Abstract
Urinary diamines are biomarkers of diisocyanate exposure. Diisocyanates are considered as skin and respiratory sensitizers and are the most frequently reported cause of occupational asthma. Herein, we report on the development and validation of an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for the measurement of five aromatic diamines, 4,4'-methylenedianiline (MDA), 2,4-toluenediamine (4TDA), 2,6-toluenediamine (6TDA), 1,5-naphthalenediamine (NDA), and p-phenylenediamine (PPDA) in human urine. The method incorporates sample preparation steps, which includes a four-hour acid hydrolysis followed by high-throughput solid phase extraction prior to chromatographic separation. Chromatographic separation was achieved using a C18 reversed phase column with gradient elution of basic mobile phases (pH 9.2). The duty cycle of the method was less than five minutes, including both the column equilibration and autosampler movement. Analytical detection was performed using positive ion atmospheric pressure chemical ionization tandem mass spectrometry (APCI-MS/MS) in scheduled multiple reaction monitoring (sMRM) mode. Excellent linearity was observed over standard calibration curve concentration ranges of three orders of magnitude with method detection limit ranging from 10 to 100 pg/mL. The inter-day and intra-day reproducibility and accuracy were within ±15%. This method is fast, accurate, and reproducible, and is suitable for assessment of exposure to the most common aromatic diisocyanates within targeted groups as well as larger population studies such as the National Health and Nutrition Examination Survey (NHANES).
Keywords: Diisocyanate exposure, urinary aromatic diamine, quantitative analysis, biomarkers, UPLC, atmospheric pressure chemical ionization, mass spectrometry
Graphical Abstract
Introduction
Isocyanates are highly reactive compounds that are widely used in commercial and consumer products. In the polyurethane industry, diisocyanates (compounds containing two isocyanate functional groups) are reacted with compounds containing hydroxyl groups, such as polyols, to form urethane linkages that are very useful in the production of polyurethane based products. The most commonly used aromatic diisocyanates are 4,4'–methylenediphenyldiisocyanate (MDI), 2,4–toluenediisocyanate (4TDI), and 2,6–toluenediisocyanate (6TDI). Other commonly used aromatic diisocyanates include 1,5-naphthalenediisocyanate (NDI) and p-phenylenediisocyanate (PPDI). MDI is most commonly used in the manufacturing of rigid foam, fibers, sealants, coatings, glues, and adhesives. Commercial TDI, available as an 80/20 mixture of 4TDI and 6TDI, is used in the manufacturing of flexible polyurethane foam found in car seats, mattresses, and cushions.1, 2 NDI and PPDI are used in the production of synthetic rubber and thermoplastic elastomers.2 Regardless of the benefits that diisocyanates offer to the polyurethane industry, exposure to these reactive compounds may lead to negative health effects. All diisocyanates are skin and respiratory sensitizers.3 Exposure to these chemicals can be lethal when inhaled at high concentrations by sensitized subjects, can elicit hypersensitivity pneumonitis and accelerated lung function loss, and is considered one of the most frequently reported causes of occupational asthma.3–5 In addition, the in vivo hydrolyzed products of diisocyanates (i.e., 4,4'-methylenedianiline (MDA), 2,4-toluenediamine (4TDA), 2,6-toluenediamine (6TDA), 1,5-Naphthanlenediamine (NDA), p-phenylenediamine (PPDA)) have been reported as hepatotoxic (MDA) and suspected human carcinogens (2,4-TDA).6–9
Environmental air monitoring is the most commonly used approach for the assessment of diisocyanate exposure.3, 10–12 However, measurement of diisocyanates content in air fails to address the true body burden. Biological monitoring is one of the most accurate approaches for measuring true body burden to toxicant exposure.13–15
A significant amount of work has been done to understand the toxicokinetics and toxicodynamics of diisocyanates in human and animal models. Hematological studies have shown that diisocyanates are bound to serum albumin16–18 and hemoglobin.19, 20 In urine, however, they are excreted as low molecular weight conjugates and can be analyzed as diisocyanate-derived diamines and/or diamine derivatives.9, 14, 21 Due to the ease of collection and non-invasive nature of urine sampling, the measurement of hydrolyzed urinary diamines has been the most widely accepted approach for the biological monitoring of diisocyanate exposure.
Over the last three decades, multiple analytical methods have been developed for the measurement of urinary aromatic diamines.13, 15, 22 The majority of the methods involve overnight hydrolysis followed by liquid-liquid extraction and sample derivation steps prior to gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS) based analysis.14, 23 Moreover, previously published analytical methods often had unacceptably long run times (~30 minutes per sample). To our knowledge, only Sakai et al. have published a relatively faster LC-MS based method to measure urinary aromatic diamines that involved shorter hydrolysis time, i.e., 1.5 hours, and eliminated sample derivatization step;24 the run time per sample was 13 minutes. However, the method was limited to only two aromatic diamines, i.e., 4TDA and 6TDA and the results reported were based on incomplete hydrolysis.24 The purpose of our study was to develop a simple, sensitive, accurate, rapid, and high-throughput ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) based analytical method for simultaneously measuring the five most common aromatic diamine biomarkers in order to better assess diisocyanate exposure within targeted groups as well as the general population.
