCID PTSD Cluster D count) was associated with less structural integrity in the left CGC (r = − 0.450, p = 0.036; Spearman's rho = − 0.397, p = n.s. trend). All other correlations were non-significant (all ps > 0.05). The overall hyperarousal (cluster D) count was not associated with novelty discrimination in the amygdala (largest Spearman's rho was − 0.148, so therefore not even a small effect). Neither total PTSD symptom count nor individual symptom cluster counts were associated with novelty discrimination in the amygdala.
4. Discussion
Consistent with prior evidence of persistent trauma-related amygdala hyperactivity, trauma-exposed women showed less habituation to familiar negative information, defined by less discrimination between novel and familiar negative information, compared to no-trauma controls. Further, in trauma-exposed women, less discrimination between novel and familiar images and a greater number of self-reported hyperarousal symptoms were associated with decreased structural integrity in the CGC, but unrelated to PHC or UF integrity. In addition, trauma-exposed people showed greater structural integrity in the PHC compared to no-trauma controls.
In line with the previous research, our data suggest that the brain's alert systems in no-trauma controls are effective in encoding familiar negative information as less threatening or ambiguous. However, the ability to habituate to threat-relevant information, which would result in greater discrimination between novel and familiar stimuli, is impaired in trauma-exposed people. This indiscriminative amygdala response pattern might be a marker of ongoing behavioral hypervigilance, which can interfere with the ability to focus on goal-oriented information and tasks in everyday life.
Further, by integrating DWI and fMRI data, we tested our hypothesis that diminished white matter integrity in affect-relevant tracts would be associated with our index of neural hypervigilance, novelty discrimination. Our data indicate that the anterior portion of the cingulum (i.e., CGC) might play a role in an over-alert amygdala response to familiar affective information. The cingulum bundle is among the most frequently identified white matter tracts showing structural abnormalities in people with trauma exposure and trauma-related symptoms (e.g., Daniels et al., 2013). A significant portion of prefrontal input travels through the short fibers in the CGC before reaching the limbic brain areas, although some long-range fibers directly connect the prefrontal cortex and the PHC (e.g., Heilbronner and Haber, 2014). Therefore, decreased CGC integrity might reflect inefficient communication between the amygdala and the cognitive control regions, resulting in, or failing to inhibit, hypervigilant (i.e., overly alert) amygdala activity. Supporting this notion, previous fMRI studies have shown decreased activity in the anterior part of the cingulate gyrus and the medial prefrontal cortex in people with trauma-related symptoms (e.g., Hughes and Shin, 2011) and other stress-related states and disorders (e.g., anxiety) (e.g., Bishop et al., 2004).
Less structural integrity of the UF was associated with less amygdala novelty discrimination for negative images, but only in no-trauma control participants. This result supports the general hypothesis that greater UF integrity would be associated with more adaptive novelty responses (i.e., supporting a role of the UF in habituation). However, the absence of this relation in the trauma group was counter to our specific hypotheses. Given the lesser novelty discrimination for negative images in the trauma group, the absence of a relationship could reflect a floor effect, as there was less variability to associate with UF integrity. In addition, if the UF is part of an effective downstream novelty discrimination or habituation process, it might be impaired in trauma-exposed people. To the degree that greater UF integrity represents efficiency in PFC-limbic communication, although structurally intact, this tract might not be recruited as efficiently (functionally) by trauma-exposed brains when viewing affective information.
Although individual variation in novelty discrimination within the trauma-exposed group was associated with differences in CGC integrity, the group difference in white matter integrity between trauma-exposed women and controls was observed in the PHC. Given that group differences were not observed in the CGC, trauma itself might not alter the structural integrity of the CGC. Rather, the variations within the CGC might reflect increased vulnerability to the development of hypervigilance following trauma exposure. Moreover, our data add to the previous evidence that integrity of distinct parts of the cingulum might be differentially associated with maladaptive affective processing (e.g., Jones et al., 2013a, Jones et al., 2013b).
