Abstract
The dopamine transporter (DAT1) gene is implicated in psychopathology risk. While the processes by which this gene exerts its effects on risk are poorly understood, a small body of research suggests that DAT1 influences early emerging negative emotionality (NE), a marker of children’s psychopathology risk. As child NE evokes negative parenting practices, the DAT1 may also play a role in gene-environment correlations. To test this model, children (N = 365) were genotyped for DAT1 and participated in standardized parent-child interaction tasks with their primary caregiver. The DAT1 9-repeat variant was associated with child negative affect expressed toward the parent during parent-child interactions, and parents of children with a 9-repeat allele exhibited more hostility and lower guidance/engagement than parents of children without a 9-repeat allele. These gene-environment associations were partially mediated by child negative affect toward the parent. Findings implicate a specific polymorphism in eliciting negative parenting, suggesting that evocative associations play a role in elevating children’s risk for emotional trajectories toward psychopathology risk.
Keywords: dopamine transporter, parenting, child negative affect, gene-environment correlation
Dopamine is an important monoamine involved in the regulation of both positive and negative emotions (Bressan & Crippa, 2005). Dopamine availability at the synaptic level is regulated by the dopamine transporter (DAT) protein, which shuttles dopamine from neuronal extracellular space into intracellular compartments (Miller & Madras, 2002). The DAT thus plays a key role in modulating dopamine-mediated behaviors. Expression of the DAT protein is shaped by genetic variation of the DAT1 gene (SLC6A3); located on chromosome 5p15.3, this gene has a 40-base pair variable number of tandem repeat (VNTR) polymorphism in the 3’-untranslated region. While this polymorphism has an array of variants in humans, ranging from 3- to 11-repeats, the 9- and 10-repeat polymorphisms are the most common and have been the focus of most research (Ueno, 2003; van Dyck et al., 2007). Although this polymorphism is located in the non-coding region, evidence suggests that it has functional effects; for example, an in vitro study using transiently transfected DAT1 9- and 10-repeat constructs in human cell lines led to significant differences in DAT protein expression (Miller & Madras, 2002). Another study found that the 9-repeat allele was associated with greater striatal DAT expression in humans (van Dyck et al., 2007), a finding that has been replicated (van de Giessen et al., 2009; although see also Heinz et al., 2000).
Association studies of DAT1 have linked this gene to various neuropsychiatric disorders, including depression (Dunlop & Nemeroff, 2007) and posttraumatic stress disorder (Segman et al., 2002). The DAT1 is also linked to childhood disorders, such as attention-deficit hyperactivity disorder (Cook et al., 1995; Durston et al., 2008) and conduct disorder (Lahey et al., 2011). Recently, genetic variants of the DAT1 have been linked to personality traits, with those with a 9-repeat allele five times more likely to exhibit angry-impulsive traits than those without this variant (Joyce et al., 2009). While research on the role of this gene in temperament and personality is in its infancy, considering these findings as a whole suggests that this gene may increase psychopathology risk by virtue of its influence on early-emerging individual differences in negative emotionality (NE). Although the role of dopamine in positive mood states and reward has received the most attention (Berridge & Robinson, 1998; Spanagel & Weiss, 1999), dopamine may also influence negative emotions and trait negative emotionality. For example, it has been argued that circulating dopamine in the prefrontal cortex predisposes to a heightened attentional focus on negative environmental stimuli (Montag et al., 2008), which may influence individual differences in the capacity to effectively regulate negative emotions. Furthermore, variation in other dopaminergic genes has been linked to heightened levels of negative affect in infants (Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001; Holmboe, Nemoda, Fearon, Sasvari-Szekely, & Johnson, 2011), further suggesting that dopaminergic genetic variation shapes NE. NE shows a substantial degree of heritability (Goldsmith, Buss, & Lemery, 1997), and has been prospectively associated with internalizing and externalizing symptoms and related disorders in children (e.g., Eisenberg et al., 2009), indicating that NE may be an important psychiatric endophenotype (Hasler, Drevets, Manji, & Charney, 2004). Identifying specific genes that influence its development is therefore a key goal for psychiatric genetics.
Research on biological influences on children’s emotional development and psychopathology is complicated by the dynamic relationships between children’s biological predispositions and the environments in which they are raised (Rutter, 1991; Rutter, 2003). Along these lines, gene-environment interaction (GXE) and gene-environment correlation (rGE) reflect how the interplay between biological and environmental influences may eventuate in psychopathology. Relevant work focused on GXE in childhood, that is, genetic differences in children’s susceptibility to particular environments, is accruing rapidly (e.g., Bakermans-Kranenburg & van IJzendoorn, 2006; Hayden et al., 2010; Sheese, Voelker, Rothbart, & Posner, 2007; Smith et al., in press), indicating that genetic influences on children’s psychopathology risk are moderated by contextual factors. For example, with specific regard to DAT1, Lahey et al. (2011) found that the influence of parenting on the development of conduct disorder varied as a function of the number of 9-repeat alleles in children. In sharp contrast, there has been much less work on identifying genetic influences on the probability of exposure to particular risk environments (Jaffee & Price, 2007). While the concept of rGEs was described many years ago (see Plomin, DeFries, Loehlin, 1977 for an early synthesis of the concept as pertains to psychology), only relatively recently has it become feasible to obtain the molecular genetic data needed to identify specific genes that may play a role in heightening risk exposure. The first measured rGE was reported by Dick et al. (2006), in which a variant of the gamma-aminobutyric acid (GABA) A receptor, alpha 2 gene (GABRA2) was associated with marital status. With respect to psychopathology risk mechanisms, Lucht et al. (2006) reported an association between perceived negative paternal parenting (reported retrospectively by adult offspring) and offspring variants of both DRD2 and GABRA6. Other groups have also reported rGEs involving parenting and DRD2 (Hayden et al., 2010; Mills-Koonce et al., 2007), although very little is known about the role of other dopaminergic genes, such as DAT1, in rGE.
