Abstract
Studies in rats, monkeys and humans have established that the medial prefrontal cortex is crucial for the ability to exert adaptive control over behavior. Here, we review studies on the role of the rat medial prefrontal cortex in adaptive control, with a focus on simple reaction time tasks that can be easily used across species and have clinical relevance. The performance of these tasks is associated with neural activity in the medial prefrontal cortex that reflects stimulus detection, action timing, and outcome monitoring. We describe rhythmic neural activity that occurs when animals initiate a temporally extended action. Such rhythmic activity is coterminous with major changes in population spike activity. Testing animals over a series of sessions with varying pre-stimulus intervals showed that the signals adapt to the current temporal demands of the task. Disruptions of rhythmic neural activity occur on error trials (premature responding) and lead to a persistent encoding of the error and a subsequent change in behavioral performance (i.e. post-error slowing). Analysis of simultaneously recorded spike activity suggests that the presence of strong theta rhythms is coterminous with altered network dynamics, and might serve as a mechanism for adaptive control. Computational modeling suggests that these signals may enable learning from errors. Together, our findings contribute to an emerging literature and provide a new perspective on the neuronal mechanisms for the adaptive control of action.
Keywords: anterior cingulate, inhibition, delta, theta, phase, dynamics, learning, timing
Adaptive control enables organisms to adjust behavior in the face of unexpected outcomes, such as when a mistake is made in performing a task. Reaction time (RT) tasks might be the simplest type of behavioral task that can be used to probe the brain basis of adaptive control. These tasks require a participant to sustain an action over an extended period of time and terminate that action, or make a second distinct action, in response to an external stimulus. The behavioral mechanisms of variability in RT performance have been studied for more than a century (Woodrow, 1914). A large number of studies have established that RT performance varies as a function of the participant's attention to the action-imperative stimulus and the duration and variability of the time period before the stimulus (i.e. the foreperiod).
A variety of clinical populations exhibit deficits in RT performance (e.g. Parkinson's disease: Evarts et al., 1981; Jahanshahi et al., 1992; Bherer et al., 2003; Alzheimer's disease: Ferris et al., 1976; Sylvain-Roy et al., 2010; ADHD: Vallesi and Shallice, 2007). Changes in RT performance also occur in normal human aging (Bherer and Belleville, 2004; van Dyck et al., 2008; Vallesi et al., 2009) and following damage in the medial frontal cortex due to stroke (Stuss et al., 2005). Moreover, there are apparent increases in impulsive (premature) responding immediately after lesions are made in the anterior cingulate cortex, a medial frontal area, in patients with intractable obsessive-compulsive disorder (Srinivasan et al., 2013). These findings are concordant with animal studies that are reviewed below. Given the clinical significance of these RT deficits, we suggest that more effort should be put into understanding the role of the medial frontal cortex in the adaptive control of RT performance.
Lesion and reversible inactivation studies have consistently found that medial parts of the frontal cortex, referred to here as the medial prefrontal cortex or mPFC, are necessary for the ability to postpone actions, i.e. to wait before acting (see Bari and Robbins 2013 for review). The challenge has been to reveal the neuronal mechanisms by which the mPFC postpones a given action. Recordings of spike activity show that the firing rates of many mPFC neurons change around the behavioral events that define transitions in the behavioral procedure (Narayanan and Laubach, 2009 and Figures 7, 8 and 10 in the present manuscript). In simple RT and time production tasks, these events are (1) pressing the lever to begin a trial, (2) the trigger stimulus, (3) releasing the lever after the stimulus (RT task) or the temporal deadline (time production task), and (4) feedback about success in performing the task (reward or time-out). As described below, each of these events is associated with substantial changes in neuronal firing rates in the mPFC and also changes in local field potentials, including prominent event-related potentials (ERPs) and significant changes in event-related spectral power (ERSP) and inter-trial coherence (ITC). (See Delorme and Makeig (2004) for review of these spectral measures.)
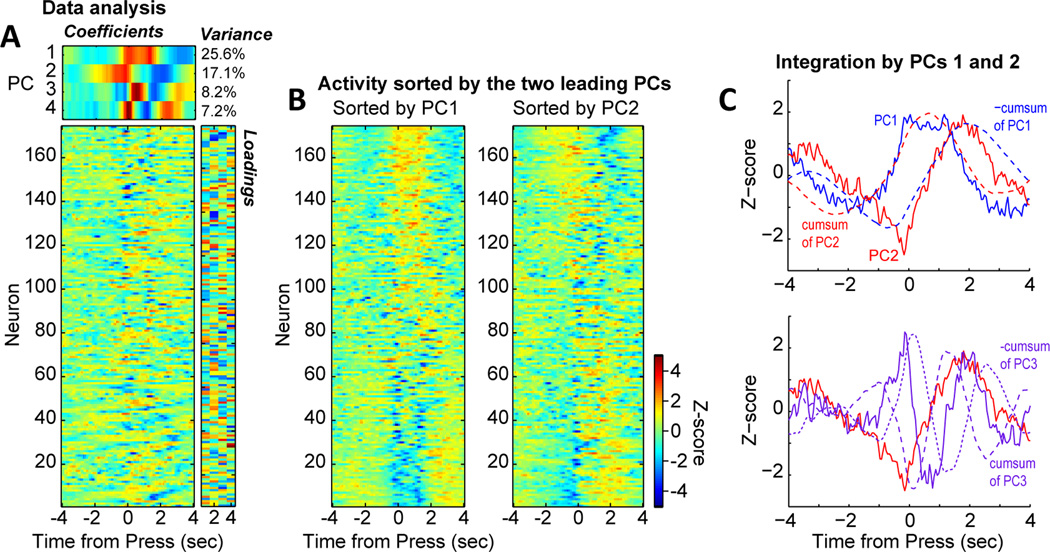
Figure 7.
Evidence for temporal integration of lever pressing by population activity in the medial prefrontal cortex. (A) Analysis of trial-averaged peri-event histograms using principal component analysis (PCA), as in Narayanan and Laubach (2009) and Bekolay et al. (2014). Activity from each neuron is shown in the matrix plot on the lower right. The PCA analysis finds the most common modes of firing (temporal patterns) from the ensemble of neurons. The temporal patterns were defined by the eigenvectors (PCs) from the analysis (upper matrix). The amount of variance accounted for by each PC was defined by the eigenvalues (noted in text next to the upper matrix). The extent to which each neuron expressed the firing patterns (i.e. were correlated with the PCs) were defined by the loadings (right matrix). (B) The neural firing patterns were sorted by the first and second leading PCs. This revealed sustained fluctuation of activity by many neurons during the delay period (PC1) and transient fluctuations around the press and release/reward events (PC2). (C) Plots of the two leading PCs showed the major firing patterns expressed by the medial prefrontal cortex neurons. PC1 is shown in solid blue and PC2 in shown in solid red. The cumulative sums of each function are plotted as dashed lines. PC1 was highly similar to the cumulative sum of PC2 and vice versa. Plots of PCs 2 and 3 did not reveal similar correspondences between the PCs and their cumulative sums.
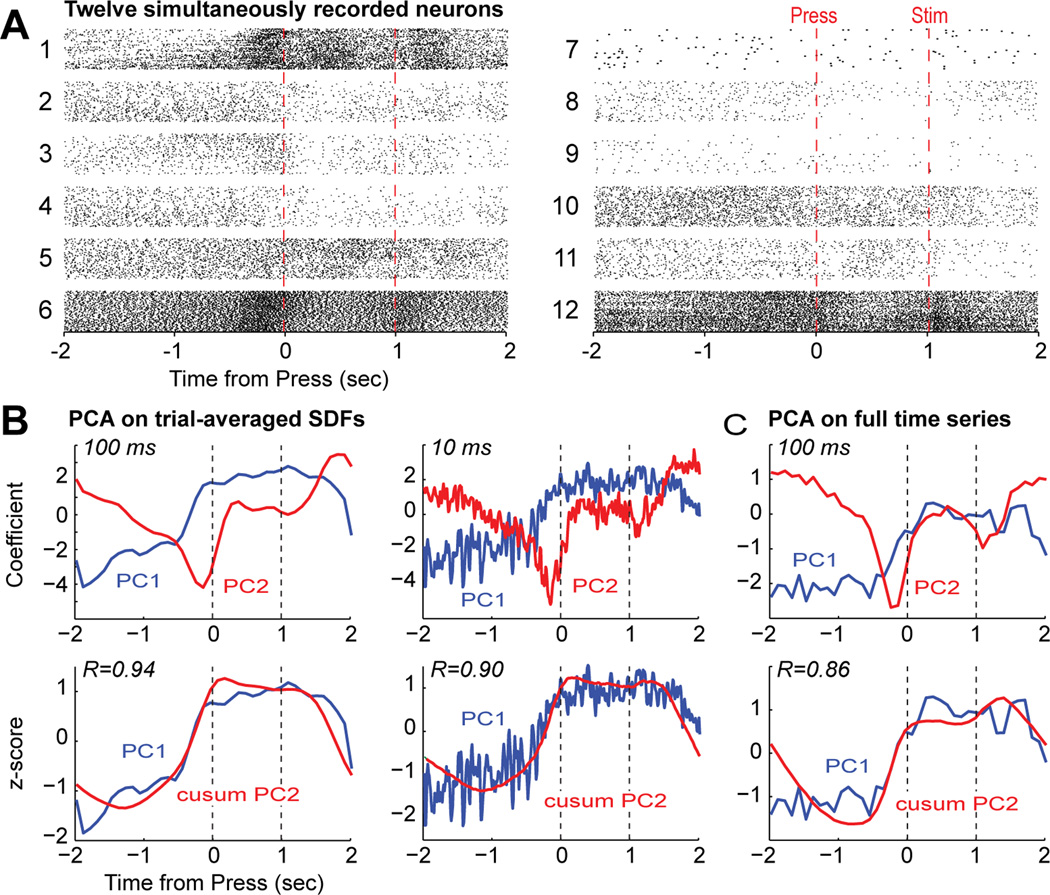
Figure 8.
Evidence for temporal integration of lever pressing by a single ensemble of mPFC neurons. (A) Raster plots for twelve simultaneously recorded neurons. Only trials with the tone stimulus and correct responding (sustained until tone and RT less than 0.6 sec) are shown. (B) Plots of the two leading PCs measured from the trial-averaged Spike Density Functions (SDFs) with a temporal resolution of 100 and 10 ms are shown in the upper plots. The same temporal patterns were identified by PCA at both time scales and these patterns were highly similar to the patterns found for the SDFs of the larger set of neurons from multiple rats (Narayanan and Laubach, 2009). Plots of the leading PC and the cumulative sum of the second PC are shown in the lower plots.. Integration occurred at both time scales. (C) Plots of the two leading PCs measured with an alternative approach using the full time series representing the neural spike train, analyzing a matrix comprised of neurons as the columns and samples (bins) as the rows (Chapin and Nicolelis, 1999; Laubach et al., 1999). The same patterns of firing rate modulation were found using this method (compare the upper plots in B and C) and the same evidence for temporal integration by the two leading PCs was also found using this analysis method.