Experimental
Standard safety precautions were followed when performing experiments, including the use of personal protective equipment, proper engineering, and administrative compliance based on the risk assessment that identified physical, health, and procedural hazards.
Materials
LCMS OPTIMA grade acetonitrile and isopropyl alcohol (IPA) were purchased from Fisher Scientific (Pittsburg, PA). LCMS grade ammonium acetate (NH4OAc) and ammonium hydroxide (NH4OH) were purchased from Sigma-Aldrich (St. Louis, MO). 1N sodium hydroxide (NaOH) and 6N hydrochloric acid (HCl) were purchased from Sigma-Aldrich. Analytical standard grade PPDA, 4TDA, 6TDA, NDA, and MDA were purchased from Sigma-Aldrich. MDA-[13C, 15N2], PPDA-[13C6], and NDA-[15N2] were purchased from IsoSciences (Kings of Prussia, PA), and 4TDA-[13C6] and 6TDA-[13C6] from Toronto Research Chemicals (Ontario, Canada).
Calibration Solutions
Separate master stocks of mixed standards solution and internal standards solution were prepared in acetonitrile. Master stocks were diluted in water to make working stocks. All calibration solutions were prepared by diluting working stock solution in 90/10 buffer mix (v/v), i.e., 90% 5 mM NH4OAc buffer (pH 9.2) and 10% 95/5 acetonitrile/100 mM NH4OAc buffer (pH 9.2). All stock solutions were stored in −70 °C freezer prior to use.
Sample Preparation
The general workflow for the sample preparation involved acid hydrolysis and automated solid phase extraction (SPE). For acid hydrolysis, 250 µL urine was mixed with 100 µL 6 N HCl and 50 µL internal standard mixture and heated at 80 °C for 4 hours. The solution was allowed to cool at room temperature and then adjusted to approximately pH 1.0 using 500 µL of 1N NaOH prior to SPE. Strata XC (30mg, 3mL) mixed-mode strong cation exchange cartridges from Phenomenex (Torrance, CA) were used for sample clean-up. SPE steps involved cartridge conditioning using 1 mL methanol followed by equilibration using 1 mL HPLC grade water. The hydrolyzed samples were then loaded and washed with 1 mL 0.1 N HCl acid followed by 2 mL methanol. Finally, analytes were eluted in 2 × 500 µL of 75/20/5% (v/v/v) methanol/IPA/NH4OH solutions. Eluents were evaporated to dryness in a TurboVap LV evaporation system from Biotage (Charlotte, NC) under nitrogen gas at 60 °C and reconstituted in 250 µL 90/10 buffer mix for UPLC-MS/MS analysis.
SPE Automation
All SPE experiments were performed on a Microlab Star Liquid Handling Workstation from Hamilton Robotics (Reno, NV). The Hamilton Star™ was built with a modified deck containing four recessed deep well vacuum chambers with one Basic Vacuum System per well. Custom 3 mL cartridge and tube holders were acquired to hold consumables necessary for vacuum application. Each column holder was reinforced with an aluminum collar gasket that permitted uninterrupted vacuum pressure. Each recessed well was capable of applying vacuum pressure to a maximum of 12 sample columns simultaneously. Hence, the system was able to handle 48 samples per batch and the total run time was approximately 1.5 hours.