Of the few studies on rGE in psychopathology, most have not examined potential mediators of the rGEs reported (i.e., mechanisms that account for the obtained association between a measured gene and environmental risk). Given that intrinsic (i.e., children’s individual differences) and extrinsic (i.e., environmental) risks for psychopathology are often correlated with one another (Rutter, 2009), rGEs represent a means of more clearly delineating how these dual sets of influences become associated with each other during development. The causal mechanisms underlying rGEs are of particular interest, as genetic factors can be related to environmental factors through an array of processes; such associations are thought to be commonly mediated by intermediate behaviors under genetic influence (Rutter, Moffitt, & Caspi, 2006; Rutter, 2007). For example, evocative rGEs refer to the process by which genetically influenced child behavior elicits contextual risk from the child’s environment, such as poor parenting practices. Thus, evocative rGE represents a process by which child genetic risk is potentially amplified through its influence on contextual risk, and may therefore have implications for preventative interventions.
In childhood, the quality of parenting children receive is a major source of contextual risk. Supportive parenting has been found to predict lower levels of child behavior problems, greater social skills, and better academic performance (Coplan, Arbeau, & Armer, 2007; Pettit, Bates, & Dodge, 1997; Stormshak, Bierman, McMahon, & Lengua, 2000). In particular, the guidance and support provided by parents to children in the context of skill acquisition and learning, also known as scaffolding (Wood, Bruner, & Ross, 1976), is associated with important child outcomes such as intelligence and academic achievement (Englund, Luckner, Whaley, & Egeland, 2004). Conversely, negative parenting practices, such as intrusiveness and hostility, have been consistently associated with child behavioral and emotional problems. For example, Caron, Weiss, Harris, and Catron (2006) found that negative parenting styles (e.g., high behavioral and psychological control) were linked to both externalizing and internalizing child psychopathology. In particular, hostile parenting may have important relevance for negative child outcomes (Sheffield Morris et al., 2002). For example, hostile parenting is strongly linked to depression in older youths (McLeod, Weisz, & Wood, 2007) and to disrupted neuroendocrine functioning in the offspring of depressed parents (Gunnar & Vazquez, 2006).
While rGE indicates that parenting behaviors may be linked to children’s genetic characteristics, little research has tested this possibility, although it has long been accepted that children are active agents in shaping the environment that parents provide (Deater-Deckard, 2000; Lee et al., 2010). For example, Lengua and Kovacs (2005) found evidence for bidirectionality of the effects of child characteristics and parenting, such that inconsistent discipline increased negative emotionality in children, and child negative emotionality evoked inconsistent discipline by parents. Additionally, maternal control is elicited by child anxiety (Eley, Napolitano, Lau, & Gregory, 2010; Moore, Whaley, & Sigman, 2004), and child disruptive disorders negatively influence parents’ ability to engage in appropriate discipline (Burke, Pardini, & Loeber, 2008). Furthermore, Ge et al. (1996) employed an adoption design to illustrate that antisocial behaviors of adopted children evoked negative adoptive parent responses, providing further evidence that passive rGEs (i.e., in the present case, genes that influence parental caregiving behaviors are also inherited by children; Plomin et al., 1977) did not solely account for associations between the heritable and environmental risk present in children at risk for disruptive behavior disorders. Thus, it is clear that child characteristics play a key role in eliciting parenting, and, to the extent that child behavior that elicits adaptive or maladaptive parenting is genetically influenced (e.g., Rhee & Waldman, 2002), evocative rGEs are likely present.
Considering the literature implicating DAT1 in children’s psychopathology risk, this gene may play a role in shaping child behaviors that, in turn, elicit poor parenting. Furthermore, the role of dopamine in bonding and affiliative behavior (Depue & Morrone-Strupinsky, 2005; Lee et al., 2010) further supports the possibility that some of the associations between children’s dopaminergic genes and adverse outcomes are mediated by genetic influences on early parent-child relationships. However, very little work has been done to explore this possibility. The current study therefore represents a novel and exploratory attempt to examine associations between the DAT1 gene and parent-child interactions, and extends our group’s efforts to identify rGEs that play a role in emerging psychopathology risk (Hayden et al., 2010). More specifically, we tested whether children with a 9-repeat allele of the DAT1 exhibited greater negative affectivity directed toward parents during standardized, observational measures of parent-child interaction. We also examined whether the DAT1 9-repeat was associated with parenting styles with key implications for adaptive and maladaptive child outcomes: parental hostility and scaffolding. Finally, we planned to attempt to identify the potential mechanism(s) underlying any rGEs by testing whether any associations between children’s DAT1 alleles and parental behavior were mediated by child negative affectivity, thus potentially supporting the presence of an evocative rGE.
Method
Participants were 365 children (197 males) from a larger sample of 567 children and their parents who were participating in a longitudinal study. The mean age of the children at the time of the current study was 72.9 months (SD = 6.0). Eligible children had no significant medical conditions or developmental delays, as well as at least one English-speaking biological parent who could also participate. Most participants were from middle-class families, as measured by Hollingshead’s Four Factor Index of Social Status (M = 44.4, SD = 10.7) (Hollingshead, 1975). Almost all children came from two-parent homes (88.8%), and were of average cognitive ability as measured by the Peabody Picture Vocabulary Test (M = 103.1, SD = 13.4) (PPVT; Dunn & Dunn, 1997). Children were Caucasian (n = 319; 87.4%), Hispanic (n = 15; 4.1%), or from a variety of other racial and ethnic backgrounds (n = 31; 8.5%).