The observed greater structural integrity of the PHC in trauma-exposed compared with no trauma-control women differs from several prior studies showing lesser PHC integrity in trauma-exposed adults (e.g., Choi et al., 2009, Fani et al., 2014), although it is consistent with at least one other (Zhang et al., 2012). There are several potential explanations for this result. First, because we were interested in trauma exposure rather than PTSD diagnosis per se, our participants all were high-functioning and represented a range of trauma symptom severity. Our sample therefore might not be directly comparable to diagnosed PTSD samples. Second, although the group difference was statistically significant, the magnitude of the FA was within the normative range for both groups. Although clearly further research that takes into account both symptom severity and developmental stage (i.e., the cingulum continues to develop into later adulthood) is necessary, it is not unreasonable that the tract that is implicated in episodic memory might be more structurally developed based on habitual and intrusive over-retrieval of episodic memories post-trauma (e.g., re-experiencing). This idea is further supported by the literature showing that the inverse of such episodic memory retrieval strength is observed in the prodrome to diseases of memory such as Alzheimer's (e.g., Ito et al., 2015).
Further, although the cross-sectional nature of the current study design does not allow testing of the causal relation between trauma exposure and differences in PHC integrity, growing evidence from cellular and molecular studies suggests that trauma exposure might augment the structural integrity of the PHC. One potential mechanism might be changes in PHC myelination caused by increased neuronal activity in the affective limbic region, as more frequent electrical impulses have been shown to facilitate myelination action by nearby oligodendrocytes (Ishibashi et al., 2006, Markham and Greenough, 2004, Wang and Young, 2014). Given that people with trauma exposure show greater neuronal activity in the parahippocampal region (Bremner, 1999, Liberzon et al., 1999, Shin et al., 2001), the amygdala (e.g., Shin et al., 2005), and the posterior cingulate gyrus (Bremner, 1999, Lanius et al., 2001, Shin et al., 2001), trauma exposure might result in increased myelination of the axons that constitute the PHC.
Another possible explanation for increased PHC integrity in the trauma-exposed group could be related to heightened levels of stress hormones, such as cortisol, following trauma, which in turn also facilitate oligodendrogenesis. A number of rodent studies have shown that severe stress promotes the production and differentiation of oligodendrocytes in the hippocampal region of the adult brain via the actions of cortisol and glucocorticoid receptors (e.g., Chetty et al., 2014, Matsusue et al., 2014). Consistent with these data from animal studies, one recent cross-sectional study in humans showed that post-traumatic stress is associated with greater hippocampal myelin content (Chao et al., 2015). However, future prospective studies are necessary to test trauma exposure as a mediator of changes in PHC myelination and structural integrity in humans.
The structural integrity of the UF, on the other hand, was not associated with trauma-exposure or neural hypervigilance to familiar information in trauma-exposed women. Although diminished tract integrity in the UF has been consistently reported in people with depression (e.g., Carballedo et al., 2012, de Kwaasteniet et al., 2013, Murphy et al., 2012, Steele et al., 2005) and anxiety (e.g., Hanson et al., 2015, Kim and Whalen, 2009, Tromp et al., 2012), the reports on the association between trauma exposure and UF integrity have been mixed (e.g., Costanzo et al., 2016, Fani et al., 2012). Given our data and previous evidence, the neurological insults from a traumatic experience (i.e., extreme stress) or the neural processes underlying trauma-related hypervigilance might be more specific to the cingulum bundle, but less pronounced in the UF.
There are several potential limitations to the current study that should be addressed in future work. First, our PTSD symptom assessment was a simple symptom count by cluster derived from the Structured Clinical Interview for DSM (SCID). Such a measure cannot differentiate between a person who experiences a particular symptom once per week from a person who experiences that symptom daily. A more nuanced measure that assesses symptom frequency and severity, such as the Clinician Administered PTSD Scale (CAPS), will be essential for a clearer understanding of the relation between behavioral hypervigilance, novelty discrimination as an index of neural hypervigilance or impaired habituation to affect, and structural integrity of relevant tracts.