UPLC-APCI-MS/MS Analysis
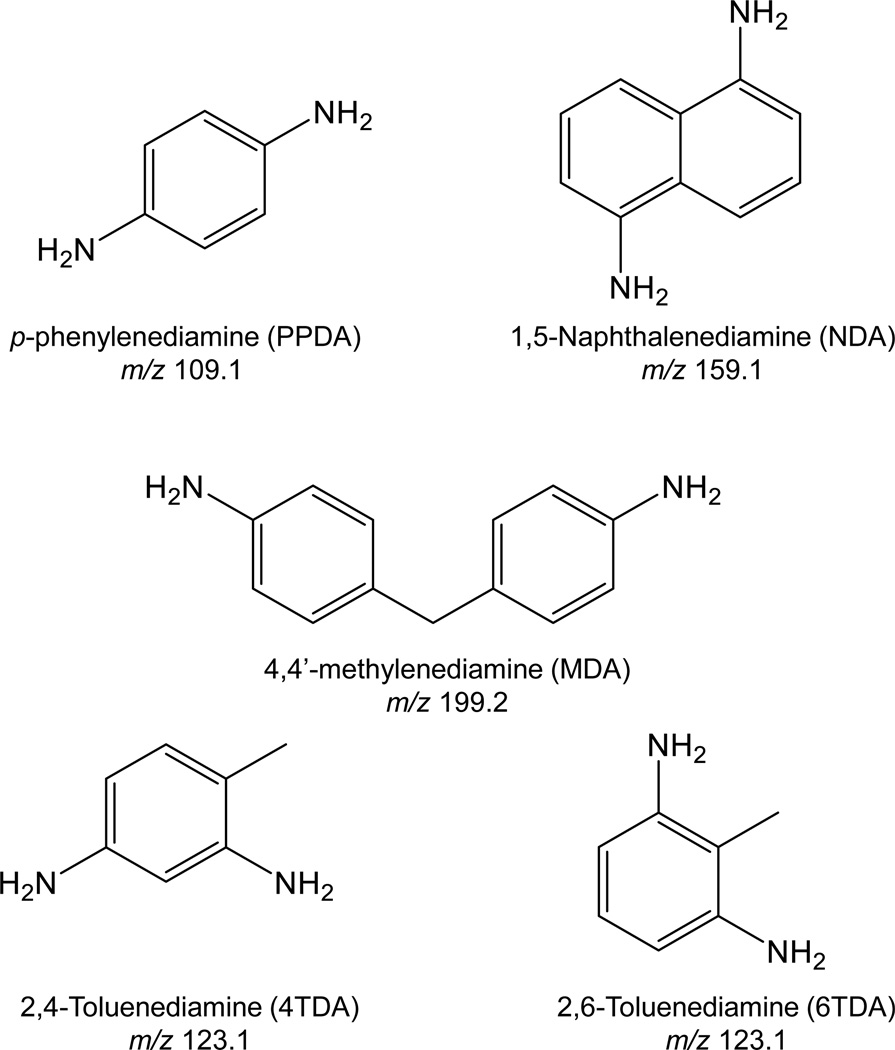
The analytical run was performed using an Acquity UPLC system (Waters Corporation, Milford, MA) equipped with ACE Excel2 SuperC18 Column (Mac-Mod Analytical, Chadds Ford, PA). The UPLC system was coupled to a 5500 triple quadrupole mass spectrometer equipped with an APCI source (AB Sciex, CA). Chemical separation was performed using the following gradient elution: initial gradient, 90% NH4OAc (mobile phase A) and 10% 95/5 acetonitrile/100 mM NH4OAc buffer (mobile phase B); 0–1.0 minute, linear gradient up to 30% B; 1.0–2.0 minutes, linear gradient up to 90% B; 2.0–2.5 minutes, linear gradient back to 15% B, and 2.5–4.0 minutes, 10% B for column equilibration. The flow rate was set at 500 µL/min throughout the run. Column and sample manager temperatures were set to 35 °C and 5 °C, respectively. Injection volume was 5 µL. The mass spectrometer was operated in positive ion APCI scheduled multiple reaction monitoring (sMRM) mode. Optimized ion source parameters were: APCI ion current 3 µA, curtain gas flow 30 (arbitrary units), GS1 gas flow 45 (arbitrary units), and probe temperature 550 °C. The chemical structures and mass-to-charge ratios of the precursor ions monitored are shown in Figure 1. Other compound dependent parameters are shown in Table 1.
Figure 1.
Chemical structures of urinary diamine metabolites of corresponding aromatic diisocyanates. Chemical structures of diisocyanates are identical except –NH2 groups in aromatic rings are substituted by –NCO groups
Table 1.
Compound specific mass spectrometric parameters for aromatic diamines
| Analytes | Ion-transitions (m/z) | DP | EP | CE | CXP | |
|---|---|---|---|---|---|---|
| Quantitation | Confirmation | |||||
| PPDA | 109.1→92 | - | 80 | 10 | 20 | 8 |
| PPDA | - | 109.1→65 | 80 | 10 | 28 | 8 |
| PPDA-13C6 | 115.1→98 | 80 | 8 | 21 | 8 | |
| NDA | 159.1→143.1 | - | 40 | 9 | 26 | 6 |
| NDA | - | 159.1→142.1 | 40 | 9 | 26 | 6 |
| NDA-15N2 | 161.1→144.1 | 40 | 10 | 27 | 6 | |
| MDA | 199.2→106 | - | 40 | 9 | 30 | 10 |
| MDA | - | 199.2→182.1 | 40 | 9 | 25 | 10 |
| MDA-15N2 13C | 202.2→108 | 40 | 9 | 30 | 10 | |
| 4TDA | 123.1→108 | 70 | 5 | 24 | 6 | |
| 4TDA | - | 123.1→106 | 70 | 5 | 20 | 6 |
| 4TDA-13C6 | 129.1→112 | 70 | 8 | 24 | 6 | |
| 6TDA | 123.1→108 | - | 70 | 5 | 20 | 6 |
| 6TDA | - | 123.1→106 | 70 | 8 | 24 | 6 |
| 6TDA-13C6 | 129.1→112 | 70 | 8 | 24 | 6 | |
DP = declusturing potential; EP = entrance potential; CE = collision energy; CXP = cell exit potential
Data Analysis
All LC-MS data were generated in Analyst 1.6.2 (Sciex, Framingham, MA) and were processed in MultiQuant 3.0.2 (Sciex, Framingham, MA).