When children and their parents came to the laboratory to take part in behavioral tasks, we collected buccal cells for genetic analysis by rubbing the inside of each child’s cheek with two collection swabs. From the larger sample of 567 children at baseline, 476 had parental consent to provide samples for genetic assessment, and 432 participated in the parent-child interaction tasks. Only those children for whom genetic and parenting data were available were included in the present analyses, yielding a sample size of 3651. Children in the current study did not differ from non-participating children on any demographic variables (child sex, socioeconomic status, cognitive ability, number of parents in the home; all ps > .05).
We used the Qiagen DNA MicroKit (Qiagen, Valencia, California, USA) to extract genomic DNA from buccal epithelial cells. Purified genomic DNA was kept at 4 °C while being analyzed and then at −80 °C for long-term storage. Polymerase chain reaction (PCR) was conducted using the Applied Biosystems thermal cycler Gene Amp 9700 (Applied Biosystems, Foster City, California, USA), and PCR products were separated on polyacrylamide gels, stained with ethidium bromide, and visualized and documented by a UV imaging system (BioRad Labs, Mississauga, Ontario, Canada). To insure accuracy of the genetic data, a technician randomly selected and reanalyzed 10% of the genetic samples. In the single case of discrepant results for the DAT1, the child’s sample was excluded. All research technicians performing genotyping were blind to other study data.
We used the following primers: 5’-TGTGGTGTAGGGAACGGCCTGAG-3’ (forward) and 5’-CTTCCTGGAGGTCACGG CTCAAGG-3’ (reverse). The PCR conditions were as follows: 5 min initial denaturation at 95 °C and 30 cycles of 30 s initial denaturation at 94 °C, 45 s annealing at 67.5 °C, 45 s extension at 72 °C, followed by 5 min of final extension at 72 °C. The 9-repeat and 10-repeat products yield a 440 bp and 480 bp fragment, respectively. Although genotypes were successfully obtained for 371 children, for the purposes of our analyses, six participants with rare variants of the DAT1 were excluded. The genotypes of the remaining 365 children were distributed as follows: 177 (48%) children had the 10/10 genotype, 153 (42%) had the 9/10 genotype, and 35 (10%) had the 9/9 genotype. This distribution is in Hardy-Weinberg equilibrium, Χ2 = .05, p = .82.
The literature is unclear concerning which DAT1 allele is associated with negative outcomes (Lee et al., 2007; Rowe et al., 1998), nor whether the repeat variants function in an additive manner. However, most previous findings relevant to child psychopathology risk (Young et al., 2002), as well as functional studies (van Dyck et al., 2005; van de Giessen et al., 2009) have contrasted children with the 9-repeat allele to those without. Furthermore, in analyses not reported in full here, we found no significant differences between children with two copies versus one copy of the 9-repeat on any study variables (all ps > .14). Therefore, to conserve space, results are presented based on contrasting children with (n = 188) and without (n = 177) a 9-repeat allele.
Teaching tasks
All 365 children and a primary caregiver (most often the mother, n = 320, 87.7%) participated in a modified version of the Teaching Tasks battery (Egeland et al., 1995). The battery consisted of four standardized parent-child interaction tasks lasting a total of 25 to 30 minutes. The tasks, which occurred in the order listed here, were designed to elicit a variety of parenting styles and child behaviors, and consisted of a guessing game, a marble maze consisting of four different trial types, pictures that had to be arranged to tell a story, and a puzzle with six different designs. We coded parental supportive presence, guidance/engagement (i.e., scaffolding), intrusiveness, and hostility using a global approach to coding, with a single rating given for each parenting behavior for each of the four tasks. Ratings were subsequently averaged across tasks to yield total scores for each parenting dimension. Ratings of parent supportive presence (α = 0.86) were based on the parent’s provision of emotional support and expression of positive regard. Parent guidance/engagement (α = 0.76) was coded based on how well the parent guided the child in completing the tasks, and the parent’s degree of engagement in working with the child. Parent intrusiveness (α = 0.65) was rated based on the extent to which the parent failed to allow autonomous child behavior. Ratings of parent hostility (α = 0.75) were based on anger, annoyance, and rejection of the child displayed by the parent. Interrater ICCs (n = 35) for supportive presence, guidance/engagement, intrusiveness, and hostility were 0.84, 0.74, 0.81, and 0.86, respectively.
In addition to parenting, child negative affect toward the parent (child NA toward parent), and overall child positive affect (PA) were coded in each task (the coding system used did not include ratings of child PA directed specifically toward the parent). Child NA toward the parent (α = 0.68) was coded based on the degree to which the child displayed anger, dislike, or hostility toward the parent. Ratings of overall child PA (α = 0.76) were based on the frequency and intensity of facial, bodily, and vocal indicators of positive emotion during the parent-child interaction tasks. Interrater ICCs (n = 35) for child NA toward parent and overall child PA were 0.73 and 0.80 respectively.
Results
Associations between DAT1 groups (formed based on whether children had a 9-repeat allele) and major study variables can be found in Table 1, and bivariate correlations among all non-genetic study variables are in Table 2. To address the issue of possible population stratification, we initially conducted all analyses with and without non-Caucasian children. Given that results were nearly identical in both cases, and that Caucasian and non-Caucasian children did not differ in terms of the proportion with at least one 9-repeat allele or in terms of child phenotype (i.e., NA directed toward their parents; ps > .73), we present findings including children from all ethnicities. Children with and without a 9-repeat allele were not significantly different on socioeconomic status or cognitive ability (as indexed by the PPVT). However, there were significantly more boys in the 9-repeat allele group. Child sex was therefore treated as a covariate for all analyses, although mediation analyses yielded a consistent pattern when analyzing boys and girls separately. With respect to behavior during parent-child interactions, the two genotype groups differed in terms of parental hostility, such that parents of children with a 9-repeat allele exhibited more hostility during the parent-child interactions (p = .05). The two genotype groups also differed in terms of parent guidance/engagement, such that parents of children with a 9-repeat allele provided less guidance (p = .01) The two groups did not differ significantly in parental support or intrusiveness. Children with a 9-repeat allele displayed significantly more NA toward their parent (p = .04) during parent-child interactions; there was no significant difference in children’s PA expressed while interacting with the parent based on the presence of a 9-repeat allele (p = .63); thus, child PA is not considered further as a potential mediator.