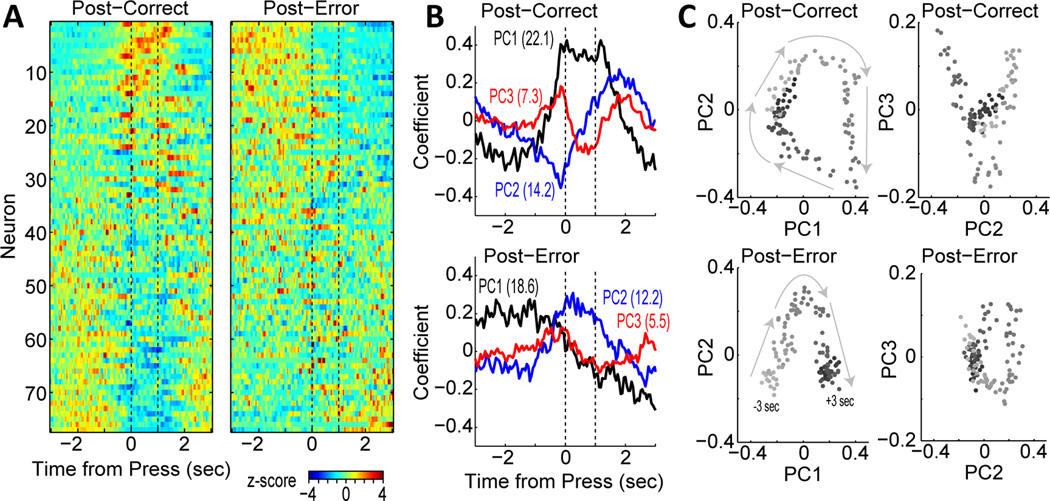
Figure 10.
Altered integration of behavioral events after errors in the task. (A) neural firing patterns for post-correct and post-error trials, sorted by the first principal component (same data set as Figure 6). Notably lacking from the post-error trials was the sustained activity during the delay period (cells at top of left plot are not common on post-error trials). (B) Plots of the three leading PCs for the post-correct and post-error trials. PCs 1 and 2 from the post-correct trials was similar to PCs 2 and 3 from the posterror trials. PC1 from the post-error trials was not observed in the PCs defined by the post-correct trials. (C) Phase plots of the two leading PCs, with time encoded in gray scale. An attractor-like structure was found on post-correct trials (upper left plot), with activity looping through a common space in the period between the trials. A similar circular structure was found in the phase plots for PCs 2 and 3 on post-error trials (lower right plot). A very different structure was found in the phase space on post-error trials (lower left plot). There was a shift across the phase space over the period of the trial, and this was due to the transition in PC1 (high before trial, low after). A neural circuit model (Bekolay et a., 2014) of these functions showed that the shift in phase space led to neurons experiencing new synaptic weights during the inter-trial interval (post-error period), a finding that suggests that such transitions could enable learning from errors.
All of these neuronal measures covary with successes and failures in performing a given task. In the simple RT task, the firing rates of some mPFC neurons are significantly different when rats press on the lever before making a correctly sustained action or an incorrectly premature action (Narayanan and Laubach, 2006). Similar differences in activity are also found in the motor cortex (Laubach et al., 2000) and can be shown to occur in the absence of any differences between correct and incorrect performance in terms of how the rats press on the lever or activate the primary muscles involved in lever pressing (Figure 4 in Laubach et al., 2000). While it is clear that the motor cortex is capable of inhibiting action, through its connections with the spinal cord (Li et al., 1990), the pregenual part of the mPFC (where our experiments have been done) is not well placed to directly influence action and is only weakly connected with the motor cortex and brainstem (Vertes, 2004; Gabbott et al., 2005). The precise role of the mPFC in the control of action has remained difficult to explain. However, the experiments reported in the present manuscript support a new interpretation of mPFC function that is able to explain why disruptions of mPFC activity lead to excessive premature responding.
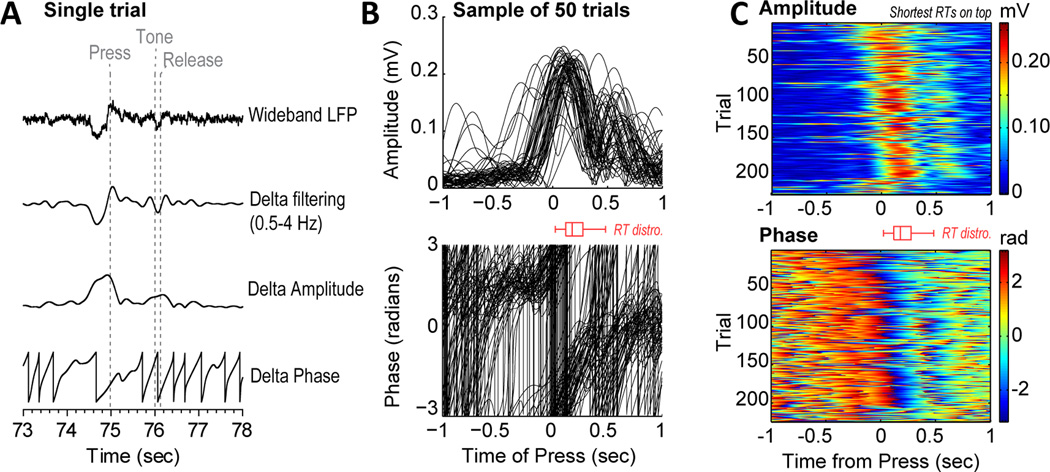
Figure 4.
Delta rhythms in the medial prefrontal cortex are phase locked when the animals initiated timed actions. (A) Example of bandpass filtering and extraction of amplitude and phase using the Hilbert transform. (B) Single trial measurements of amplitude (top) and phase (bottom) from a typical field potential recording. (C) Raster plots for amplitude and phase on correct trials. Same recording as B. Trials are sorted by response latency. Boxplots (red) in B and C show the distribution of RTs.
In the present manuscript, we review the current literature on the role of the medial frontal cortex in the adaptive control of action. First, we review previous findings from studies of adaptive control in rats, monkeys and humans and several potential computational models that might explain how the mPFC implements control over action. Second, we review our own studies on the role of low-frequency brain rhythms and corresponding changes in population spike activity that are associated with the initiation of timed actions. Our findings contribute to an emerging literature that suggests that the medial frontal cortex is a key brain region for detecting action-imperative stimuli, triggering appropriate timed actions, and enabling adjustments in performance.
Towards a physiologically realistic model for adaptive control by the medial PFC
A key idea that has emerged over the past two decades is that neurons in the mPFC, especially the anterior cingulate cortex, encode the recent history of successes and failures in performing a given task (Shima and Tanji, 1998; Shidara and Richmond, 2002; Ito et al., 2003; Amiez et al., 2005). mPFC neurons are thought to process these signals in parallel with network-level rhythmic activity that is associated with adaptive control, especially in the theta range (Cavanagh et al., 2012). Reversible inactivation of the mPFC impairs the expression of performance adjustments, such as changing plans of action (Shima and Tanji, 1998), switching between exploration and exploitation (Amiez et al., 2006), and post-error slowing (Narayanan and Laubach, 2008). Yet, the precise neuronal mechanisms for adaptive control have remained elusive. A crucial issue is to link neuronal spike activity to macro-level events that have been reported in many human studies and showing that these macro-level events are comparable across species (Narayanan et al., 2013).
A number of human studies have established that the mPFC is involved in the adaptive control of behavior (see Ridderinkhof et al., 2004 for review and Shackman et al., 2011 for an alternative perspective). A core initial finding was the discovery of error-related evoked potentials in EEG recordings from humans performing choice RT tasks (Falkenstein et al., 1991; Gehring et al., 1993). These signals were interpreted as markers of performance monitoring and adjustment (Gehring et al., 1993), were localized to the anterior cingulate cortex using EEG (Dehaene et al., 1994) and fMRI (Carter et al., 1998) based methods, and were associated with underlying theta band (4–8 Hz) oscillations (Luu et al., 2004). An early alternative interpretation emerged that proposed that the signals reflect the difficulty, or conflict, associated with the choice made by the participant (Carter et al., 1998). That is, trials with difficult discriminations tend to have higher error rates and these conditions selectively activate the anterior cingulate cortex. However, single-unit recordings in the monkey mPFC, specifically the dorsal ACC, did not support this view and found that, instead, neurons in this part of the brain encode information about behavioral outcomes (Ito et al., 2003). More recent studies have emphasized the role of these signals in processing feedback about the success of action (e.g. Sallet et al., 2013), and feedback-related potentials have recently been found in the rat mPFC (Warren et al., 2014).
Single-unit recordings in humans have supported the view that the mPFC rapidly evaluates actions as successful or erroneous (Sheth et al., 2012; Bonini et al., 2014). Studies of human EEG from this group have indicated that these regions in humans are central to action monitoring (Burle et al., 2008). In addition, quantitative analyses of action and feedback related ERPs support the idea that mid-frontal signals encode variations in performance and monitor ongoing actions (Meckler et al., 2010, 2011, 2013). This interpretation has been supported by single unit and field potential recordings in the monkey anterior cingulate cortex (Ito et al., 2003; Emeric et al., 2008). A recent combined recording and inactivation study suggests that these signals are necessary for the expression of adaptive control (Kuwabara et al., 2014).
Studies in rodent models are needed to investigate the neural circuits that underlie adaptive control. Our laboratory has studied this issue using a rat model (Narayanan et al., 2006) and found that similar neural correlates of outcome monitoring exist across species (Narayanan et al., 2013). Our studies have focused on a rostral cingulate region, specifically the prelimbic area (aka area 32; (Vogt and Paxinos, 2014)). This cingulate region is found in all mammals (see Laubach, 2012 for review) and has clear anatomical homologies across species (Vogt et al., 2013; Vogt and Paxinos, 2014). Furthermore, by using reversible inactivation methods (Narayanan et al., 2006; Allen et al., 2008), we found that this cortical region is necessary for the encoding of past and current behavioral outcomes by the firing rates of neurons in the primary motor cortex and for the ability to adjust behavior after errors, i.e. to exhibit post-error slowing in a simple RT task (Narayanan and Laubach, 2008). Similar signals were later found in an mPFC-dependent delayed alternation task (Horst and Laubach, 2009, 2012). Exactly how these signals work to achieve adaptive control has not been fully resolved.