Results and Discussion
Hydrolysis
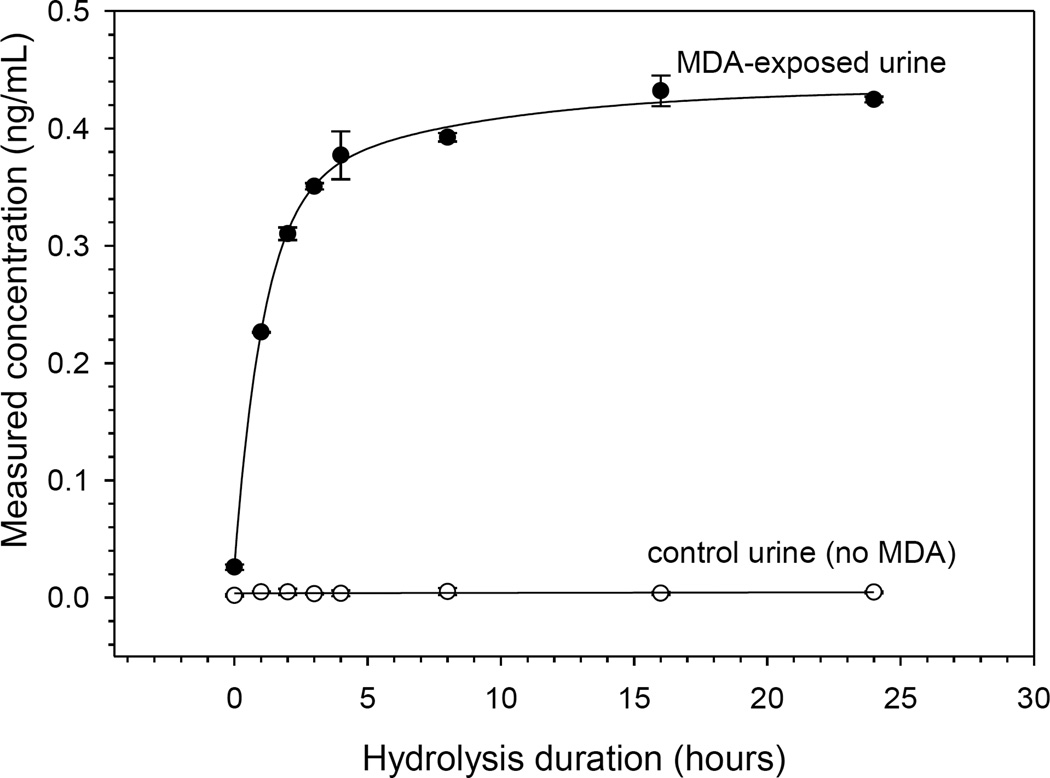
Biological monitoring of exposure to diisocyanates has primarily involved analyzing isocyanate-derived diamines released by acid or base hydrolysis of urine or plasma samples.15 Upon exposure, aromatic diisocyanates are converted in vivo into corresponding diamines. These diamines form conjugates with a variety of functional groups on macromolecules including, hydroxyl, sulfhydryl, and amino groups. In addition, diamines can undergo N-acetylation to form mono- and di-acetylated diamine metabolites, which are readily excreted in urine.9 Acid hydrolysis of urine can breakdown both conjugated and acetylated diamines into a free form while base hydrolysis is only effective for conjugated diamines.21 In this study, acid hydrolysis was used so that the total concentration of diamine metabolites in urine could be measured effectively. Shown in Figure 2 are temporal hydrolysis profiles of MDA concentration in both positive and negative control urine samples over a 24-hour period. These results show a linear increase in concentration of MDA in the positive control urine sample for about 3 hours, at which point the concentration begins to plateau up to 24 hours. No MDA response was observed in negative control urine. As a result, all experiments in this study were performed using 4-hour acid hydrolysis. Additionally, to observe the stability of aromatic diamines under a given hydrolytic condition, spiked urine samples were hydrolyzed over a 24-hour period. No significant difference in absolute response was observed for any analyte (results not shown), indicating that urinary aromatic diamines were stable for at least 24 hours under these hydrolytic conditions.
Figure 2.
Temporal acid hydrolysis profiles of an MDA-exposed urine sample (solid circles) and non-exposed control urine (open circles). Error bars shown are standard deviations for triplicate samples.
Solid Phase Extraction
Solid phase extraction was performed by taking advantage of strong cation-exchange-, π-π interaction-, and hydrophobic-interaction-based mechanisms offered by the Strata-XC SPE cartridge. Listed in Table 2 are pKa1, pKa2, and logP for aromatic diamines. Based on pKa almost all aromatic diamines should be positively charged at a pH below 2.4 according to the Henderson-Hasselbalch approximation. Therefore, below pH 2.4, maximum recovery is expected for these diamines, which are primarily retained by the SPE sorbent through electrostatic interactions. Upon comparison of total spiked recovery at pHs 1, 2, and 3 highest recoveries were obtained at pH 1. At this pH, four out of five aromatic diamines had average recoveries of 100%, except for PPDA where the average recovery was 91%. At pH 2 and 3, PPDA recovery was lower than the other analytes, with recovery of less than 50% at pH 3. Of all analytes, MDA had the highest recovery at all pHs, with average recovery of at least 94% even at pH 3 although it had low pKa values. This observation can be explained based on the higher logP values of MDA that favors hydrophobic interaction offered by the SPE sorbent. In contrast, PPDA had least hydrophobic interactions (low logP & high pKa2) and hence suffered from low recoveries at pHs 2 and 3. Percentage spike recovery was calculated based on the following standard equation using four pre-spiked and four post-spiked urine samples at each of the three pHs (Table 3):
| (1) |
Table 2.