Table 1.
Demographic and study variables by child DAT1 genotype
| Child DAT1 genotype |
|||||||
|---|---|---|---|---|---|---|---|
|
DAT1 10/10 (n = 177) |
DAT1 9/9 and 9/10 (n = 188) |
d |
|||||
| Variable | M | SD | n | M | SD | n | |
| Child sex, male* | 85 (48%) |
112 (60%) |
|||||
| PPVT | 102.58 | 13.08 | 103.52 | 13.80 | .07 | ||
| SES | 43.77 | 10.13 | 44.88 | 11.23 | .10 | ||
| PCI support | 4.36 | 0.56 | 4.27 | 0.59 | .16 | ||
| PCI guidance* | 4.23 | 0.57 | 4.08 | 0.50 | .28 | ||
| PCI hostility* | 1.08 | 0.23 | 1.14 | 0.31 | .22 | ||
| PCI intrusiveness | 1.62 | 0.73 | 1.59 | 0.68 | .04 | ||
| PCI child PA | 2.82 | 0.62 | 2.79 | 0.72 | .04 | ||
| PCI child NA toward parent* |
1.21 | 0.40 | 1.30 | 0.47 | .21 | ||
DAT1, dopamine transporter gene; NA, negative affect; PA, positive affect; PCI, parent-child interaction task; PPVT, Peabody Picture Vocabulary Test; SD, standard deviation; SES, socioeconomic status, as indexed by Hollingshead Four Factor Index of Social Status (Hollingshead, 1975).
p ≤ .05.
Table 2.
Bivariate correlations among non-genetic study variables
| r | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. |
| 1. Child sex | - | .09 | −.08 | .01 | .06 | −.06 | −.06 | .08 | .02 |
| 2. PPVT | - | .04 | .06 | .17** | .02 | −.16** | .05 | −.08 | |
| 3. SES | - | .08 | .10 | −.07 | −.06 | −.09 | .01 | ||
| 4. PCI support | - | .73** | −.47** | −.20** | .08 | −.20** | |||
| 5. PCI guidance/ engagement |
- | −.37** | −.46** | .10* | −.19** | ||||
| 6. PCI hostility | - | .23** | −.01 | .29** | |||||
| 7. PCI intrusiveness | - | −.14** | .06 | ||||||
| 8. PCI child PA | - | −.09 | |||||||
| 9. PCI child NA toward parent |
- | ||||||||
Note: Child sex coded as 0 = boys, 1 = girls.
p < .05,
p < .01.
Given that the two DAT1 allelic groups differed in terms of child NA toward the parent, we wanted to test whether child NA mediated the association between children’s DAT1 9-repeats and parenting. There were significant associations between the hypothesized mediator and the outcome (i.e., child NA toward the parent and parental hostility, r = .26, p = .001, and parental guidance/engagement, r = −.22, p = .001). Contemporary theories of mediation assert that associations between the distal predictor or IV and the outcome need not reach significance even when mediation is present (MacKinnon & Fairchild, 2009), although child DAT1 genotype and parenting were associated in the case of parental hostility and guidance/engagement. The bootstrap sampling procedure and macro developed by Preacher and Hayes (2004, 2008) were used to test mediation. This procedure estimates both mean direct (c) and mean indirect (i.e., mediated, c’) effects, as well as confidence intervals (CIs) obtained from multiple samples (set to 5000 for our analyses). The estimated effect is not statistically significant (at p < .05) if the estimated CIs obtained by the bootstrapping procedure contain the number zero. This mediation method is similar to more traditional approaches that use multiple regression, but holds many advantages, including greater robustness with regard to small sample sizes and violations of normality (Preacher & Hayes, 2004, 2008).