Second, our stimulus set does not permit an independent test of the role of stimulus arousal level, as the stimuli were intentionally selected to represent scenes commonly encountered in daily life, which usually are less extreme on the arousal spectrum. In our view this limitation to our analyses does not hinder our results, as the phenomenon of interest (hypervigilance, or readiness for threat that does not exist) must be measured in the absence of threat (e.g., highly arousing information), rather than as a reaction to clear and present threat. Nonetheless, future work might test the full range of both the arousal and valence parameters with a goal toward better understanding what constitutes threat for trauma-exposed people.
Third, we did not assess use of hormonal contraceptives nor menstrual cycle phase, although sex hormones have an influence on affective processing as well as trauma-related symptoms. Based on our experience with recruitment for studies in which we do assess OC use and menstrual phase, it is not likely that the distribution of women using OCs in our current sample varies by group and therefore would impact group results. Further, because most of the key analyses involved within subjects contrasts (e.g., novel versus familiar), within group effects are likely to have been minimized. However, assessment of these factors is important for experimental control and will enhance the precision of future results.
Our results expand upon and integrate prior work by testing the interactions between structural and functional candidate neurobiological mechanisms of behavioral hypervigilance. In so doing, this work contributes to an integrated neural model of maladaptive affective processes in trauma-exposed people. Our results suggest that the anterior cingulum might play an important role in diminished discrimination between novel and familiar affective information in the amygdala, therefore, potentially contributing to tonic and exhausting behavioral hypervigilance following trauma exposure. Impaired habituation to affective information is a likely mechanism underlying less novelty discrimination (e.g., Wright et al., 2001). Although habituation to the second presentation of a stimulus is very fast, the literature support that the amygdala in particular does not respond as robustly on the second presentation of affective information (e.g., Balderston et al., 2011). On the other hand, the second presentation is unlikely to represent complete habituation, thus future studies might test the trajectory of habituation to affective images normatively and in trauma exposure.
Acknowledgments
This research was supported in by the National Institutes of Health [, NIMHD MD007599]; and the National Institute on Drug Abuse [2R24 DA012136].
Footnotes
The task difference was due to experimenter error. There were no differences by task type in amygdala activation or in reaction time, which would be the two variables potentially affected, ps > 05. In addition, when we entered task type as a covariate in our planned analyses, the results did not change. We therefore report analyses without task type as a covariate.
References
- Abe O., Yamasue H., Kasai K., Yamada H., Aoki S., Iwanami A., Ohtani T., Masutani Y., Kato N., Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. Neuroimaging. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Alexander A.L., Hurley S.A., Samsonov A.A., Adluru N., Hosseinbor A.P., Mossahebi P., do Tromp P.M., Zakszewski E., Field A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connec. 2011;1(6):423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano J.M., Dickerson B.C., Barrett L.F. Sex differences in the persistence of the amygdala response to negative material. Soc. Cogn. Affect. Neurosci. 2014;9(9):1388–1394. doi: 10.1093/scan/nst127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston N., Schultz D., Helmstetter F. The human amygdala plays a stimulus specific role in the detection of novelty. NeuroImage. 2011;55(4):1889–1898. doi: 10.1016/j.neuroimage.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Ball R., Ranieri W.F. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beevor C.E. On the course of the fibres of the cingulum and the posterior parts of the corpus callosum and fornix in the marmoset monkey. Philos. Trans. R. Soc. Lond. B. 1891;182:135–199. [Google Scholar]