One influential idea was proposed in a recent modeling study by Alexander and Brown (2011). They proposed that the mPFC serves as an 'action-outcome predictor'. They developed a connectionist model using standard learning rules. The model was able to integrate past outcomes in order to predict future outcomes and reproduced established markers for adaptive control, such as the Error-Related Negativity (ERN) and firing rate correlates from monkey physiology. While this connectionist model has done a lot to inspire theory and experiments on the mPFC, the neurons in it were modeled only in terms of firing rate, and not precise temporal patterns, and so it is unclear how the model can account for macro-level network signals such as the ERN (Falkenstein et al., 1991; Gehring et al., 1993) or post-error theta (Luu et al., 2004) that are based on precise synchronizations of firing rates over large pools of neurons
As a first step towards addressing this issue, a spiking network model of the mPFC was developed through collaboration between one of us (ML) and the Eliasmith group (Bekolay et al., 2014). The model was based on analysis of the population statistics of spike activity in the rat mPFC during the simple RT task (Narayanan and Laubach, 2008, 2009). A key finding was that neuronal population activity in the mPFC integrates information about actions and the passage of time (Narayanan and Laubach, 2009) and accounts for successes and failures in obtaining desired outcomes by exhibiting persistent spiking activity (Narayanan and Laubach, 2008). We were able to reproduce such population spike activity by chaining together two pools of recurrently connected neurons (i.e. integrator networks). The first integrator network tracked the fact that the trial had begun and the second integrator network tracked the time that had elapsed since the start of the trial (Fig 4 in Bekolay et al., 2014).
The design of the computational model proposed by Bekolay et al. (2014) is similar in many ways to an emerging view in the interval timing literature: timing depends on an encoding of state transitions in the behavioral procedure. Although somewhat similar accounts to explain temporal performance have been proposed by behavioral models of timing (e.g., Killeen and Fetterman, 1988; Machado, 1997), the transitions proposed by such models were suggested to occur at a broader and ill-defined behavioral level. Evidence of such encoding by the brain at either a behavioral or cognitive level is still lacking.
Transitions between states in the procedure are denoted by salient behavioral events, such as stimuli, the animal's actions, and the delivery (or absence) of the reward. These events are usually referred to as “time markers” if they can serve as clues to the passage of time (Caetano and Church, 2009). The RT and time production tasks that we have used are comprised of several states: disengagement from the task (pre-trial), waiting for the stimulus or the temporal deadline, acting after the stimulus or deadline, and receiving the reward or experiencing a time-out. Each of these states is bracketed by salient behavioral events: pressing the lever, receiving the stimulus, responding to the stimulus or at the end of the temporal deadline, and receiving the reward. Event-related potentials were found after each of these events (see Figures 2–6 below), and were accompanied by major changes in neuronal spike activity, as quantified using multivariate methods such as PCA (see Figures 7, 8 and 10 below).
Figure 2.
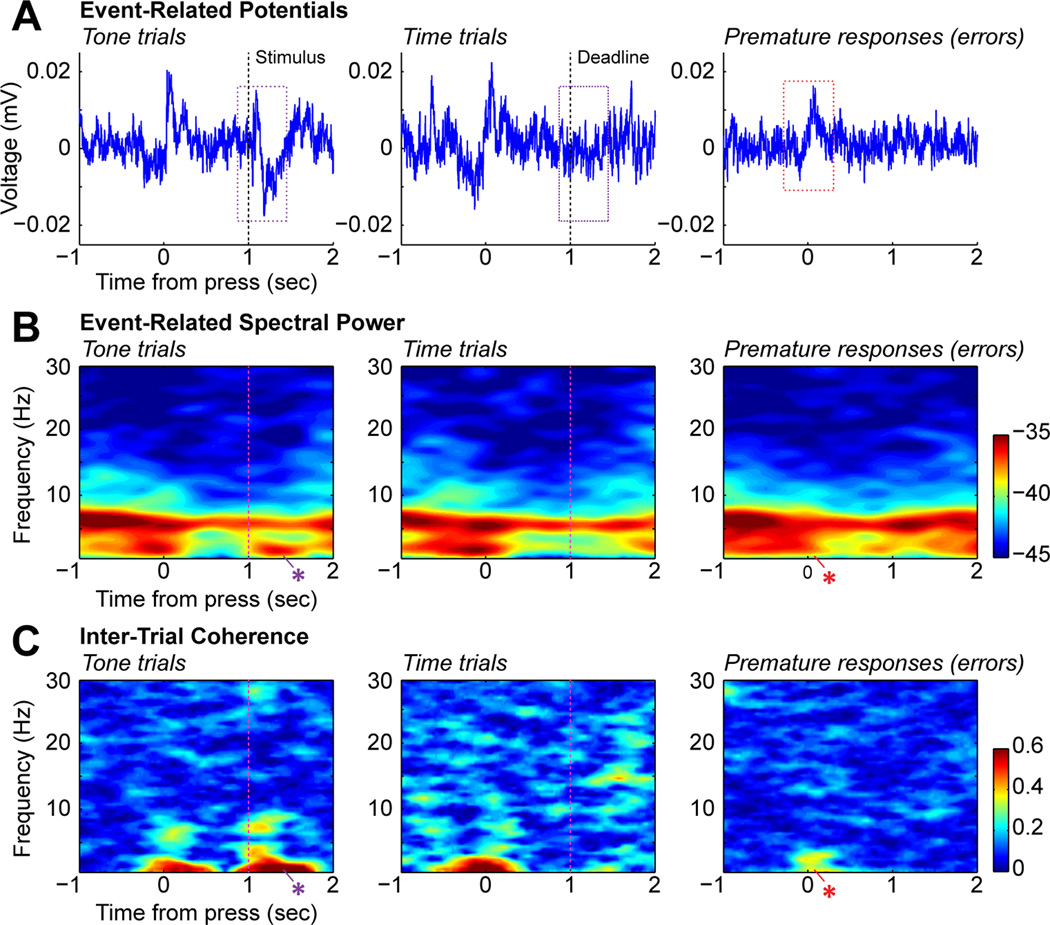
Spectral analysis of field potentials from the medial prefrontal cortex of rats performing Kornblum's (1973) task. (A) Event-related potentials from trials with correct responses on tone and time trials (left and middle) and trials with premature responses (right). The color boxes denote the period of ERP analysis. There was a clear modulation of the ERP when the rat pressed on the lever to start the trials (0 sec, all trials) and again when the stimulus was presented (1 sec on tone trials). (B) Eventrelated spectral power for the three types of trials. There was persisting power in the theta range (4–8 Hz) throughout the trial and there was elevated power in the delta range (below 4Hz) when the rat pressed the lever before correct trials. Delta power also increased when the rat released the lever after the tone (blue asterisk). Delta power was notably reduced prior to premature errors (red asterisk). (C) Inter-trial coherence, or ITC, for the same types of trials. ITC measures the correlation in the phase angles over trials. High levels of ITC suggest that phase locking occurred around the task events. There was phase locking in the delta range when the rats pressed the lever before correct responses, but not before premature responses (red asterisk, right panel). There was also delta phase locking when the rats released the lever in response to the tone (blue asterisk, left panel) and theta phase locking after the stimulus.
Figure 6.
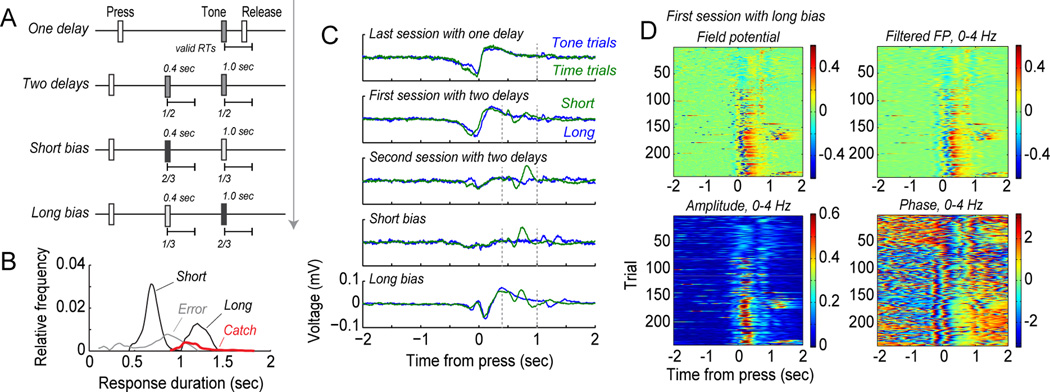
Delta phase locking in the medial prefrontal cortex tracks changes in the expected timing of action. (A) Behavioral testing used to evaluate temporal expectancy. Rats were trained to sustain lever presses for 1 sec to earn rewards. Then, they were tested in three sessions using Kornblum's (1973) procedure, with tones presented on half of the trials, in three sessions with a novel delay of 0.4 sec on half of trials, two sessions with tones at 0.4 sec on two-thirds of trials (short bias sessions), and finally two sessions with tones at 0.4 sec on one-third of trials (long bias sessions). (B) Response durations from a test session run after the final session with bias for the long foreperiod. This session was biased for short foreperiods and included no stimulus on 10% of the trials (catch trials). The rat responded at the time of the long foreperiod on the catch trials. This finding suggests that the rat learned to wait for 1 sec and then release the lever, and was not willing to wait longer for the stimulus. (C) Example of event-related potentials (ERPs) from one rat from the series of testing sessions. The large ERP associated with lever pressing diminished when the rat experienced the sessions with two potential stimulus times. An ERP was detected in those sessions when the stimulus occurred at 0.5 sec (green traces). The press-related ERP was enhanced when rats were tested in the long-bias sessions (bottom row). (D) The enhancement of delta range modulation developed within the first long-bias test session. The field potential shifted from showing limited modulation to showing strong modulation of amplitude and phase around trial 60.
The successful model was based on an earlier model from Singh and Eliasmith (2006), called a double integrator network. The crucial aspect of the Bekolay model was that reward feedback terminated activity in the second integrator network. Without such feedback, the second integrator remained in an elevated state and denoted the failure to perform the task correctly on the previous trial. A phase analysis of this network showed that correct and incorrect performance resulted in the model entering into a different part of the state space (Fig 9 in Bekolay et al., 2014). Neurons would thus experience different synaptic states following correct and incorrect performance, and this process could enable learning from errors. New modeling efforts are needed to address this issue using dynamic synapses with plasticity capable of implementing reinforcement learning algorithms.