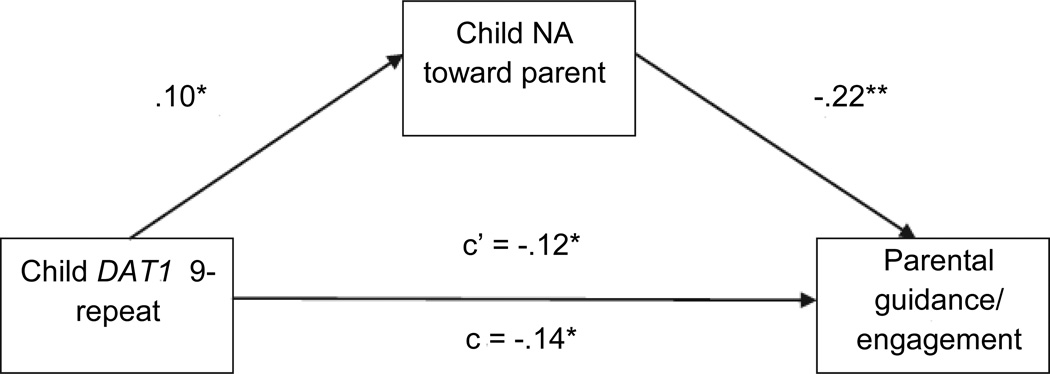
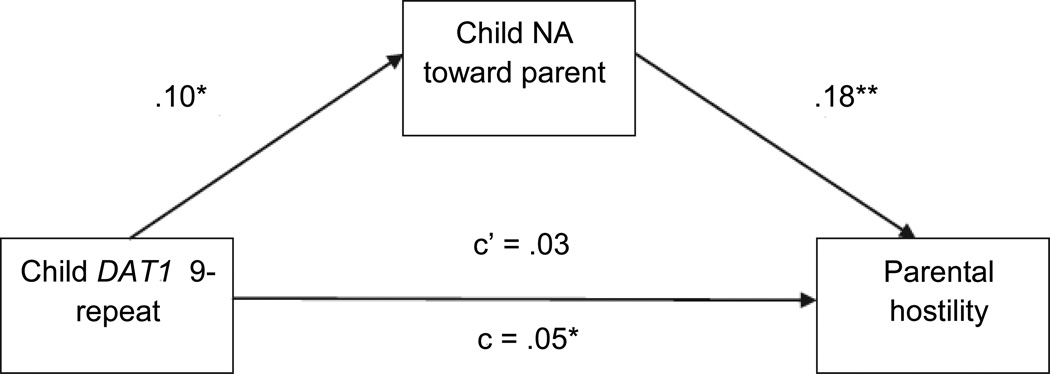
Two initial models were run using these procedures, the first focusing on parental guidance/engagement and the second focusing on parental hostility (Figure 1). With regard to the former, the bootstrapping procedure yielded a significant estimate of the indirect effect of DAT1 genotype on parental guidance/engagement after including child NA toward parent in the model (p < .05). Given that the effect of DAT1 genotype on parental guidance/engagement decreased (but did not become zero) with the inclusion of the mediator, it appears that child NA toward the parent partially mediated the relationship between child DAT1 genotype and parental guidance/engagement (Figure 1). The bootstrapping procedure also yielded a significant estimate of the indirect effect of DAT1 genotype on parental hostility after including child NA toward parent in the model (p < .05). The effect of DAT1 genotype on parental hostility decreased, becoming nonsignificant with the inclusion of the mediator, indicating that child NA toward parent partially mediated the relationship between child DAT1 genotype and this parenting dimension (Figure 2).
Figure 1. Mediated effect of child dopamine transporter (DAT1) genotype on parental guidance/engagement by child negative affect (NA) toward parent.
Note: Child DAT1 coded as 0 = 10/10 genotype, 1 = 9/9 or 9/10 genotype; parental guidance/engagement coded during parent-child interaction task; c = total effect of child DAT1 genotype on parental guidance/engagement; c’ = effect of child DAT1 genotype on parental guidance/engagement after including child NA toward parent in the model. *p < .05, **p < .01.
Figure 2. Mediated effect of child dopamine transporter (DAT1) genotype on parental hostility by child negative affect (NA) toward parent.
Note: Child DAT1 coded as 0 = 10/10 genotype, 1 = 9/9 or 9/10 genotype; parental hostility coded during parent-child interaction task; c = total effect of child DAT1 genotype on parental hostility; c’ = effect of child DAT1 genotype on parental hostility after including child NA toward parent in the model. *p < .05, **p < .01.
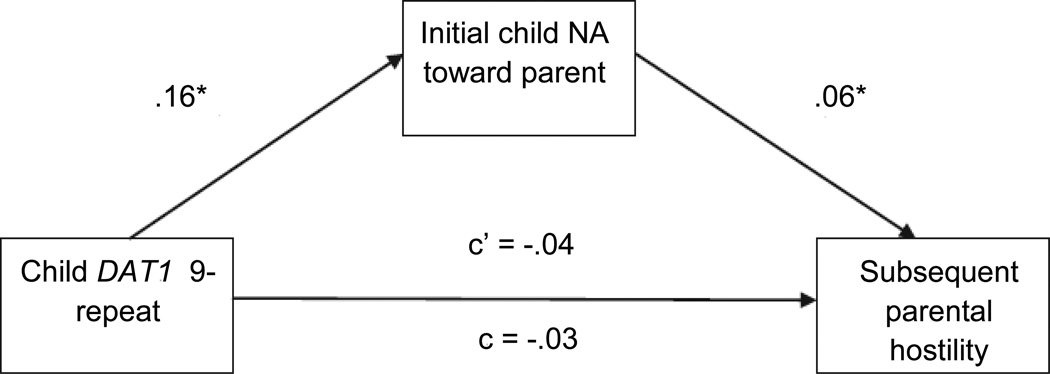
We next conducted two supplementary analyses aimed at better establishing temporal associations between children’s NA toward their parents and parenting behavior. For these analyses, children’s NA during the first part of the parenting battery (i.e., during the guessing game and maze tasks) was examined as a mediator of associations between DAT1 and parent guidance/engagement and hostility during the second half of the battery (picture story and puzzle), controlling for the equivalent parenting behavior during the first part of the battery and child NA toward the parent during the second half of the battery (i.e., the effects of initial parenting and later child behavior on parenting during the latter half of the parenting assessment were controlled). As these analyses were designed to follow-up significant findings from initial mediation models, one-tailed tests were used. The bootstrap sampling procedure and macro developed by Preacher and Hayes (2004, 2008) were again used to test mediation. In the model predicting parental guidance/engagement, child DAT1 genotype was not significantly related to child NA during the first part of the battery (p = .16), so this analysis is not described further. The model predicting parental hostility is presented in Figure 3. The bootstrapping procedure yielded CIs that did not contain ‘0’ indicating significant mediation.
Figure 3. Mediated effect of child dopamine transporter (DAT1) genotype on later parental hostility by initial child negative affect (NA).
