The Eunice Kennedy Shriver National Institute of Child Health and Human Development Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial (SUPPORT)1 compared the effect of oxygen saturation targets on retinopathy of prematurity (ROP) or death among infants born at 24 to 27 weeks’ gestational age. One thousand three hundred sixteen infants were randomized to lower (85%–89%) vs higher (91%–95%) target oxygen saturation. Rates of severe retinopathy of prematurity or death were not different (28.3% and 32.1%, respectively; relative risk with lower target, 0.90; 95% [CI], 0.76 –1.06; P = .21). Lower oxygen targets reduced severe retinopathy of prematurity among survivors (8.6% vs 17.9%; relative risk, 0.52; 95% CI, 0.37 –0.73; P < .001). However, an unanticipated and unexplained finding was increased mortality in the lower saturation group (19.9% vs 16.2%; relative risk, 1.27; 95% CI, 1.01–1.60; P= .04). The study included neurodevelopmental assessment at 2 years of age; at that assessment we noted a disproportionate loss of small for gestational age (SGA) infants. This observation promoted us to assess whether there was an interaction between oxygen target group and growth status.
Methods
Methods and outcomes of SUPPORT have been reported previously.1 In this post hoc analysis, we compared survival rates and causes between SGA (<10% on the Olsen curves)2 and appropriate for gestational age (AGA) infants by assigned target saturation groups using Kaplan-Meier survival analyses. We repeated the analyses in the original cohort using Poisson regression in a generalized estimating equations model that controlled for stratification by clinical center, gestational age strata (24 0/7 to 25 6/7 weeks vs 26 0/7 to 27 6/7 weeks), and familial clustering (as multiple births were randomized to the same treatment group) with the addition of a term-testing interaction between the randomized oxygen target group and growth status.
Results
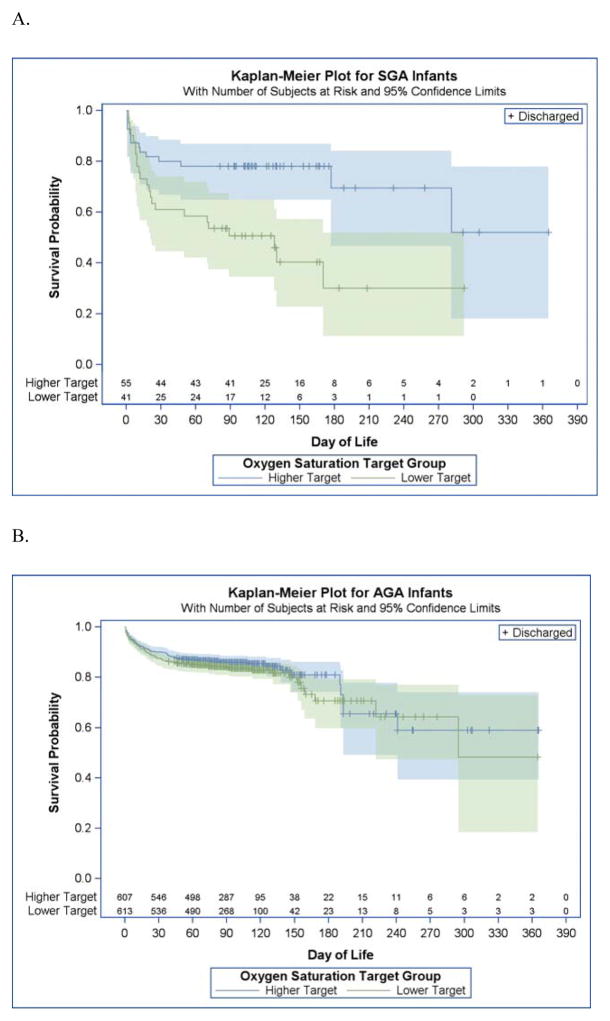
Of the 1316 infants enrolled in SUPPORT, 237 infants died (Table). Thirty-seven of 96 SGA infants died (38.5%), while 200 of 1220 AGA infants (16.4%) died (P < .01). Mortality did not differ significantly for AGA infants between both saturation groups (lower target 17.6% vs higher target 15.2%; P = .17). In contrast, SGA infants had more than twice the mortality in the lower vs the higher target group (lower target 56.1% vs higher target 25.5%; P < .01; interaction term P= .06) (Figure). Severe retinopathy of prematurity was reduced in the lower saturation group in AGA infants (8.5% vs 16.5%; relative risk 0.55; 95% CI, [0.30–0.78]; P < .001), with a numerical difference in the SGA survivors that did not reach statistical significance (12.5% vs 35.1 relative risk 0.59; 95% CI, [0.17–2.07]; P = .41). The leading causes of death in the AGA infants were respiratory distress syndrome and infection, while the leading causes of death in SGA infants were respiratory distress syndrome and bronchopulmonary dysplasia. There was no difference in mortality from necrotizing enterocolitis (14% SGA vs 16% AGA).
Table.
Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial Population Characteristics and Causes of Death by Growth Status
| Population Characteristics | Infants, No./Total No. (%) | P value for Significance | |
|---|---|---|---|
| SGA (n = 96) | AGA (n = 1220) | ||
| Birth weight, mean (SD), g | 516.3 (89.3) | 854.8 (76.8) | <.0001 |
| Gestational age at birth, mean | 26.1 (1.1) | 26.2 (1.1) | .33 |
| Multiple birth | 14/96 (14.6) | 323/1220 (26.5) | .01 |
| Male | 46/96 (47.9) | 666/1220 (54.6) | .21 |
| Race/ethnicity | .03 | ||
| Non-Hispanic black | 38/96 (39.6) | 451/1220 (37.0) | |
| Non-Hispanic white | 46/96 (47.9) | 475/1220 (38.9) | |
| Hispanic | 8/96 (8.3) | 251/1220 (20.6) | |
| Other/Unknown | 4/96 (4.2) | 43/1220 (3.5) | |
| Antenatal steroids (any) | 91/96 (94.8) | 1174/1219 (96.3) | .45 |
| Death | |||
| In the delivery room | 1/96 (1.0) | 5/1220 (0.4) | .38 |
| Prior to discharge | 37 (38.5) | 200 (16.4) | <.01 |
| Causes of death | |||
| Respiratory distress syndrome | 8 (21.6) | 43 (21.5) | |
| Bronchopulmonarydysplasia | 8 (21.6) | 18 (9) | |
| Intraventricularhemorrhage | 4 (10.8) | 20 (10) | |
| Infection | 2 (5.4) | 42 (21) | |
| Necrotizing enterocolitis or perforation | 5 (13.5) | 38 (19) | |
| Malformation | 1 (2.7) | 12 (6) | |
| Pulmonary hypoplasia | 2 (5.4) | 4 (2) | |
| Other | 7 (18.9) | 23 (13.5) | |
Abbreviations: AGA, appropriate for gestational age, SGA, small for gestational age.
Figure.
Kaplan-Meier Survival Curves for Small and Appropriate for Gestational Age Infants in the Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial by Randomized Oxygen Saturation Strata A, small for gestational age infants randomized to the lower oxygen target (shown in green) had significantly poorer survival than those randomized to the higher oxygen target (shown in blue). B, appropriate for gestational age infants had similar mortality between the 2 oxygen strata.
Discussion
This post hoc study found evidence of an interaction between SGA infants and lower oxygen targets associated with increased mortality, which, to our knowledge, has not been reported before. Appropriate for gestational age infants did not experience the same mortality in the lower saturation group. It is known that SGA infants have higher mortality and worse outcomes compared with AGA infants of the same gestation3. Furthermore, growth restriction has been shown to increase the risk of pulmonary hypertension in the setting of bronchopulmonary dysplasia4–6. These reports mirror our finding of bronchopulmonary dysplasia as a leading mortality cause in the SGA cohort. We view these analyses as hypothesis generating. An important opportunity to do this is in the planned Neonatal Oxygenation Prospective Meta-analysis, which includes data from 4800 infants with a prespecified analysis by growth status. We speculate that SGA infants may experience hypoxia in utero that destabilizes respiratory control or affects pulmonary vascular resistance, increasing vulnerability to lower saturation targets.
Acknowledgments
Funding/Support: The National Institutes of Health and the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network provided grant support for the study, and supplemental funding via R03HD078528.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: This article represents the views of the authors and does not necessarily represent the views of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network.
Additional Contributions: The members of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network who contributed to this article include BJ Stoll, P. Sanchez, K. Watterberg, S. Shankaran, and R. Higgins. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which performed the storage, management, and analyses of the data.
Author Contributions: Dr Gantz had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the analyses.
Study concept and design: Walsh, Martin, Carlo.
Acquisition, analysis, or interpretation of data: Walsh, Di Fiore, Gantz, Carlo, Finer.
Drafting of the manuscript: Walsh, Di Fiore, Martin.
Critical revision of the manuscript for important intellectual content: Di Fiore, Gantz, Carlo, Finer.
Statistical analysis: Walsh, Gantz.
Obtained funding: Walsh, Martin.
Administrative, technical, or material support: Di Fiore, Carlo.
Study supervision: Martin.
References
- 1.Carlo WA, Finer NN, Walsh MC, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;363(21):1959–1969. doi: 10.1056/NEJMoa0911781. Medline:20472937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. Medline:20100760. [DOI] [PubMed] [Google Scholar]
- 3.Zeitlin J, El Ayoubi M, Jarreau PH, et al. MOSAIC Research Group. Impact of fetal growth restriction on mortality and morbidity in a very preterm birth cohort. J Pediatr. 2010;157(5):733–739. doi: 10.1016/j.jpeds.2010.05.002. Medline:20955846. [DOI] [PubMed] [Google Scholar]
- 4.Check J, Gotteiner N, Liu X, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33(7):553–557. doi: 10.1038/jp.2012.164. Medline:23328924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014;165(5):909–914. doi: 10.1016/j.jpeds.2014.07.040. Medline:25189821. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson L, Haglund B, Odlind V, Altman M, Ewald U, Kieler H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015;104(3):259–263. doi: 10.1111/apa.12888. Medline:25469645. [DOI] [PubMed] [Google Scholar]