Figure 9.
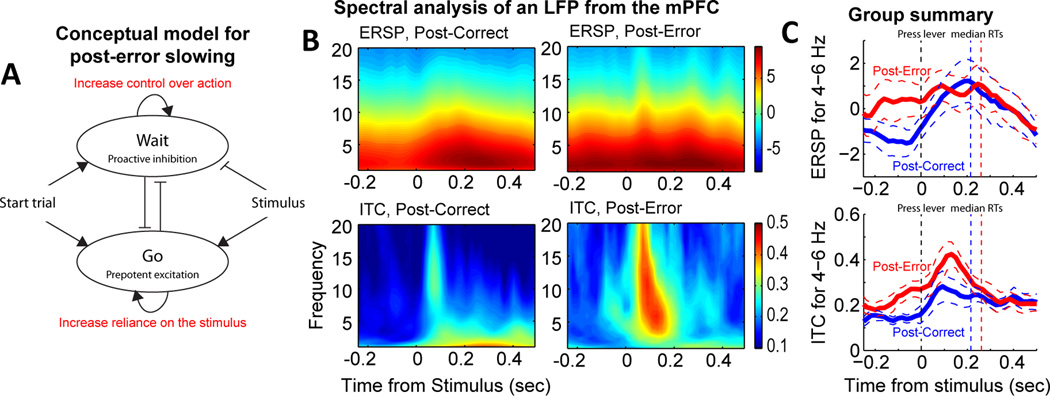
Neural correlates of performance adjustments in the medial prefrontal cortex. (A) Conceptual model for adjustments in performance strategy. Errors could lead to rats increasing control over action (“inhibition”) and/or increasing stimulus processing (attention, integration). (B) Spectral analysis of field potentials showed evidence for both types of changes in processing. Comparisons of event-related spectral power on post-correct and post-error trials showed increased power at low frequencies just before the stimulus (−0.2 to 0 sec). Phase locking to the stimulus was enhanced on post-error trials, spanning the rages of theta and beta. (C) Group summary for spectral power and phase locking in the range of theta (4–8 Hz) for post-correct (blue) and post-error (red) trials. Dashed lines represent 95% confidence bands based on recordings of 28 field potentials recorded in 5 rats.
An additional extension of spiking network models to incorporate field potentials is needed for studying adaptive control. Post-error theta rhythms are commonly observed in humans performing similar speeded response tasks (Cavanagh et al., 2012). These signals might serve as a mechanism for adaptive control (Cavanagh and Frank, 2014). A recent collaborative study between our laboratory and a human research group found common markers for adaptive control in rats and humans (Narayanan et al., 2013). Developing computational models of these signals will require spiking network implementations of physiologically defined neurons and field simulations, e.g. (Lempka et al., 2011). Computational models of adaptive control must move beyond the slow time scales that are captured by firing rate models. These models may reproduce ERP level findings but cannot account for fast rhythmic activity, such as theta, or precisely coherent spike activity associated with these rhythms (Narayanan et al., 2013). We hope that our studies will inspire serious computational efforts at addressing these issues.
A plea for simple tasks that can be used across species
Several tasks are classically associated with studies of mPFC/ACC function in humans, including the Stroop task (Pardo et al., 1990) and Erikson flanker task (Botvinick et al., 1999). Animal studies have used a much wider range of behavioral designs, and these are often determined by species-specific issues (e.g. maze tasks in rodents, eye movement tasks in primates). Findings from many animal studies are difficult to relate to studies on the human mPFC, in part due to differences in behavioral design. Our approach has been to use a simple type of behavioral task that can be performed in a highly similar way across species. Deficits in simple reaction time performance (with simple meaning no choice about the response) are apparent in stroke patients (Stuss et al., 2005) and are apparent immediately after cingulotomies are made (Srinivasan et al., 2013). Importantly, animals such as rats and monkeys can readily learn to perform these tasks (Laubach et al., 2000), and this allows for studying neural correlates of RT variability and adaptive control without resorting to months of behavioral training.
Behavioral methods for studying the neural basis of RT performance in experimental animals were developed starting in the 1960s (e.g. Stebbins, 1962; Stebbins and Lanson, 1962; Stebbins and Miller, 1964)). These studies led to some of the first single-unit recordings made in the motor (Evarts, 1966) and medial prefrontal (Niki and Watanabe, 1979) cortices. Using similar methods in rodents, a number of groups found that lesions and reversible inactivations of the mPFC (specifically, the prelimbic area) impaired the ability of animals to sustain actions over delay periods (Broersen and Uylings, 1999; Risterucci et al., 2003; Narayanan et al., 2006). A related study (Brown et al., 1991) found that a more dorsal mPFC region, the medial agranular cortex (also known as the secondary motor cortex), controls bias over lateralized responses to visual stimuli and is necessary for speeded performance. This cortical area is not part of the anatomically defined anterior cingulate cortex. A more recent study by our group (Smith et al., 2010) used the simple RT task and found similar slowing of RTs occurred when this cortical region was inactivated. These changes in behavioral occurred in the absence of changes in premature responding. Together, these studies establish that there is regional specialization within the rodent mPFC for the control of action initiation, with the ventral (anterior cingulate) regions having a selective role in limiting inappropriate actions and the dorsal (medial agranular) regions having a selective role in the initiation of action.
The validity of these findings for the human brain has been established by a clinical study that used a modified simple RT task to study the immediate effects of damage near the cingulate bundle on action initiation (Srinivasan et al., 2013). They found the same behavioral deficit found in the rat studies cited above: Patients were unable to sustain an action over an extended delay and made excessive premature responses. The functional significance of this deficit was recently characterized in a comprehensive review (Bari and Robbins, 2013) as an impairment in the ability to postpone action, a core deficit of inhibitory control. In other words, the mPFC is necessary for the ability to wait (Narayanan et al., 2006).
But waiting alone cannot explain the effects of damage in the mPFC. In a clever study, Risterucci and colleagues (2003) used a standard simple RT design, in which rats had to release a lever in response to a visual stimulus that was presented after one of four potential delays. Lesions led to increased premature responding and eliminated the delay-dependent speeding of the RT (i.e. shorter RT after longer delay). Eventually, the rats were able to perform the task at pre-surgical levels. However, by slightly changing the timing of the delay periods, the premature response deficit was re-expressed. These findings suggest that in the absence of mPFC control, rats are able to learn to withhold responding but are not able to adapt to dynamic changes in a given behavioral procedure.
Inspired by this study, we tested rats with several variants on the simple RT design (Narayanan et al., 2006), i.e. rats were tested in sessions with fixed or variable delay periods and with fixed or variable intensity stimuli. Evidence for an inhibitory role of the mPFC was found in sessions with variable delays: Rats were actually quicker to respond after short delays with mPFC inactivated. These results suggested that a form of response inhibition was generated by the mPFC early in the delay period. This interpretation was later confirmed by recording single-unit activity in the mPFC and by inactivating the mPFC while recording in the primary motor cortex (Narayanan and Laubach, 2006). Taken together, our findings suggested that the mPFC generates a control signal that is used to control the timing of action and acts on the primary motor cortex to influence behavior.
Whereas many studies implicate mPFC in inhibitory control in tasks that require specific actions to be sustained over extended periods of time (Chudasama et al., 2003, 2012; Risterucci et al., 2003; Narayanan and Laubach, 2006; Narayanan et al., 2006; Srinivasan et al., 2013), this region does not appear to be involved in delayed discounting (Cardinal et al., 2001). Delay discounting (or delayed gratification) tasks involve presenting a highly valued outcome after an extended delay and a lower value outcome after a minimal delay. In contrast to the mPFC, lesions and manipulations of monoamines (serotonin and norepinephrine) in the orbital frontal cortex have clear effects on delayed discounting (Winstanley et al., 2004, 2006; Sun et al., 2010). The neuronal basis of these effects has not been examined using paired recordings in the medial and orbital frontal cortex, and this should be a topic of future research.
Anatomical studies have reported minimal direct anatomical connections between the mPFC and primary motor cortex (Donoghue and Wise, 1982; Vertes, 2004; Hoover and Vertes, 2007). Therefore, a temporal control signal generated in the mPFC would likely need to use other circuits to influence the motor cortex, perhaps by way of a reciprocally connected cortical area or by more complex circuits through the basal ganglia and/or thalamus (Narayanan and Laubach, 2006). To investigate the “direct cortical pathway” hypothesis, a study from our group compared findings from mPFC inactivations with results from reversibly inactivating the secondary motor cortex (the “rostral forelimb area” located within the medial agranular cortex) (Smith et al., 2010). Inactivating the secondary motor area did not increase premature responding. Instead, the rats tended to respond more slowly, similar to Brown et al. (1991). These studies suggest that the secondary motor cortex (medial agranuarl cortex) is a direct cortical pathway between the mPFC and primary motor cortex in rodents.
In addition to studies of mPFC using RT and time production tasks (reviewed above), we also used reversible inactivation methods to examine the role of the mPFC in a classic test of spatial working memory, the delayed alternation task (Horst and Laubach, 2009). We found that mPFC inactivation alters measures of temporal processing in that task and, more recently, found cells that fired as a function of the animal's pace in performing the task (Horst and Laubach, 2012). Related studies found that mPFC neurons fire to action-imperative stimuli in the delayed alternation task (Caetano et al., 2012) and during the initiation of consummatory behavior (licking) when correct responses are reinforced (Horst and Laubach, 2013). Our neural circuit model (Bekolay et al., 2014) incorporated these response properties (stimulus, action and outcome driven cells with recurrent connectivity) and was able to reproduce the common modes of firing by populations of mPFC neurons in the simple RT task (Narayanan and Laubach, 2009) and the effects of previous outcomes on RT performance, specifically post-error slowing (Narayanan and Laubach, 2008). Finally, we have described how individual neurons in the aged mPFC, as well as population activity in this area, fail to respond to imperative stimuli in a delayed alternation task, which leads to an inability of older rats to time their actions properly in the task when compared to younger rats (Caetano et al., 2012). These studies establish that our findings in the RT and time production tasks generalize to other mPFC-dependent tasks, and support the view that the mPFC is crucial for the temporal control of action.