Note: Child DAT1 coded as 0 = 10/10 genotype, 1 = 9/9 or 9/10 genotype; parental hostility coded during parent-child interaction task; c = total effect of child DAT1 genotype on parental hostility; c’ = effect of child DAT1 genotype on parental hostility after including child NA toward parent in the model. Initial levels of parental hostility and subsequent levels of child NA toward the parent were included as covariates. p <.05; one-tailed tests used in this model only.
Discussion
We examined whether there was an association between child DAT1 genotype and parenting assessed during standardized parent-child interaction tasks, and found that children with a 9-repeat allele received greater parental hostility and lower levels of parental guidance and engagement during parent-child interactions. Tests of mediation indicated that associations between children’s DAT1 genotype and these two parenting dimensions were partially mediated by child NA directed toward the parent during parent-child interactions. Our findings provide preliminary support for the presence of evocative rGEs, whereby the association between children’s DAT1 genotypes and parenting is partially accounted for by the effect of this gene on children’s negative affect during parent-child interactions, which may elicit parental hostility and diminished guidance and engagement. Our findings implicate the 9-repeat of the DAT1 gene in eliciting maladaptive parenting, an important, potentially malleable environmental variable that may possibly compound children’s underlying genetic vulnerability, although additional work is needed to test the latter possibility (i.e., the extent to which children at high genetic risk for adverse outcomes are differentially impacted by poor parenting styles, which is addressed later in this section). Child NA toward the parent only partially mediated the relationship between children’s DAT1 genotype and parenting; hence, other mechanisms, such as passive rGEs, may also be operating, or children’s DAT1 variants may influence parenting through its effects on child behaviors not measured in the present study. It is likely that child genetic risk is expressed through an array of pathways (Bakermans-Kranenburg & van IJzendoorn, 2011). In some cases, genetic effects may be relatively direct; that is, genes may influence biochemical processes that increase individual predispositions toward maladaptive emotional and cognitive responses to environmental stimuli (Munafò, Brown, & Hariri, 2008). However, genetic variation may also influence outcomes through relatively indirect processes, such as evocative rGEs.
We found that children with a 9-repeat allele exhibited greater NA toward parents during parent-child interactions. How genotypic variation at DAT1 shapes differences in child NA remains unclear, and given the lack of published research on the role of DAT and DAT1 in child emotional development, our discussion of processes and mechanisms is speculative. It could be that DAT1 influences child NA by virtue of the role of dopamine in general emotional processing (Badgaiyan, Fischman, & Alpert, 2009). For example, Sevy et al. (2006) found that a decrease in central dopaminergic activity resulted in poor emotion-based decision making, which suggests that variation in DAT1 may exert its effects at the interface of cognition and emotion. More specifically, dopamine appears to play a role in the regulation of anger and impulsivity; for example, functional imaging findings support the contention that dopamine availability influences anger and impulsivity (Forbes et al., 2009). These findings are supported by a small but generally supportive literature on genetic associations between the DAT1 and relevant emotional and behavioral phenotypes. Carriers of the 9-repeat allele have been found to have a fivefold increased likelihood of exhibiting angry-impulsive traits (Joyce et al., 2009). Since this allele appears to lead to greater striatal synaptic dopamine (Heinz et al., 2000), this finding is consistent with the notion that efficient reuptake of dopamine from the synaptic cleft, facilitated by the dopamine transporter protein, is key in the effective regulation of anger and impulsivity. In addition, administering a dopamine receptor antagonist has been shown to selectively disrupt the recognition of facial anger (Lawrence, Calder, McGowan, & Grasby, 2002). These findings indicate that dopamine may not only be a biological substrate related to anger and impulsivity, it may also influence the extent to which children are able to process affective and social cues that their behavior is inappropriate or otherwise unacceptable, thus facilitating adaptive modification of such behavior. Considering that dopamine facilitates the development of close interpersonal bonds (Depue & Morrone-Strupinsky, 2005), it is also possible that DAT1 variants contribute to the extent to which children are impaired in their capacity to develop close bonds with caregivers. A relatively weak dopaminergically-mediated parent-child bond might, over time, result in parent-child interactions characterized by heightened negativity. In summary, there are likely multiple pathways through which genotypic variation at DAT1 may contributes to childhood externalizing symptoms (Young et al., 2002), although further work is clearly needed.
The children who took part in the current project are part of a larger, ongoing study of childhood risk for psychopathology. These children were previously assessed at age 3, raising the question of whether the obtained rGEs were present in children’s earlier interactions with their parents. We did not find evidence for an rGE between DAT1 and caregiver hostility or guidance/engagement at the earlier assessment using observational measures of parent-child interactions similar to those reported here, nor did we find associations between this gene and child NA. However, children with a copy of the 9-repeat allele had mothers who expressed significantly more negativity and less warmth when discussing their children during an interview. Mothers of children with a 9-repeat allele of the DAT1 also reported engaging in more negative parenting styles at trend-level. While speculative, we propose that this pattern of findings is suggestive of a transactional process that unfolds over time. More specifically, our findings are consistent with the possibility that there is a genetically-driven process that has fairly weak effects when children are young, which is why relatively few genetic associations were obtained with age 3 child behavior. This process may escalate over time, such that by age 6, children with the 9-repeat allele exhibit both greater NA toward their parent, and receive greater hostility and less guidance/engagement from parents. Such a pattern is consistent with a cumulative reciprocity model of parent-child influence (e.g., Rothbaum & Weisz, 1994), whereby negative child behaviors and parents’ negative caregiving become increasingly interconnected over time. This process has the potential to strengthen throughout middle childhood, leading to even poorer relationships with parents and greater risk for psychopathology during preadolescence and adolescence. Aside from the development of psychopathology, the nature of parent-child interactions can shape children’s relationships with others in negative ways. For example, children who experience exchanges of reciprocal negative affect during interactions with their parents demonstrate low peer competency, are more verbally aggressive, and are less socially skilled overall (Carson & Parke, 1996). Thus, children with a 9-repeat allele of DAT1 may also be at elevated risk for poor peer relations in later development, another known risk factor for maladaptive outcomes. Determining whether such processes unfold during later childhood and adolescence is an important future step for this research.