Chemical properties of aromatic diamines.28
| Analytes | pKa1 | pKa2 | logP |
|---|---|---|---|
| PPDA | 3.04 | 6.46 | 0.32 |
| 6TDA | 2.80 | 5.28 | 0.83 |
| 4TDA | 2.91 | 5.58 | 0.83 |
| NDA | 3.20 | 4.41 | 1.30 |
| MDA | 3.92 | 4.83 | 2.41 |
Table 3.
Effect of sample pH on solid phase extraction. Percentage spike recovery of urine samples spiked at various pHs (n=4)
| Analytes | Percentage Spike Recovery | ||
|---|---|---|---|
| pH=1 | pH=2 | pH=3 | |
| PPDA | 90.7 ± 5.7% | 61.3 ± 2.7% | 49.1 ± 1.2% |
| 6TDA | 100.4 ± 7.0% | 79.3 ± 2.9% | 85.6 ± 2.5% |
| 4TDA | 100.2 ± 8.4% | 77.3 ± 8.2% | 79.3 ± 3.9% |
| NDA | 100.4 ± 4.3% | 78.1 ± 6.0% | 81.6 ± 4.7% |
| MDA | 100.4 ± 5.0% | 96.4 ± 7.1% | 94.0 ± 4.7% |
UPLC-APCI-MS/MS
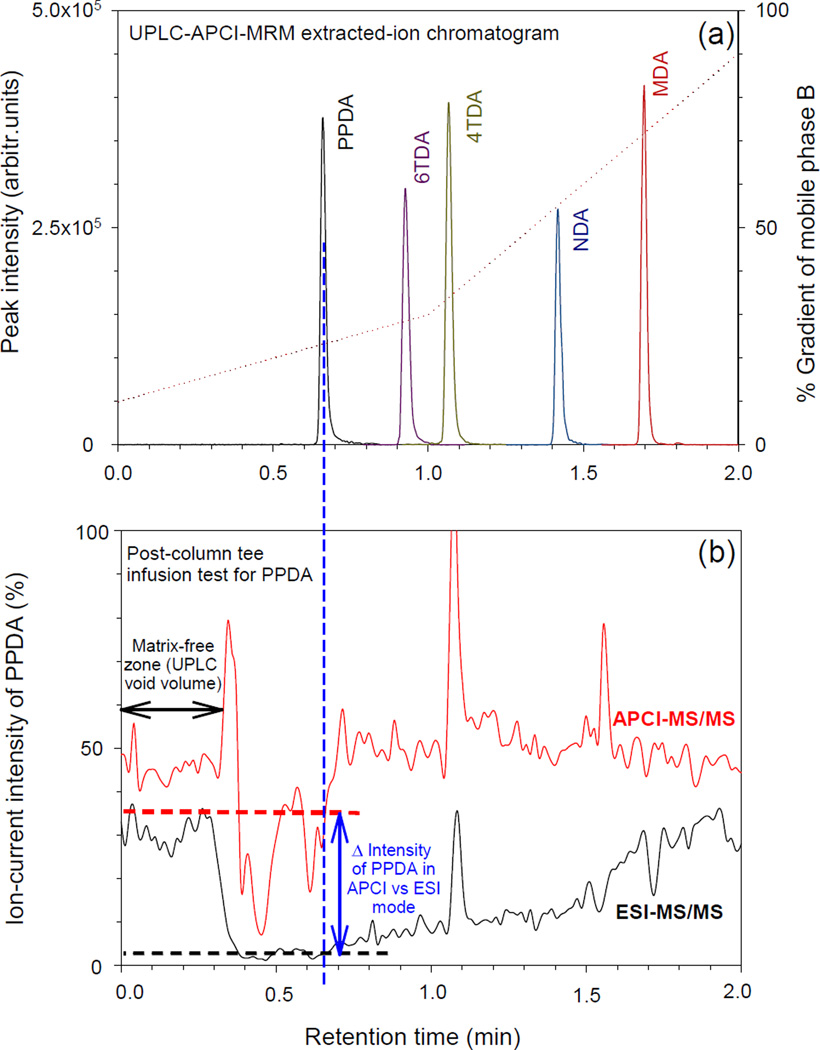
Chromatographic separation was performed at pH 9.2 using an ammonium acetate buffer in both aqueous and organic mobile phases. According to the Henderson-Hasselbalch approximation, all target compounds exist as neutral molecules at pH 9.2 (refer to Table 2 for pKa values) and hence should be well retained by reversed-phase columns. With a gradient elution, from 10% to 90% organic mobile-phase composition, these compounds are eluted sequentially according to their logP values. Figure 3a shows a typical extracted-ion chromatogram of standard solutions overlaid with a mobile-phase gradient plot of the percentage change in composition of the organic mobile phase (mobile phase B). These results show that PPDA, the compound with the lowest logP value, elutes early while MDA, the compound with the highest logP, has the longest retention time. All analytes were ionized using positive ion APCI. APCI ionizes low molecular mass neutral molecules more efficiently based on gas phase ion-molecule reactions, and is inherently less susceptible to matrix suppression effects compared to electrospray ionization (ESI). Matrix suppression effects associated with APCI and ESI modes were compared using post-column tee infusion of urine from a UPLC into a standard solution from an auxiliary syringe (Figure 3b). The urine matrix used for this evaluation was processed through the same sample preparation steps as an unknown sample including acid hydrolysis and SPE. In absence of the urine matrix, the total ion-current of PPDA generated in APCI mode was about 1.5 times higher than in ESI mode as seen by comparing ion intensities in the matrix-free zone in Figure 3. At 0.4 min both APCI and ESI PPDA signal levels were suppressed as the urine front arrived at the ionization source, however the suppression corresponding to the retention time of PPDA (0.67 min) was greatest for ESI. In ESI mode, ion-suppression of PPDA was 90% compared to 25% in APCI mode resulting in a PPDA signal 11 times higher using APCI than ESI with a urine matrix. Similarly, the overall signal produced by APCI for 4TDA, 6TDA, NDA, and MDA in urine matrix were 6, 5.5, 2.5, and 1.5 times higher than in ESI.