The medial prefrontal cortex encodes errors prospectively and retrospectively
In the previous section, we presented data describing how mPFC controls actions that must maintained over extended periods of time. Single unit recording from our group has shown that medial prefrontal neurons consistently encode errors – or movements that occur too early in time and do not lead to reward. The first evidence for this came from Laubach et al. (2000). In this study, neural ensemble activity was recorded in the primary motor and medial frontal cortices during the initial acquisition of the simple RT task. An encoding of the accuracy of the delayed response developed in the primary motor cortex over the period of training. Most interestingly, the cells fired differently before the initiation of correct and incorrect responses (prospective error encoding). Simultaneous recordings of sensors on the manipulandum (tilt-sensor on the lever) and electromyographical (EMG) activity from muscles involved in lever pressing found no evidence for these signals being driven by differences in movement (Laubach et al., 2000).
Based on the study by Risterucci et al. (2003) and our own reversible inactivation study (Narayanan et al., 2006), we decided to follow on Laubach et al. (2000) by recording in the primary motor cortex and mPFC using variations on the standard simple RT task. These studies consistently revealed evidence for prospective error encoding by neurons in both cortical areas and further revealed the mPFC also participates in retrospective error encoding in the RT task (Narayanan and Laubach, 2008). That is, some mPFC neurons fired persistently after mistakes were made in the task and maintained their post-error firing rates throughout the inter-trial interval and subsequent trial until the next reward was earned. These neural signals were accompanied by the expression of post-error slowing by the rats, in which the rats took significantly more time to initiate actions when a mistake (premature or too late) was made on the previous trial. Crucially, inactivation of the mPFC eliminated this post-error slowing (Narayanan and Laubach, 2008). Similar encoding of prospective and retrospective outcome-related information was later found in delayed alternation tasks (Horst and Laubach, 2012, 2013; Hyman et al., 2013). Using population analysis methods similar to those used by our group (Narayanan and Laubach, 2009; Caetano et al., 2012), Hyman et al. (2013) found a pattern of firing across neurons that was related to the previous behavioral outcome. A similar population activity pattern in the simple RT task was recently reported from simulated neuronal ensembles in the spiking network model reported in Bekolay et al. (2014). These population level signals suggest that outcomes may be encoded by network-level activity within the mPFC.
To examine this issue, we initiated a collaborative study with a human EEG group, James Cavanagh and Michael Frank. The rationale for this collaboration was Cavanagh et al. (2009, 2012), which reported that errors led to selective increases in theta oscillations when humans performed a set of common tasks used to study adaptive control. Our collaborative study involved us applying data analysis methods from the Cavanagh studies to our recordings of field potentials from the rat mPFC and primary motor cortex and making new recordings of human EEG as participants performed the simple RT task used in the rodent studies described above (based on Kornblum, 1973 and used by Srinivasan et al., 2013). Two results from this project were that (1) rats and humans show the same neural markers of adaptive control, with increases in theta oscillations on post-error trials that were correlated with RT adjustments, and (2) mPFC inactivation eliminates correlates of adaptive control in the primary motor cortex.
A theoretical perspective on how the mPFC might control the motor system to achieve control over RT performance was proposed by Summerfield and Yeung (2013). A standard assumption in RT studies is that actions are initiated when firing rates in the motor cortex pass a threshold. Based on this idea and one of their own studies (Wyart et al., 2012), Summerfield and Yeung proposed that post-error adjustments might arise by oscillatory processing in the mPFC resulting in an increase in the threshold required for triggering action in the motor cortex. They proposed that a mechanism based on a global regulation of cortical excitability underlies this process. If the mPFC entrains the phase of cortical oscillations, then changes in mPFC activity could alter the ability of the motor circuit to become activated following presentation of an action-imperative stimulus. Support for this idea was found in the rodent portion of our study (Narayanan et al., 2013) as (1) there was increased spike-field coherence in the theta (4–8 Hz) range in the mPFC on post-error trials and (2) inactivation of mPFC eliminated theta-range oscillations and spike-field coherence on post-error trials in the motor cortex. The underlying idea is that these oscillations could represent long-range communication between areas (Fries, 2005; Womelsdorf and Fries, 2007). Investigation of this idea would require recording from several brain areas at once to study the propagation of oscillation across functionally connected neuronal networks (Paz et al., 2009; Fujisawa and Buzsáki, 2011).
How does the mPFC become aware of the need to implement adaptive control in the first place? A special feature of the mPFC region that we have studied, the prelimbic cortex, is that it is extensively interconnected with the agranular insular cortex (Gabbott et al., 2003). This cortical area encodes visceral and gustatory sensory information in rats and humans (Cechetto and Saper, 1987; Allen et al., 1991; Yasui et al., 1991; King et al., 1999; see Cechetto, 2014 for review). The prelimbic area also densely projects to a number of subcortical reward-related centers, such as the dorsal and ventral striatum, the dopaminergic midbrain and the lateral hypothalamus (Sesack et al., 1989; Vertes, 2004; Gabbott et al., 2005). A potential mechanism for linking reward-feedback (possibly encoded in the insula) with adaptive control (encoded in the mPFC) might therefore arise by interconnections between the two cortical areas (as first proposed in Laubach, 2011) as well as the convergence of the agranular insular and prelimbic cortices onto the same groups of cells in parts of the basal ganglia, such as the striatum and subthalamic nucleus. The subthalamic nucleus, in particular, receives input from dorsal medial frontal regions, and has been shown to regulate premature responding during RT tasks (Baunez and Robbins, 1999).
Simple reaction time performance depends on stimulus detection and time estimation
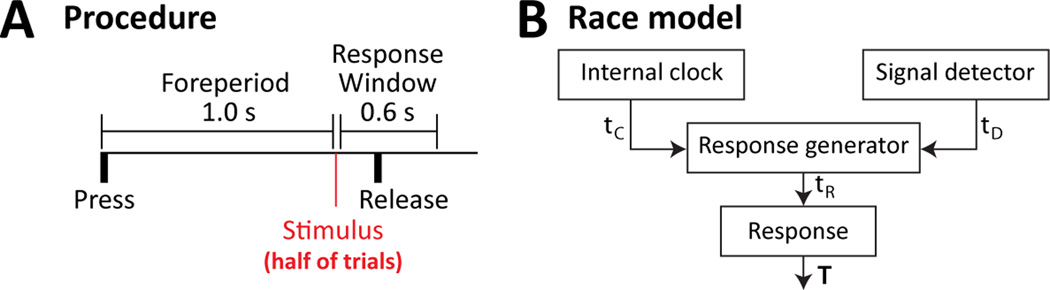
In the section above, we have described how medial prefrontal cortex participates in adaptive control and is able to adjust performance following mistakes in task performance. To understand the precise nature of performance adjustments in speeded response tasks, including simple RT tasks, it is necessary to review the behavioral processes that are thought to underlie RT variability. Classic studies led to the idea that RT performance depends on a competition between stimulus detection and time estimation (Ollman and Billington, 1972; Kornblum, 1973). These studies required participants to make a response (lever or button press) until a stimulus was presented after a fixed time interval, aka foreperiod (Figure 1A). The stimulus occurred on half of the trials, and the participants had to estimate the time interval between the response and the moment the stimulus would appear to initiate action in the trials with no stimulus presentation. In the deadline model of Ollman and Billington (1972), participants employ a temporal criterion to respond (after some amount of time has elapsed) and break from waiting if the trigger stimulus is detected. An alternative model is the race model of Kornblum (1973) in which there is a competition between an “internal clock” (that triggers action after the end of the interval) and a “signal detector” (that triggers action when the stimulus is detected) (Figure 1B). These models are similar in many respects to later models of choice RT performance that assume that stimulus detection is gated by temporal processing and is adjusted after mistakes are made (Laming, 1979a, 1979b). A key finding for the validity of these models in human studies (such as Kornblum, 1973) was that participants showed reduced RT variability on the trials with the stimulus, resulting in a peak in the RT distribution that is not found on the stimulus-free trials.
Figure 1.
Rats, like humans, perform simple reaction time tasks as predicted by Kornblum's (1973) race model. (A) Behavioral procedure. Rats press on a lever to begin each trial and have to release the lever one sec later to earn a liquid reward (B) Kornblum's race model for explaining the contribution of time estimation and stimulus detection to response time variability.
Several rodent studies implicate medial frontal networks in exactly this kind of temporal processing. Inactivation of medial prefrontal cortex impairs the performance of time estimation tasks (Kim et al., 2009; Narayanan et al., 2012). Moreover, single neurons and neuronal population activity (analyzed using principal component analysis) exhibit ‘ramping’ components as rats time discrete temporal intervals (Kim et al., 2013; Xu et al., 2014), similar to the ramp and hold components described in the simple RT task by Narayanan and Laubach (2009). Another key player seems to be the dopamine system, which has been shown to participate in temporal processing (Meck, 1996; Buhusi and Meck, 2005). Systemic injections in rats (Maricq and Church, 1983; Meck, 1983) or oral administration in humans (Lake and Meck, 2013) of dopamine agonists and antagonists seem to speed up and slow down the subjective passage of time, respectively. In particular, these temporal processes appear to be powerfully influenced by D1 dopamine receptors. Depleting VTA dopamine or focally blocking prefrontal D1 receptors impairs temporal control of action (Narayanan et al., 2012). Furthermore, optogenetically inhibiting prefrontal neurons expressing D1 receptors in mice impairs temporal control, while stimulating these neurons slightly improved temporal control (Narayanan et al., 2012). Another recent rodent study found evidence for dopaminergic circuits being specifically involved in temporal processing during RT performance. Parker et al. (2013) found that depleting the source of prefrontal dopamine in the ventral tegmental area did not impact overall RT performance, but eliminated delay-dependent speeding. Furthermore, focally blocking D1, but not D2, dopamine receptors within the medial prefrontal cortex ‘flattened’ the RT distribution, such that RTs were shorter at short, but not long, delays. These studies provide insight into how rodents guide their behavior in time, a fundamental and highly conserved behavior (Buhusi and Meck 2005). Future studies should examine how neuromodulatory molecules such as dopamine, acetylcholine, and norepinephrine influence RT performance. This effort is essential to developing new therapeutic strategies for Parkinson’s disease, schizophrenia, Alzheimer’s disease, ADHD, and other neuropsychiatric diseases (Parker et al., 2013).
Neural activity in the medial PFC reflects stimulus detection and action timing
The behavioral studies reviewed above suggest shared bases for temporal processing in RT tasks in rodents and humans. Recent work from our group discovered strikingly similar extracellular potentials in both rodents and humans during RT performance (Narayanan et al., 2013). Indeed, rodent field potentials and human EEG shared several major features: (1) prominent stimulus-related transients, (2) transients associated with the start of the trial, and (3) alterations in these signals on error trials (Figure 2A). These extracellular potentials provide a unique window into information processing by cortical networks, as rhythms and frequencies may entrain large populations of neurons with cognitive signals (Buzsáki, 2006). Prefrontal theta oscillations can be linked with other regions, such as the ventral tegmental area and the hippocampus (Hyman et al., 2005; Jones and Wilson, 2005; Siapas et al., 2005; Benchenane et al., 2011; Fujisawa and Buzsáki, 2011). Combined with our own data suggesting theta coherence between medial prefrontal cortex and motor cortex, these findings suggest that low-frequency oscillations integrate task-relevant information across brain regions.