Jaffee and Price recently (2007) noted that careful measurement of the environment may play a critical role in the successful identification of rGEs; as observational measures of parenting may show stronger predictive validity for child outcomes (Zaslow et al., 2006), their use is a major strength of our study. There were, however, a number of limitations. First is the issue of population stratification, which may increase the likelihood of false positive associations, although there is debate regarding the extent to which it represents a threat to the validity of association studies (Hutchison, Stallings, McGeary, & Bryan, 2004; Wacholder, Rothman, & Caporaso, 2002). It is possible that the VNTR locus in the DAT1 gene is in linkage disequilibrium with another sequence or structural variant that is responsible for the obtained associations with child behavior and parenting (Dick et al., 2011). Furthermore, while our sample size is large for one using observational measures, it is relatively small for a genetic association study. In addition, no data were available for parent genotype; not having these data made it impossible for us to examine the role of passive rGEs, which may play a role in driving the associations between child NE and parenting found in this study (Lee et al., 2010). Lastly, the data presented here are cross-sectional, although the interplay between child genetic risk, child behavior, parenting, and negative child outcomes requires longitudinal investigation. However, preliminary tests examining parent-child interaction over the course of our parenting task battery tentatively supported the notion of child-to-parent effects, at least in the case of parental hostility. We are currently collecting additional longitudinal data that will permit more conclusive tests of our larger model of DAT1 and emerging psychopathology over time.
Future directions for research on rGE and its translation into intervention
The identification of rGEs suggests the possibility of preventative efforts aimed at identifying those at greatest genetic risk for environment precipitants of psychopathology. However, it is clearly implausible that the effects of parent hostility and lower guidance/engagement (scaffolding) on negative child outcomes (Caron et al., 2006; Englund et al., 2004; Sheffield Morris et al., 2002) are driven exclusively by children’s NA related to having a 9-repeat allele of DAT1. An array of factors undoubtedly play a role in shaping positive and negative parenting practices, only some of which are related to child genetic factors. It is therefore unclear whether preventative efforts targeting the parenting of children with a 9-repeat allele would show additional value above and beyond broad interventions focused on improving parenting in the general population, unless it becomes clear that poor parenting has an especially potent impact on these children. This implies the presence of a GXE effect within the context of an rGE, which we did not find in our sample for the DAT1. Future work on measured rGE should systematically incorporate tests of GXE toward the long-term goal of determining the feasibility of targeted preventions based on child genetic factors. Additionally, the robustness of rGEs must be determined before it makes sense to apply such findings to preventative efforts. Our group recently provided evidence in support of findings initially published by Propper et al (2008) implicating children’s DRD2 alleles in eliciting supportive parenting; we hope that other research groups will attempt replication of the present findings, as well as other published rGEs in the literature.
A better understanding of the genetic bases of child behaviors that evoke environmental risk may be beneficial if interventions differ in effectiveness as a function of the causal processes involved in shaping the targeted behavior. More specifically, if some forms of child NA are driven primarily by genetic processes, rather than environmental triggers, standard parent-child interventions may be less effective, and interventions focused more exclusively on increasing children’s strategies for regulating NA might be indicated. It may also be the case that child behaviors that are strongly genetically influenced might require especially intensive psychosocial interventions, or behavioral interventions augmented with pharmacological treatment. While clearly speculative, this notion is consistent with findings that suggest that psychopathology severity, which is thought to be a marker of greater genetic loading for the disorder, often indicates the need for more intensive intervention strategies or combination therapies (e.g., DeRubeis et al., 2005; Elkin et al., 1989; Khan, Brodhead, Kolts, & Brown, 2005). However, we emphasize that whether research on rGE can be successfully used to develop personalized interventions is an empirical question that has yet to be tested, and is unlikely to be resolved in the immediate future.
It is also important to determine the magnitude of the associations of specific genes with environmental risk. Most genes have small effects on disorders and other complex behaviors (e.g., Clarke, Flint, Attwood, & Munafò, 2010; Kendler, 2005), and it stands to reason that associations with environmental variables will likely be weaker still, as these outcomes are even more distal from the biological actions of genes. In the present study, the child DAT1 9-repeat allele accounted for a small amount of variance in parenting (i.e., .5–1% of the variance). If it becomes clear that genes with evocative or active effects on the environment are associated with only marginally increased environmental risk, the implications for prevention are limited unless multifactorial models of rGEs can be developed. Such models could potentially account for a greater degree of variance in environmental risk exposure, if, for example, the cumulative effect of genes that influence a common biological pathway implicated in behavior can be modeled. However, the ability to develop such models has been limited by the complexity of epistatic models. Epistasis, the interaction between genes, is likely ubiquitous in shaping complex traits like NA (Moore, 2003), yet the capacity to develop adequate models is hampered by the difficulties inherent to studying how genetic processes interact in brain tissue. While an array of approaches have been used to model genetic risk (Chen et al., 2011; Hill, Goddard, & Visscher, 2008; Jones & Szatmari, 2002; Lettre, Lange, & Hirschhorn, 2007), it remains unclear which most accurately captures the nature of genetic interactions in shaping brain systems. Furthermore, developing polygenic models of the complex behaviors involved in rGE necessitates large sample sizes. Unfortunately, sample size tends to be inversely associated with the quality of the measures of phenotypes and environments, which is also critically important for progress to be made in this field (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Jaffee & Price, 2007; Uher & McGuffin, 2010). Optimal strategies for balancing these considerations continue to be a source of debate in the field (e.g., Duncan & Keller, 2011).