Figure 3.
(a) Extracted-ion chromatograms of aromatic diamines collected using UPLC-APCI-MRM as well as percentage composition of mobile phase B against retention time shown as dotted line. (b) Representative post-column tee infusion test showing that ion-current of PPDA produced by APCI in urine matrix is 11 times higher than ESI at its retention time.
Quantitative Measurement and Analytical Figures of Merit
Quantitative measurements were performed using calibration curves that were extended over a concentration range of 0.01–10 ng/mL for MDA and 0.05–50 ng/mL for the other analytes. Coefficients of determination for all curves exceeded 0.995 when fitted by linear regression on 1/x weighted data.
To validate the use of non-urine based calibrators for quantification, a matrix validation experiment was performed. For this experiment, the calibration curve slopes obtained using calibrators mixed in urine and mixed in the calibration solution (i.e., 90/10 buffer mix) were compared. The percentage difference in the slopes for each analyte was below 5%, ranging from 1.4% to 4.7%. Robustness of this method to matrix differences is primarily attributed to use of stable isotope analogs for internal standardization, which behave closely to the native analog.
The intra-day accuracy and precision of the method were assessed by analyzing urine samples spiked at low, intermediate, and high concentration with respect to the calibration range (Table 4). Samples were prepared in triplicates. Inter-day accuracy and precision was calculated from 15 total replicate analyses of spiked urine samples at three different concentrations and were analyzed on five different days over a five-week period. The intra- and inter-day bias for all analytes ranged from −0.78 to −10.2% and −2.0 to 13.7% respectively, while the RSD ranged from 1.0–6.0% and 2.06–8.3% respectively (Table 4).
Table 4.
Intra-day and inter-day accuracy (% bias) and precision (% RSD) of spiked urines at three different concentrations. Samples were analyzed in triplicate. Inter-day data were estimated from spiked urine samples analyzed for five different days over several weeks (n=15)
| Intra-Day | Inter-Day | ||||||
|---|---|---|---|---|---|---|---|
| Analyte | Actual Concentration (ng/mL) |
Calculated Concentration (ng/mL) |
Bias (%) |
RSD (%) |
Calculated Concentration (ng/mL) |
Bias (%) |
RSD (%) |
| PPDA | 0.50 | 0.53 | 6.0 | 6.0 | 0.54 | 8.0 | 8.30 |
| 2.45 | 2.20 | −10.2 | 4.0 | 2.78 | 13.5 | 6.58 | |
| 24.5 | 25.1 | 2.5 | 3.7 | 26.2 | 6.9 | 3.58 | |
| 6TDA | 0.48 | 0.47 | −2.1 | 3.8 | 0.45 | −6.3 | 7.60 |
| 2.45 | 2.40 | −2.0 | 4.5 | 2.50 | 2.0 | 4.44 | |
| 24.4 | 25.2 | 3.3 | 1.5 | 27.4 | 12.3 | 2.06 | |
| 4TDA | 0.49 | 0.44 | −10.2 | 2.0 | 0.51 | 4.1 | 3.42 |
| 2.44 | 2.39 | −2.0 | 2.4 | 2.52 | 3.3 | 4.49 | |
| 24.4 | 24.6 | 0.90 | 2.7 | 27.0 | 10.7 | 2.59 | |
| NDA | 0.50 | 0.47 | −6.0 | 2.3 | 0.51 | 2.0 | 7.14 |
| 2.51 | 2.58 | 2.8 | 2.6 | 2.33 | −7.2 | 2.28 | |
| 25.1 | 25.3 | 0.8 | 2.7 | 24.0 | −4.4 | 2.41 | |
| MDA | 0.16 | 0.162 | 1.25 | 2.7 | 0.17 | 6.3 | 5.09 |
| 0.51 | 0.525 | 2.94 | 1.0 | 0.58 | 13.7 | 2.76 | |
| 5.10 | 5.06 | −0.78 | 2.5 | 5.00 | −2.0 | 5.09 | |
Shown in Table 5 are limits of detection (LODs) and limits of quantitation (LOQs) of aromatic diamines in urine samples. LODs and LOQs were estimated based on 3S0 and 10S0 respectively, where S0 is standard deviation as the concentration approached zero concentration in standard deviation versus concentration plot.25 The estimated detection limits are sub ng/mL for all analytes with the lowest value (10 pg/mL) for MDA and the highest value (100 pg/mL) for PPDA. Since injection volume was 5 µL, the on-column injected detection limit was between 0.05 pg (or 50.4 pmol) for MDA and 0.5 pg (or 924 pmol) for PPDA. Past studies have shown that the concentration of urinary aromatic diamines in occupationally exposed (diisocyanate) subjects are ≥ 200 pg/mL for 4TDA, 6TDA, NDA, and MDA with the lowest detectable background concentration of 50 pg/mL for MDA and 100 pg/mL for 4TDA, 6TDA, and NDA.26 These values are significantly higher than the LODs calculated using our method. Hence, the method reported here has sufficient sensitivity to provide effective assessment of biomarkers of both occupational and environmental exposure to aromatic diisocyanates.