Our studies suggest that low-frequency oscillations (below 12 Hz) encode critical aspects of RT performance. For instance, using Kornblum's (1973) task, we compared field potentials on trials when rats responded to a stimulus at the end of the delay period (tone trials) and trials when the rats initiated responding based on timing alone (time trials). An evoked potential was apparent just after the stimulus, which did not occur on the time trials (Figure 2A), was accompanied by phase locking in the delta range (<4 Hz) (left plot in Figure 2C). These responses were accompanied by persistently elevated theta activity (4–8 Hz) (Figure 2B) and delta-range phase locking occurred at the start of both types of trials (left and center plots in Figure 2C). These neural markers of stimulus detection and action timing were notably lacking on trials when the rat responded prematurely (right plots in Figure 2), which suggests that those signals could serve as reference points to properly time the moment at which the action needs to be performed. To study how action was distinct on stimulus-triggered and timed actions, we examined event-related field potentials (Figure 3). These field potentials from mPFC were quite distinct on stimulus and time trials. On the latter trial type, the animals initiated responses that were guided by a temporal rule, based on an internal response 'deadline'. As expected, responses after the stimulus had a clear evoked potential and those guided by deadlines did not (Figure 3B).
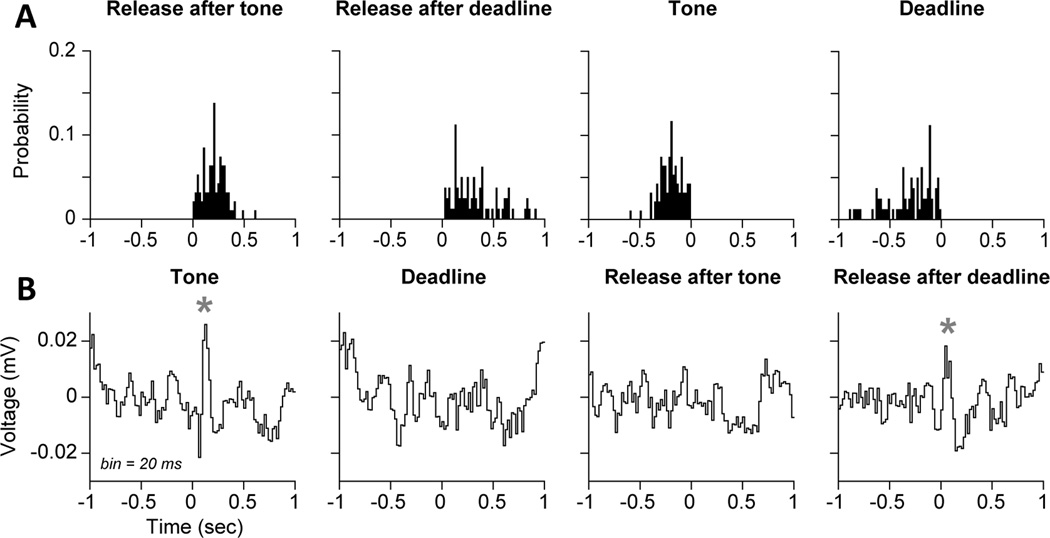
Figure 3.
Event-related potentials dissociate stimulus-triggered and timed actions. (A) Behavioral events are shown. The times of lever release are shown in the left pair of plots relative to the stimulus (tone trials) and deadline (time trials). The times of the tone and deadline in the right pairs of plots relative to the lever release on tone and time trials. (B) Field potentials from the mPFC are shown for the four events (tone, deadline, release after tone, and release after deadline). The tone, but not the deadline, was associated with an evoked potential. Release after the deadline, but not after the tone, was associated with an evoked potential. The evoked events could serve as time markers indicating transitions in the behavioral procedure from waiting to acting.
Our finding of delta phase entrainment being associated with the initiation of action timing is reminiscent of a study by Stefanics et al. (2010) that reported that “human delta oscillations” can mediate effects of expectation on the RT. Indeed, these low-frequency events are associated with variations in RT performance. Using bandpass filtering and the Hilbert transform (Figure 4A), we measured the amplitude and phase of delta rhythms (or “delta-band activity” as suggested by a reviewer of this paper) on a trial by trial basis (Figure 4B) and then plotted stacked images of the signals, using false color image plots, that were sorted by the animals' RT. A consistent finding was that the amplitude of the delta rhythm started earlier before trials with the shortest RTs (top plot in Figure 4C) and was accompanied by a phase reset (bottom plot in Figure 4C). These events are coterminous with the press-related ERPs in Figure 2 (time 0) and major changes in spike activity (Narayanan and Laubach, 2009), as reviewed below. It is important to point out that the signals were not simply the product of bandpass filtering and Fourier-based analysis. Similar events could be detected using a non-Fourier method, Empirical Mode Decomposition (Huang et al., 1998) (Figure 5).
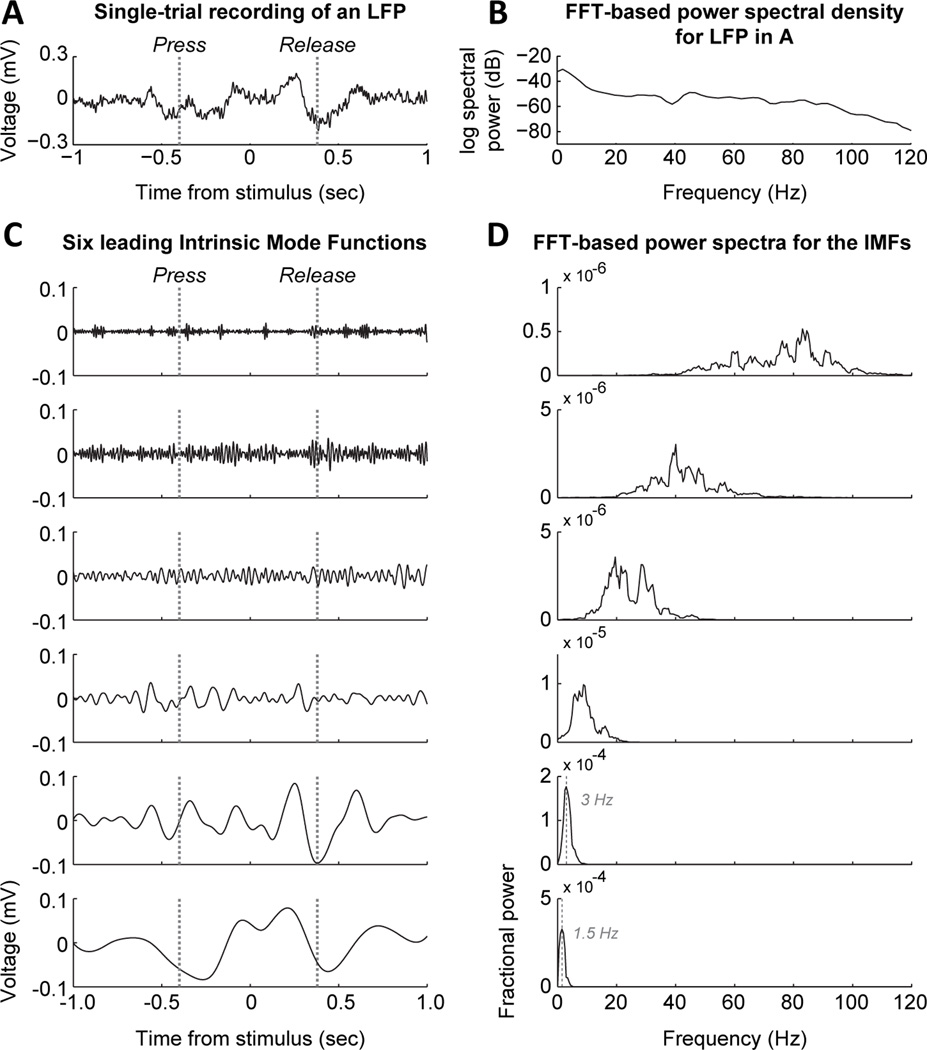
Figure 5.
Validation of low-frequency "delta" activity using a non-Fourier method. (A) Single-trial recording of a field potential from the prelimbic area during the simple RT task. (B) Standard FFT-based power spectrum for the signal in A, plotted on a log scale for power. Power was especially concentrated below 10 Hz and there was a peak in the gamma range. (C) Intrinsic Mode Functions (IMFs) found using a Empirical Mode Decomposition (Huang, 1989). This technique finds a set of harmonic functions ("waves") with the same number of extrema (max and min values) and zero crossings (sign reversals). The algorithm can be applied to a continuous recording (time series) of any type and will only return IMFs that satisfy the constraints defined above. This particular recording showed evidence for six wavelike components. The two lowest frequency components were similar to those found by using a traditional approach in LFP analysis of bandpass filtering and the Hilbert transform. (D) FFT-based power spectra for the six IMFs in C. The two lowest frequency components had peaks at 1.5 and 3 Hz, respectively, within the traditional “delta” range. The higher components were in the typical LFP ranges called theta, beta, low gamma, and high gamma. This analysis shows that the low-frequency rhythms described in Figures 2–4 and 6 are not simply the consequence of using the standard approach to LFP data analysis and the rhythmic components, especially in the delta range can be detected on single trials.
We evaluated if the delta range activity was sensitive to changes in the behavioral procedure by recording over a series of behavioral test sessions with changes in the expected timing of the imperative stimulus (Figure 6A). That is, rats were trained with a single, fixed delay period (1 sec) and then experienced sessions with two equally likely delays (0.4 or 1 sec) and sessions with one delay more likely (2/3 of trials) than the other (1/3 of trials). These latter sessions were called short and long “bias” sessions. Comparisons of the event-related potentials (ERPs) synchronized to the initiation of action timing (lever press) showed clear changes in the size of the ERP over the period of testing (Figure 6B). The ERP was largest when there was a single delay period and was reduced in magnitude as the animals experienced the two potential delays and in sessions with “short delay bias”. However, the ERP re-emerged in sessions with “long delay bias”. These changes were apparent in the first testing session with long delay bias (Figure 6C). Plots of amplitude (top row) and phase (bottom row) from those sessions revealed a gradual development of increased amplitude and phase consistency when the rats pressed the lever and learned that the stimulus was more likely to occur at the long delay. Of note, because we do not have a monotonically varying foreperiod, we could not assess continuously varying temporal expectancies in this study – future studies should address this issue.