- Behrens T.E.J., Berg H.J., Jbabdi S., Rushworth M.F.S., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A.D. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat. Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Blackford J.U., Avery S.N., Cowan R.L., Shelton R.C., Zald D.H. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc. Cogn. Affect. Neurosci. 2011;6:621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., Kennedy W.A., Rauch S.L., Buckner R.L., Strauss M.M., Hyman S.E., Rosen B.R. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bremner J.D. Does stress damage the brain? Biol. Psychiatry. 1999;45(7):797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Carballedo A., Amico F., Ugwu I., Fagan A.J., Fahey C., Morris D., Meaney J.F., Frodl T. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159(5):537–548. doi: 10.1002/ajmg.b.32060. [DOI] [PubMed] [Google Scholar]
- Catani M., Dell'Acqua F., Bizzi A., Forkel S.J., Williams S.C., Simmons A., Murphy D.G., Thiebaut de Schotten M. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012;48(10):1262–1287. doi: 10.1016/j.cortex.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Chao L.L., Tosun D., Woodward S.H., Kaufer D., Neylan T.C. Preliminary evidence of increased hippocampal myelin content in veterans with posttraumatic stress disorder. Front. Behav. Neurosci. 2015;9:333. doi: 10.3389/fnbeh.2015.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemtob C., Roitblat H., Hamada R., Carlson J., Twentyman C. A cognitive action theory of post-traumatic stress disorder. J. Anxiety Disord. 1988;2:253–275. [Google Scholar]
- Chetty S., Friedman A.R., Taravosh-Lahn K., Kirby E.D., Mirescu C., Guo F., Krupik D., Nicholas A., Geraghty A.C., Krishnamurthy A., Tsai M.-K., Covarrubias D., Wong A.T., Francis D.D., Sapolsky R.M., Palmer T.D., Pleasure D., Kaufer D. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatry. 2014;19:1275–1283. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Jeong B., Rohan M.L., Polcari A.M., Teicher M.H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:386–396. [PubMed] [Google Scholar]
- Constans J.I. Information-processing biases in PTSD. In: Vasterling J.J., editor. Neuropsychology of PTSD: Biological, Cognitive and Clinical Perspectives. Guildford Press; New York: NY: 2005. [Google Scholar]
- Costanzo M.E., Jovanovic T., Pham D., Leaman S., Highland K.B., Norrholm S.D., Roy M.J. White matter microstructure of the uncinate fasciculus is associated with subthreshold posttraumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed service members. Neurosci. Lett. 2016;618:66–71. doi: 10.1016/j.neulet.2016.02.041. [DOI] [PubMed] [Google Scholar]
- Dalgleish T., Moradi A.R., Taghavi M.R., Neshat-Doost H.T., Yule W. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol. Med. 2001;31:541–547. doi: 10.1017/s0033291701003567. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma – a systematic review and meta-analysis. Depress. Anxiety. 2013;216:207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- De Groot M., Vernooij M.W., Klein S., Ikram M.A., Vos F.M., Smith S.M., Niessen W.J., Andersson J.L. Improving alignment in tract-based spatial statistics: evaluation and optimization of image registration. NeuroImage. 2013;76:400–411. doi: 10.1016/j.neuroimage.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot M., Ikram M.A., Akoudad S., Krestin G.P., Hofman A., van der Lugt A., Niessen W.J., Vernooij M.W. Tract-specific white matter degeneration in aging: the Rotterdam study. Alzheimers Dement. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011. [DOI] [PubMed] [Google Scholar]
- de Kwaasteniet B., Ruhe E., Caan M., Rive M., Olabarriaga S., Groefsema M., Heesink L., van Wingen G., Denys D. Relation between structural and functional connectivity in major depressive disorder. Biol. Psychiatry. 2013;74(1):40–47. doi: 10.1016/j.biopsych.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juhasz C., Muzik O., Maqbool M., Chugani D.C., Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., King T.Z., Jovanovic T., Glover E.M., Bradley B., Choi K., Ely T., Gutman D.A., Ressler K.J. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., King T.Z., Reiser E., Binder E.B., Jovanovic T., Bradley B., Ressler K.J. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology. 2014;39:1206–1213. doi: 10.1038/npp.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., King T.Z., Shin J., Srivastava A., Brewster R.C., Jovanovic T., Bradley B., Ressler K.J. Structural and functional connectivity in posttraumatic stress disorder: associations with FKBP5. Depress. Anxiety. 