Table 5.
Method LODs and LOQs in urine samples. LODs and LOQs were calculated as 3S0 and 10S0, respectively, where S0 represents standard deviation as the concentration approached zero concentration.
| Analytes | Method LOD (ng/mL) |
Method LOQ (ng/mL) |
|---|---|---|
| PPDA | 0.10 | 0.33 |
| 6TDA | 0.03 | 0.10 |
| 4TDA | 0.03 | 0.10 |
| NDA | 0.03 | 0.10 |
| MDA | 0.01 | 0.03 |
Stability Testing
Temporal stability tests were performed on spiked urine samples at temperatures simulating different sample handling and storage conditions. For this test, spiked urine samples were pooled, aliquoted and stored at three separate temperature conditions (i.e., room temperature, 4 °C, and −20 °C). At various time intervals (i.e., 0, 4, 8, 16, 24, 48, 72, 96, 120, 144, 168 hours) simulating samples under these different temperature conditions, urine aliquots were transferred from their respective temperature location to a −70 °C freezer. Sample storage at −70 °C was the lowest temperature available for us to store urine to minimize sample loss following the time interval. For sample analysis, all samples were removed from the −70 °C and analyzed within the same run. Results showed that aromatic diamine stabilities in collected urine samples require refrigeration within four hours of sample collection, potentially due to loss from surface adsorption/decomposition in polyethylene sample storage cryovials. At room temperature, all analytes were stable for at least four hours and then concentrations dropped down by approximately 80% over seven days, particularly for PPDA, 4TDA and 6TDA, while NDA and MDA were less affected (≤ 50%). Samples stored at 4 °C and −20 °C experienced less than 20% loss.
Additionally, freeze-thaw stability was assessed for spiked urine samples for seven freeze-thaw cycles. The average calculated concentration of all analytes was within 20% indicating adequate stability for up to seven freeze-thaw cycles.
Speed of Analysis and Sample Throughput
Including column equilibration and autosampler movement, the duty cycle of this UPLC-MS/MS method is 4.5 minutes. Maximum throughput for this method is 333 samples per day, which yields 1666 analytical results. In contrast, analytical methods reported elsewhere require at least 15 minutes or longer23, 24, 27 and are limited to their maximum throughput to 96 samples per day or less. This indicates that the method presented here is at least three times faster than other analytical methods currently being used for analyzing aromatic diamines in urine samples. In addition, almost all methods reported in the literature require liquid-liquid extraction (LLE) followed by a sample derivatization step prior to analysis. Typically, LLE is a low-throughput technique compared to automated SPE, and compound derivatization involves another labor-intensive quantitative variable that limits overall sample throughput and speed of the assay. In contrast, our assay utilizes fully automated high-throughput SPE (48 samples per batch in approximately 1.5 hours) and eliminates the time intensive derivatization step, making the method straightforward, fast, and well suited for high-throughput clinical and research laboratories.
Conclusion
A simple and the most comprehensive UPLC-APCI-MS/MS method has been developed and validated for the simultaneous measurement of total concentration of five aromatic diamine biomarkers of exposure to corresponding diisocyanate in human urine. The method incorporates simplified sample preparation, shortened analytical run time, improved sensitivity, high accuracy and precision, high sample recovery, and is well suited for our high-throughput routine biomonitoring laboratory as well as other clinical/research laboratories. The method is currently being used in our laboratory for biomonitoring of aromatic diisocyanate exposures in the general U.S. population as part of NHANES.
Acknowledgments
The authors acknowledge Dr. K. Udeni Alwis and Dr. Hongzhu Liao for their initial input on analytical approaches.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Cocker J. Occup. Med. 2007;57:391–393. doi: 10.1093/occmed/kql148. [DOI] [PubMed] [Google Scholar]
- 2. http://www.hse.gov.uk/aboutus/meetings/iacs/acts/watch/130105/p4annex1.pdf.