In summary, our studies revealed that rhythmic activity in the mPFC underlies the execution of timed actions. ERPs are coterminous with the initiation and termination of timed behaviors (Figures 2 and 3), These events are accompanied by delta-band activity that is phase locked to the initiation of timing behavior (Figure 4). The delta-band activity can be captured by non-Fourier methods such as Empirical Mode Decomposition that are designed to detect “wave-like” events (Figure 5). The ERPs and delta-band activity are sensitive to changes in the expected timing of action in the behavioral task (Figure 6). To our knowledge, these findings are the first reports of rhythmic network activity in the mPFC having a role in the control of precisely timed actions.
Neural population activity in the mPFC tracks the initiation of a timed action
In the previous section we have described macro-level phenomena underlyign the control of timed action. Here, we show how neuronal population activity varies with regard to the ERPs and delta-band activity shown in Figures 2–6. Quantitative analysis of population spiking activity (Narayanan and Laubach, 2009) suggests that two distinct signals are emitted by the mPFC at the start of the trial (Figure 7). One signal is a slow modulation of firing rate, starting just before the initiation of the timed action. The other signal is more sharply modulated at the time of the action. These two signals appear to be interdependent. That is, plots of the cumulative sums of the first two population functions, but not the higher order functions, closely resemble each other (Figure 7C). These signals are not specific to maintaining a lever press during the simple RT and time production tasks. Similar population functions were found in the mPFC as rats waited for the end of the delay period in a delayed alternation task (Caetano et al., 2012) and engaged in extended bouts of reward consumption in a delayed alternation task and variable interval procedure (Horst and Laubach, 2013).
One potential pitfall for the analysis of population activity that we have reported is that the results are based on firing estimates that are averaged over trials and from multiple animals. This approach was based on Paz et al. (2005). It is possible that the same population functions would not be found if single-animal or single-trial data were used. To examine this issue, we analyzed data from single animals using the same PCA methods and found that the same two leading modes of population activity could be be detected (Figure 8A,B). To address the issue of using trial-averaged data, we used an alternative PCA method (Chapin and Nicolelis, 1999; Laubach et al., 1999) that was based on the full time series for each neuron in a given ensemble (Figure 8C). We found that the same two leading modes of population activity could be detected using this alternative approach. Finally, it was possible to reproduce the firing patterns using simulated spike trains from the double-integration computational model developed in Bekolay et al. (2014). (See Figures 7–8 in that manuscript.)
Our PCA analysis revealed that major changes in spiking activity in the mPFC were coterminous with the macro-level events described in the previous section. These findings suggests that the expression of ERPs and delta-band phase locking to the initiation of behavioral timing is linked to changes in populations activity. To our knowledge, this is a novel result for the mPFC. We are not aware of any published study that reports changes in spiking activity accompany changes in established markers of network synchronization such as ERPs and delta-band synchronization. Two previous studies, one experimental (Narayanan and Laubach, 2009) and the other based on a spiking network model (Bekolay et al., 2013), suggest that the neuronal activity patterns are generated by an integrator network that can track the passage of time since the animal has entered into the delay period. As such, our results provide a novel mechanism for denoting behavioral time markers in terms of neuronal activity that could be used to control action timing, as proposed by Caetano and Church (2009).
Rhythmic neural activity in the mPFC encodes performance adjustments after errors
The studies described above establish that neuronal spike activity in mPFC has a role in stimulus and temporal processing and in encoding previous and forthcoming behavioral outcomes during the performance of simple RT tasks. How do these signals enable adaptive control over performance? That is, how are performance adjustments implemented by the mPFC? We propose a conceptual model (Figure 9A) in which errors can lead to two types of changes in processing, which could occur together, and that would lead to post-error slowing. First, errors could lead to increased control over action, for example by increasing the threshold needed to trigger action on post-error trials (Summerfield and Young, 2013). Second, errors could increase reliance on the stimulus, leading subjects to pay more attention to the stimulus (or increase resources for stimulus detection and integration). Both of these processes imply that post-error slowing reflects a change in the performance strategy.
To determine if there was support for these two types of changes in processing following an error, we compared spectral amplitude and power on post-correct and post-error trials using the same data sets and analysis methods as in Narayanan et al. (2013). We found evidence both for pre-stimulus increases in the amplitude of low-frequency “theta” fluctuations and for increased phase locking to the action-imperative stimulus on post-error trials. Similar results were also found for theta-range field potential fluctuations in the motor cortex (not shown). These field potential signals were generally coherent with spiking by mPFC neurons and depended on the integrity of the mPFC network, i.e. reversible inactivation of mPFC disrupts the expression theta locking in the motor cortex and leads to the loss of adaptive control over action (Narayanan et al., 2013). These processes could serve as a mechanism for adaptive control (Cavanagh and Frank, 2014).
The increased low-frequency rhythm before the stimulus could reflect changes in spiking during the initiation of the trial, as revealed in our PCA-based population analysis (Figures 7–8), and lead to increased phase locking to the stimulus. Such changes in spike activity would then reflect a change in the behavioral strategy in the task, and might result from altered dynamics and learning among the neuronal ensemble. Analysis of neuronal population activity supported this view. There was a major difference in spiking before trials were initiated (Figure 10A,B). The firing rates of many neurons were modulated around the lever press on post-correct trials (left plot in Figure 10A). By contrast, neurons fired persistently before the start of the post-error trials (right plot in Figure 10B). These differences in neural activity resulted in the leading components of variance being highly distinct as a function of the previous trial outcome. On post-correct trials, the population functions were the same as in Narayanan and Laubach (2009), which reported data from sessions with a single (fixed) delay and with two equally likely delays, post-correct trials. On post-error trials, the leading component reflected the persistent activity associated with the recent error.
These differences in population activity resulted in distinct dynamics associated with the post-correct and post-error trials. Phase plots of these functions revealed distinct neural dynamics associated with the outcome of the previous trial (Figure 10C). Interactions between the two leading components on post-correct trials resulted in a closed triangular trajectory through phase space, with the neural population starting from and returning to the same region of phase space before and after the trial. Phase plots of the higher components were complex and lacked clear structure. By contrast, post-error trials exhibited a “hop” across phase space, starting and ending in distinct locations within the space defined by the neural activity.
The dynamics were reproduced by the spiking network model in Bekolay et al. (2014). As the complete set of synaptic weights could be gleaned from the model, it was possible to demonstrate that errors resulted in the network experiencing activity states that are not experienced after correct responses. These activity patterns engaged sets of distinct synapses with activity levels that were not found on post-correct trials. As a result, it is possible to learn from errors given that the network dynamics associated with correct and incorrect responding were different. The observed in vivo dynamics of the mPFC and the neural circuit model, together, provide a novel account for how the mPFC may learn from mistakes while enabling adaptive control over action.
Conclusions
The mPFC is crucial for the control of action initiation and the ability to adjust performance after mistakes are made. Two recent studies (Narayanan et al., 2013; Srinivasan et al., 2013) used a classic simple RT task (Kornblum, 1973) and established that these functions are evolutionarily conserved. A key neural marker for adaptive control is low-frequency rhythmic activity, in the delta (<4 Hz) and theta (4–12 Hz) range that can be measured in EEG recordings in humans and field potential recordings in experimental animals. Changes in these brain rhythms are associated with changes in neuronal spike activity that encodes previous and forthcoming behavioral outcomes. Based on the studies discussed in this review, it seems that significant progress can now be made on understanding the neural mechanisms for the adaptive control of action and determining if changes in these processes underlie prominent diseases such as Parkinson's and Alzheimer's diseases and a variety of psychiatric illnesses associated with mPFC dysfunction.
Highlights.
This manuscript reviews the role of the medial prefrontal cortex in the adaptive control of action based on multi-electrode recordings done in rodents.
We report field potential data that reveal a role for the medial prefrontal cortex (mPFC) in stimulus detection and time estimation.
We describe event-related potentials in the mPFC that are triggered by the animal's actions and action-imperative stimuli. These potentials might act as “time markers” that denote transitions between waiting to acting.
We relate these network-level signals to neuronal spike activity and report that low-frequency fluctuations (<4 Hz) in field potentials are associated with changes in population activity.
Finally, we propose a mechanism by which these neuronal signals might enable updating performance strategies after mistakes are made.