2016;33:300–307. doi: 10.1002/da.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Wright C.I., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res. Bull. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Hahn T., Dresler T., Plichta M.M., Ehlis A.C., Ernst L.H., Markulin F., Polak T., Blaimer M., Deckert J., Lesch K.P., Jakob P.M., Fallgatter A.J. Functional amygdala-hippocampus connectivity during anticipation of aversive events is associated with Gray's trait “sensitivity to punishment”. Biol. Psychiatry. 2010;68(5):459–464. doi: 10.1016/j.biopsych.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 2015;27:1611–1619. doi: 10.1017/S0954579415000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner S.R., Haber S.N. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. J. Neurosci. 2014;34:10041–10054. doi: 10.1523/JNEUROSCI.5459-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T., Rotshtein P., Hadar U. Emotion–perception interplay in the visual cortex: “the eyes follow the heart”. Cell. Mol. Neurobiol. 2001;21(6):733–752. doi: 10.1023/a:1015156222101. [DOI] [PubMed] [Google Scholar]
- Hu H., Zhou Y., Wang Q., Su S., Qiu Y., Ge J., Wang Z., Xiao Z. Association of abnormal white matter integrity in the acute phase of motor vehicle accidents with post-traumatic stress disorder. J. Affect. Disord. 2016;190:714–722. doi: 10.1016/j.jad.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Hughes K.C., Shin L.M. Functional neuroimaging studies of post-traumatic stress disorder. Expert. Rev. Neurother. 2011;11(2):275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T., Dakin K.A., Stevens B., Lee P.R., Kozlov S.V., Stewart C.L., Fields R.D. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Sasaki M., Takahashi J., Uwano K., Yamashita F., Higuchi S., Goodwin J., Harada T., Kudo K., Terayama Y. Detection of early changes in the parahippocampal and posterior cingulum bundles during mild cognitive impairment by using high-resolution multi-parametric diffusion tensor imaging. Psychiatry Res. Neuroimaging. 2015;231:346–352. doi: 10.1016/j.pscychresns.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Christiansen K.F., Chapman R.J., Aggleton J.P. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Doidge A.N., Meyer M., Lawrence N., Lawrence A.D., Jones D.K. Subgenual cingulum microstructure supports control of emotional conflict. Cereb. Cortex. 2016:1–13. doi: 10.1093/cercor/bhw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis M., van Rooij S.J., do Tromp P.M., Fox A.S., Rademaker A.R., Kahn R.S., Kalin N.H., Geuze E. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology. 2015;40:2434–2442. doi: 10.1038/npp.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Whalen P.J. The structural integrity of an amygdala–prefrontal pathway predicts trait anxiety. J. Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Jeong D.U., Sim M.E., Bae S.C., Chung A., Kim M.J., Chang K.H., Ryu J., Renshaw P.F., Lyoo I.K. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Kimble M.O., Fleming K., Bandy C., Kim J., Zambetti A. Eye tracking and visual attention to threating stimuli in veterans of the Iraq war. J. Anxiety Disord. 2010;24(3):293–299. doi: 10.1016/j.janxdis.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Technical Report A-8. University of; Florida, Gainesville, FL: 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Gupta M.A., Neufeld R.W., Gati J.S., Menon R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Taylor S.F., Amdur R., Jung T.D., Chamberlain K.R., Minoshima S., Koeppe R., Fig L.M. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Markham J.A., Greenough W.T. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1(4):351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue K., Aibara D., Hayafuchi R., Matsuo K., Takiguchi S., Gonzalez F.J., Yamano S. Hepatic PPARγ and LXRα independently regulate lipid accumulation in the livers of genetically obese mice. FEBS Lett. 2014;588(14):2277–2281. doi: 10.1016/j.febslet.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M., Lueken U., Wittchen H.U., Kirschbaum C. The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int. J. Psychophysiol. 2011;79(2):118–126. doi: 10.1016/j.ijpsycho.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Murphy M.L., Carballedo A., Fagan A.J., Morris D., Fahey C., Meaney J., Frodl T. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol. Psychiatry. 2012;72(8):663–670. doi: 10.1016/j.biopsych.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Naifeh J.A., North T.C., Davis J.L., Reyes G., Logan C.A., Elhai J.D. Clinical profile differences between PTSD-diagnosed military veterans and crime victims. J. Trauma Dissociation. 2008;9(3):321–334. doi: 10.1080/15299730802139139. [DOI] [PubMed] [Google Scholar]