- 3.Lockey JE, Redlich CA, Streicher R, Pfahles-Hutchens A, Hakkinen PJ, Ellison GL, Harber P, Utell M, Holland J, Comai A, White M. J. Occup. Environ. Med. 2015;57:44–51. doi: 10.1097/JOM.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dykewicz MS. J. Allergy Clin. Immun. 2009;123:519–528. doi: 10.1016/j.jaci.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Merget R, Marczynski B, Chen Z, Remberger K, Raulf-Heimsoth M, Willroth PO, Baur X. Eur. Respir. J. 2002;19:377–380. doi: 10.1183/09031936.02.00244702. [DOI] [PubMed] [Google Scholar]
- 6.Report on Carcinogens. (13th) http://ntp.niehs.nih.gov/ntp/roc/content/profiles/toluenediisocyanates.pdf.
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemical to Humans. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. 1999 [PMC free article] [PubMed] [Google Scholar]
- 8.McGill DB, Motto JD. N. Engl. J. Med. 1974;291:278–282. doi: 10.1056/NEJM197408082910604. [DOI] [PubMed] [Google Scholar]
- 9.Timchalk C, Smith FA, Bartels MJ. Toxicol. Appl. Pharmacol. 1994;124:181–190. doi: 10.1006/taap.1994.1022. [DOI] [PubMed] [Google Scholar]
- 10.Purnell CJ, Walker RF. Analyst. 1985;110:893–905. doi: 10.1039/an9851000893. [DOI] [PubMed] [Google Scholar]
- 11.Karoly WJ, Flatley JJ, Stevenson RD, Bowers JD. J. Occup. Environ. Hyg. 2004;1:789–798. doi: 10.1080/15459620490885644. [DOI] [PubMed] [Google Scholar]
- 12.Sennbro CJ, Ekman J, Lindh CH, Welinder H, Jönsson BAG, Tinnerberg H. Ann. Occup. Hyg. 2004;48:415–424. doi: 10.1093/annhyg/meh035. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg C, Savolainen H. J. Chromatogr. A. 1986;367:385–392. doi: 10.1016/s0021-9673(00)94859-3. [DOI] [PubMed] [Google Scholar]
- 14.Budnik LT, Nowak D, Merget R, Lemiere C, Baur X. J. Occup. Med. Toxicol. 2011;6 doi: 10.1186/1745-6673-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocker J. Ann. Occup. Hyg. 2011;55:127–131. doi: 10.1093/annhyg/meq083. [DOI] [PubMed] [Google Scholar]
- 16.Johannesson G, Sennbro CJ, Willix P, Lindh CH, Jönsson BAG. Arch. Toxicol. 2004;78:378–383. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- 17.Lind P, Dalene M, Tinnerberg H, Skarping G. Analyst. 1997;122:51–56. doi: 10.1039/a606148f. [DOI] [PubMed] [Google Scholar]
- 18.Luna LG, Green BJ, Zhang F, Arnold SM, Siegel PD, Bartels MJ. Toxicol. Rep. 2014;1:743–751. doi: 10.1016/j.toxrep.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gries W, Leng G. Anal. Bioanal. Chem. 2013;405:7205–7213. doi: 10.1007/s00216-013-7171-z. [DOI] [PubMed] [Google Scholar]
- 20.Sabbioni G, Hartley R, Henschler D, Höllrigl-Rosta A, Koeber R, Schneider S. Chem. Res. Toxicol. 2000;13:82–89. doi: 10.1021/tx990096e. [DOI] [PubMed] [Google Scholar]
- 21.Sepai O, Henschler D, Sabbioni G. Carcinogenesis. 1995;16:2583–2587. doi: 10.1093/carcin/16.10.2583. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg C, Savolainen H. Analyst. 1986;111:1069–1071. doi: 10.1039/an9861101069. [DOI] [PubMed] [Google Scholar]
- 23.Marand Å, Karlsson D, Dalene M, Skarping G. Analyst. 2004;129:522–528. doi: 10.1039/b403439b. [DOI] [PubMed] [Google Scholar]
- 24.Sakai T, Morita Y, Kim Y, Tao YX. Toxicol. Lett. 2002;134:259–264. doi: 10.1016/s0378-4274(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 25.Taylor J. Quality Assurance of Chemical Measurements. New York: Lewis Publishers; 1987. [Google Scholar]
- 26.Sennbro CJ, Littorin M, Tinnerberg H, Jönsson BAG. Int. Arch. Occ. Env. Hea. 2005;78:541–546. doi: 10.1007/s00420-005-0619-5. [DOI] [PubMed] [Google Scholar]
- 27.Sennbro CJ, Lindh CH, Tinnerberg H, Gustavsson C, Littorin M, Welinder H, Jönsson BAG. Biomarkers. 2003;8:204–217. doi: 10.1080/1354750031000090660. [DOI] [PubMed] [Google Scholar]
- 28. http://www.chemicalize.org/ [Google Scholar]