Acknowledgements
Support for the research discussed in this review was provided to ML by the US National Science Foundation (194344, 1121147), US National Institutes of Health (AG030004), the American Federation for Aging Research, and the Tourette Syndrome Foundation. NSN is currently funded by the US National Institutes of Health (NS078100). The authors would like to thank Trevor Bekolay, Linda Amarante, and two anonymous reviewers for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander WH, Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14:1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171:30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph J-P, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex N Y N 1991. 2006;16:1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 1999;141:57–65. doi: 10.1007/s002130050806. [DOI] [PubMed] [Google Scholar]
- Bekolay T, Laubach M, Eliasmith C. A Spiking Neural Integrator Model of the Adaptive Control of Action by the Medial Prefrontal Cortex. J Neurosci. 2014;34:1892–1902. doi: 10.1523/JNEUROSCI.2421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bherer L, Belleville S. Age-related differences in response preparation: the role of time uncertainty. J Gerontol B Psychol Sci Soc Sci. 2004;59:P66–P74. doi: 10.1093/geronb/59.2.p66. [DOI] [PubMed] [Google Scholar]
- Bherer L, Belleville S, Gilbert B. Temporal preparation strategy may inflate RT deficit in patients with Parkinson’s disease. J Clin Exp Neuropsychol. 2003;25:1079–1089. doi: 10.1076/jcen.25.8.1079.16725. [DOI] [PubMed] [Google Scholar]
- Bonini F, Burle B, Liégeois-Chauvel C, Régis J, Chauvel P, Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Uylings HBM. Visual attention task performance in Wistar and Lister Hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 1999;94:47–57. doi: 10.1016/s0306-4522(99)00312-7. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM, Robbins TW. Response-related deficits following unilateral lesions of the medial agranular cortex of the rat. Behav Neurosci. 1991;105:567–578. doi: 10.1037//0735-7044.105.4.567. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T. Error negativity does not reflect conflict: a reappraisal of conflict monitoring and anterior cingulate cortex activity. J Cogn Neurosci. 2008;20:1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford University Press, USA; 2006. [Google Scholar]
- Caetano MS, Church RM. A comparison of responses and stimuli as time markers. Behav Processes. 2009;81:298–302. doi: 10.1016/j.beproc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano MS, Horst NK, Harenberg L, Liu B, Arnsten AFT, Laubach M. Lost in transition: aging-related changes in executive control by the medial prefrontal cortex. J Neurosci Off J Soc Neurosci. 2012;32:3765–3777. doi: 10.1523/JNEUROSCI.6011-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci Off J Soc Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJB. Theta lingua franca: a common midfrontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol. 2014;99:326–331. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MAL. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–140. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. J Neurosci Off J Soc Neurosci. 2012;32:10915–10924. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins T. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: Cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol. 1966;29:1011–1027. doi: 10.1152/jn.1966.29.6.1011. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Teräväinen H, Calne DB. Reaction time in Parkinson’s disease. Brain J Neurol. 1981;104:167–186. doi: 10.1093/brain/104.1.167. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Ferris S, Crook T, Sathananthan G, Gershon S. Reaction time as a diagnostic measure in senility. J Am Geriatr Soc. 1976;24:529–533. doi: 10.1111/j.1532-5415.1976.tb03277.x. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error Detection and Compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164:444–456. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. Working with memory: evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. J Neurophysiol. 2012;108:3276–3288. doi: 10.1152/jn.01192.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. Reward-related activity in the medial prefrontal cortex is driven by consumption. Front Neurosci. 2013;7:56. doi: 10.3389/fnins.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NE, Shen Z, Long SR, Wu MC, Shih HH, Zheng Q, Yen N-C, Tung CC, Liu HH. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc R Soc Lond Ser Math Phys Eng Sci. 1998;454:903–995. [Google Scholar]
- Hyman JM, Whitman J, Emberly E, Woodward TS, Seamans JK. Action and outcome activity state patterns in the anterior cingulate cortex. Cereb Cortex N Y N 1991. 2013;23:1257–1268. doi: 10.1093/cercor/bhs104. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance Monitoring by the Anterior Cingulate Cortex During Saccade Countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Brown RG, Marsden D. Simple and Choice Reaction Time and the Use of Advance Information for Motor Preparation in Parkinson’s Disease. Brain. 1992;115:539–564. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychol Rev. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghim J-W, Lee JH, Jung MW. Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci Off J Soc Neurosci. 2013;33:13834–13847. doi: 10.1523/JNEUROSCI.1443-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, Jung MW. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci. 2009;3:38. doi: 10.3389/neuro.08.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF. Human forebrain activation by visceral stimuli. J Comp Neurol. 1999;413:572–582. [PubMed] [Google Scholar]
- Kornblum S. Simple reaction time as a race between signal detection and time estimation: A paradigm and model. Atten Percept Psychophys. 1973;13:108–112. [Google Scholar]
- Kuwabara M, Mansouri FA, Buckley MJ, Tanaka K. Cognitive control functions of anterior cingulate cortex in macaque monkeys performing a Wisconsin Card Sorting Test analog. J Neurosci Off J Soc Neurosci. 2014;34:7531–7547. doi: 10.1523/JNEUROSCI.3405-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Meck WH. Differential effects of amphetamine and haloperidol on temporal reproduction: dopaminergic regulation of attention and clock speed. Neuropsychologia. 2013;51:284–292. doi: 10.1016/j.neuropsychologia.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Laming D. Autocorrelation of choice-reaction times. Acta Psychol (Amst) 1979a;43:381–412. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Laming D. Choice reaction performance following an error. Acta Psychol (Amst) 1979b;43:199–224. doi: 10.1016/0001-6918(79)90032-5. [DOI] [PubMed] [Google Scholar]
- Laubach M. A Comparative Perspective on Executive and Motivational Control by the Medial Prefrontal Cortex. Neural Basis Motiv Cogn Control. 2012:95. [Google Scholar]
- Laubach M, Shuler M, Nicolelis MAL. Independent component analyses for quantifying neuronal ensemble interactions. J Neurosci Methods. 1999;94:141–154. doi: 10.1016/s0165-0270(99)00131-4. [DOI] [PubMed] [Google Scholar]
- Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature. 2000;405:567–571. doi: 10.1038/35014604. [DOI] [PubMed] [Google Scholar]
- Lempka SF, Johnson MD, Moffitt MA, Otto KJ, Kipke DR, McIntyre CC. Theoretical analysis of intracortical microelectrode recordings. J Neural Eng. 2011;8:045006. doi: 10.1088/1741-2560/8/4/045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Florence SL, Kaas JH. Areal distributions of cortical neurons projecting to different levels of the caudal brain stem and spinal cord in rats. Somatosens Mot Res. 1990;7:315–335. doi: 10.3109/08990229009144711. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychol Rev. 1997;104:241–265. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology (Berl) 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Meckler C, Allain S, Carbonnell L, Hasbroucq T, Burle B, Vidal F. Motor inhibition and response expectancy: a Laplacian ERP study. Biol Psychol. 2010;85:386–392. doi: 10.1016/j.biopsycho.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Meckler C, Allain S, Carbonnell L, Hasbroucq T, Burle B, Vidal F. Executive control and response expectancy: a Laplacian ERP study. Psychophysiology. 2011;48:303–311. doi: 10.1111/j.1469-8986.2010.01077.x. [DOI] [PubMed] [Google Scholar]
- Meckler C, Carbonnell L, Hasbroucq T, Burle B, Vidal F. To err or to guess: an ERP study on the source of errors. Psychophysiology. 2013;50:415–421. doi: 10.1111/psyp.12020. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–1895. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci U S A. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Ollman RT, Billington MJ. The Deadline model for simple reaction times. Cognit Psychol. 1972;3:311–336. [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Alberico SL, Miller AD, Narayanan NS. Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. 2013;255:246–254. doi: 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Measuring correlations and interactions among four simultaneously recorded brain regions during learning. J Neurophysiol. 2009;101:2507–2515. doi: 10.1152/jn.91259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Natan C, Boraud T, Bergman H, Vaadia E. Emerging Patterns of Neuronal Responses in Supplementary and Primary Motor Areas during Sensorimotor Adaptation. J Neurosci. 2005;25:10941–10951. doi: 10.1523/JNEUROSCI.0164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Sallet J, Camille N, Procyk E. Modulation of feedback-related negativity during trial-and-error exploration and encoding of behavioral shifts. Front Neurosci. 2013;7:209. doi: 10.3389/fnins.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The Integration of Negative Affect, Pain, and Cognitive Control in the Cingulate Cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Singh R, Eliasmith C. Higher-dimensional neurons explain the tuning and dynamics of working memory cells. J Neurosci Off J Soc Neurosci. 2006;26:3667–3678. doi: 10.1523/JNEUROSCI.4864-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Horst NK, Liu B, Caetano MS, Laubach M. Reversible Inactivation of Rat Premotor Cortex Impairs Temporal Preparation, but not Inhibitory Control, During Simple Reaction-Time Performance. Front Integr Neurosci. 2010;4:124. doi: 10.3389/fnint.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Asaad WF, Ginat DT, Gale JT, Dougherty DD, Williams ZM, Sejnowski TJ, Eskandar EN. Action initiation in the human dorsal anterior cingulate cortex. PloS One. 2013;8:e55247. doi: 10.1371/journal.pone.0055247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC. Response latency as a function of amount of reinforcement. J Exp Anal Behav. 1962;5:305–307. doi: 10.1901/jeab.1962.5-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC, Lanson RN. Response latency as a function of reinforcement schedule. J Exp Anal Behav. 1962;5:299–304. doi: 10.1901/jeab.1962.5-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC, Miller JM. Reaction time as a function of stimulus intensity for the monkey. J Exp Anal Behav. 1964;7:309–312. doi: 10.1901/jeab.1964.7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanics G, Hangya B, Hernádi I, Winkler I, Lakatos P, Ulbert I. Phase Entrainment of Human Delta Oscillations Can Mediate the Effects of Expectation on Reaction Speed. J Neurosci. 2010;30:13578–13585. doi: 10.1523/JNEUROSCI.0703-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Yeung N. Oh, rats! Post-error behavioral adjustment in creatures great and small. Nat Neurosci. 2013;16:1715–1716. doi: 10.1038/nn.3577. [DOI] [PubMed] [Google Scholar]
- Sun H, Green TA, Theobald DEH, Birnbaum SG, Graham DL, Zeeb FD, Nestler EJ, Winstanley CA. Yohimbine increases impulsivity through activation of cAMP response element binding in the orbitofrontal cortex. Biol Psychiatry. 2010;67:649–656. doi: 10.1016/j.biopsych.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvain-Roy S, Bherer L, Belleville S. Contribution of temporal preparation and processing speed to simple reaction time in persons with Alzheimer’s disease and mild cognitive impairment. Brain Cogn. 2010;74:255–261. doi: 10.1016/j.bandc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Vallesi A, McIntosh AR, Stuss DT. Temporal preparation in aging: a functional MRI study. Neuropsychologia. 2009;47:2876–2881. doi: 10.1016/j.neuropsychologia.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T. Developmental dissociations of preparation over time: deconstructing the variable foreperiod phenomena. J Exp Psychol Hum Percept Perform. 2007;33:1377–1388. doi: 10.1037/0096-1523.33.6.1377. [DOI] [PubMed] [Google Scholar]
- Van Dyck CH, Avery RA, MacAvoy MG, Marek KL, Quinlan DM, Baldwin RM, Seibyl JP, Innis RB, Arnsten AFT. Striatal dopamine transporters correlate with simple reaction time in elderly subjects. Neurobiol Aging. 2008;29:1237–1246. doi: 10.1016/j.neurobiolaging.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. Cingulate area 32 homologies in mouse, rat, macaque and human: cytoarchitecture and receptor architecture. J Comp Neurol. 2013;521:4189–4204. doi: 10.1002/cne.23409. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct. 2014;219:185–192. doi: 10.1007/s00429-012-0493-3. [DOI] [PubMed] [Google Scholar]
- Warren CM, Hyman JM, Seamans JK, Holroyd CB. Feedback-related negativity observed in rodent anterior cingulate cortex. J Physiol Paris. 2014 doi: 10.1016/j.jphysparis.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci Off J Soc Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Woodrow H. The measurement of attention. Psychol Monogr. 1914;17:i–158. [Google Scholar]
- Wyart V, de Gardelle V, Scholl J, Summerfield C. Rhythmic Fluctuations in Evidence Accumulation during Decision Making in the Human Brain. Neuron. 2012;76:847–858. doi: 10.1016/j.neuron.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang S, Dan Y, Poo M. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci U S A. 2014;111:480–485. doi: 10.1073/pnas.1321314111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]