- Protopopescu X., Pan H., Tuescher O., Cloitre M., Goldstein M., Engelien W., Epstein J., Yang Y., Gorman J., LeDoux J., Silbersweig D., Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol. Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Sanjuan P.M., Thoma R., Claus E.D., Mays N., Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. Neuroimaging. 2013;214:260–268. doi: 10.1016/j.pscychresns.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Oxford University Press. New York; NY: 2006. Fiber pathways of the brain. [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L., Mueller S.G., Wang Z., Marmar C.R., Weiner M.W., Neylan T.C. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Whalen P.J., Pitman R.K., Bush G., Macklin M.L., Lasko N.B., Orr S.P., Rauch S.L. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Orr S.P., Carson M., Rauch S.L., Macklin M.L., Lasko N.B., Peters P.M., Metzger L.J., Dougherty D.D., Cannistraro P., Alpert N.M., Fischman A.J., Pitman R.K. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Wright C.I., Cannistraro P., Wedig M.M., McMullin K., Martis B., Macklin M.L., Lasko N.B., Cavanagh S.R., Krangel T.S., Orr S.P., Pitman R.K., Whalen P.J., Rauch S.L. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto: CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Steele J.D., Bastin M.E., Wardlaw J.M., Ebmeier K.P. Possible structural abnormality of the brainstem in unipolar depressive illness: a transcranial ultrasound and diffusion tensor magnetic resonance imaging study. J. Neurol. Neurosurg. Psychiatry. 2005;76(11):1510–1515. doi: 10.1136/jnnp.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki W.A. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: organization of cortical inputs and interconnections with amygdala and striatum. Semin. Neurosci. 1996;8:3–12. [Google Scholar]
- Tromp D.P., Grupe D.W., Oathes D.J., McFarlin D.R., Hernandez P.J., Kral T.R., Lee J.E., Adams M., Alexander A.L., Nitschke J.B. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch. Gen. Psychiatry. 2012;69:925–934. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuescher O., Protopopescu X., Pan H., Cloitre M., Butler T., Goldstein M., Root J.C., Engelien A., Furman D., Silverman M., Yang Y., Gorman J., LeDoux J., Silbersweig D., Stern E. Differential activity of rostral cingulate and brainstem in panic disorder and PTSD. J. Anxiety Disord. 2011;25(2):251–257. doi: 10.1016/j.janxdis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bulk B.G., Somerville L.H., van Hoof M.J., van Lang N.D., van der Wee N.J., Crone E.A., Vermeiren R.R. Amygdala habituation to emotional faces in adolescents with internalizing disorders, adolescents with childhood sexual abuse related PTSD and healthy adolescents. Dev. Cogn. Neurosci. 2016;21:15–25. doi: 10.1016/j.dcn.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Vogt L.J., Perl D.P., Hof P.R. Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J. Comp. Neurol. 2001;438(3):353–376. doi: 10.1002/cne.1320. [DOI] [PubMed] [Google Scholar]
- von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Young K.M. White matter plasticity in adulthood. Neuroscience. 2014;276:148–160. doi: 10.1016/j.neuroscience.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Weierich M.R., Wright C.I., Negreira A., Dickerson B.C., Barrett L.F. Novelty as a dimension in the affective brain. NeuroImage. 2010;49:1–20. doi: 10.1016/j.neuroimage.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Kemp A.H., Felmingham K., Barton M., Olivieri G., Peduto A., Gordon E., Bryant R. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Wright C.I., Fischer H., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yoon S.A., Weierich M.R. Salivary biomarkers of neural hypervigilance in trauma-exposed women. Psychoneuroendocrinology. 2016;63:17–25. doi: 10.1016/j.psyneuen.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li W., Shu N., Zheng H., Zhang Z., Zhang Y., He Z., Hou C., Li Z., Liu J., Wang L., Duan L., Jiang T., Li L. Increased white matter integrity of posterior cingulate gyrus in the evolution of post-traumatic stress disorder. Acta Neuropsychiatr. 2012;24:34–42. doi: 10.1111/j.1601-5215.2011.00580.x. [DOI] [PubMed] [Google Scholar]