Implications for developmental psychopathology
Recent work (e.g., Martel et al., 2010) indicates that personality is a key mediator of the effects of genetic vulnerability on children’s psychopathology. While we did not examine psychopathological outcomes specifically, our findings implicate a specific variant in increasing children’s NA toward caregivers, a tendency that is almost certainly a marker of children’s trait negative emotionality, a known general risk for the development of an array of disorders (Clark, 2005; Eisenberg et al., 2009). However, it has also been proposed that child NA may serve a more complex role as a marker of children’s sensitivity to contextual factors, increasing the probability of both positive and negative outcomes in a context-dependent manner (Belsky & Pluess, 2009). While we found some evidence for evocative effects of child NA on negative parenting styles, associations were moderate, indicating that some children who express high levels of NA toward caregivers are not exposed to negative caregiving. Gaining a fuller understanding of parental characteristics that predict a decreased tendency to respond to negative child behavior with negative behavior of their own will help psychopathologists build more comprehensive transactional models of risk and resilience (Belsky & Barends, 2003). Incorporating parental genetic information may contribute toward this goal; such data were unfortunately not available to us at the time of this study.
Consistent with our findings, individual genes are held to have small effects on emotional behavior and personality. However, the context of evocative rGEs provides a means by which the influence of specific genes on negative outcomes may become amplified over time by virtue of eliciting environmental risk. Given that genetic and environmental risks are often correlated (Rutter, 2009), the identification of mediators of rGE clarifies the processes by which these risks become associated. Indeed, such risks may become increasingly interrelated as environmental risk plays a dynamic, regulatory role on gene expression during development via an array of epigenetic mechanisms (Cameron, Parent, Champagne, Fish, Ozaki-Kuroda & Meaney, 2005; Meaney & Szyf, 2005; Mill, 2011). Scientists’ understanding of complexity of these mechanisms is continually evolving, and work that delineates such processes in developmental psychopathology is in its infancy. While it is currently unclear how to best incorporate information on epigenetic mechanisms, especially in research on humans, developing appropriate measures of such processes will be an important step toward taking research on GXE and rGE in developmental psychopathology beyond merely demonstrating statistical associations and toward an approach that speaks to biological processes (Mill, 2011).
In summary, we found evidence for an rGE involving children’s DAT1 9-repeat allele and parental hostility and guidance/engagement. Our findings are compatible with the larger literature indicating that rGEs are mediated by personality and behavior (see Jaffee & Price, 2007, for a review), in that the association between the 9-repeat allele and parenting was partially mediated by child NA toward parents during parent-child interactions. While further longitudinal work is needed to support the full model we propose, these findings represent an additional contribution to the small but growing literature on the role of rGEs in developmental psychopathology.
Acknowledgments
This research was supported by a Young Investigator award from NARSAD and an Early Researcher Award from the Ontario Ministry of Research and Innovation to Elizabeth P. Hayden, a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources, and a National Institute of Mental Health grant R01 MH069942 to Daniel N. Klein.
Footnotes
Children for whom DNA and parenting data were available had significantly lower parental hostility than those without DNA, t(68.38) = 2.54, p < .05. No differences in other study variables were found (all ps >.18).
References
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament in 12-month-old infants. Journal of Child Psychology and Psychiatry. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. NeuroImage. 2009;47:2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity in predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Belsky J, Barends N. Personality and parenting. In: Bornstein MH, editor. Handbook of Parenting Volume 3: Being and Becoming a Parent. 2ND. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2002. pp. 415–438. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behavior –review of data from preclinical research. Acta Psychiatrica Scandinavia. 2005;111(Supple. 427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Burke JD, Pardini DA, Loeber R. Reciprocal relationships between parenting behavior and disruptive psychopathology from childhood through adolescence. Journal of Abnormal Child Psychology. 2008;36:679–692. doi: 10.1007/s10802-008-9219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Parent C, Champagne FA, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carlborg O, Haley CS. Epistasis: Too often neglected in complex trait studies? Nature Reviews Genetics. 2004;5:618–625. doi: 10.1038/nrg1407. [DOI] [PubMed] [Google Scholar]
- Caron A, Weiss B, Harris V, Catron T. Parenting behavior dimensions and child psychopathology: specificity, task dependency, and interactive relations. Journal of Clinical Child and Adolescent Psychology. 2006;35:34–45. doi: 10.1207/s15374424jccp3501_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Parke RD. Reciprocal negative affect in parent-child interactions and children’s peer competency. Child Development. 1996;67:2217–2226. Retrieved from http://www.wiley.com/bw/journal.asp?ref=0009-3920&site=1. [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA. Temperament as a unifying basis for personality and psychopathology. Journal of Abnormal Psychology. 2005;114:505–521. doi: 10.1037/0021-843X.114.4.505. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. Retrieved from: http://www.apa.org/pubs/journals/abn/index.aspx. [PubMed] [Google Scholar]
- Chen C, Chen C, Moyzis R, Stern H, He Q, Li H, et al. Contributions of dopamine-related genes and environmental factors to highly sensitive personality: A multi-step neuronal system-level approach. PLoS ONE. 2011;6:e21636. doi: 10.1371/journal.pone.0021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Flint J, Attwood AS, Munafò MR. Association of the 5-HTTLPR genotype and unipolar depression: a meta-analysis. Psychological Medicine. 2010;40:1767–1778. doi: 10.1017/S0033291710000516. [DOI] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. American Journal of Human Genetics. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Arbeau KA, Armer M. Don’t fret, be supportive! Maternal characteristics linking child shyness to psychosocial and school adjustment in kindergarten. Journal of Abnormal Child Psychology. 2008;36:359–371. doi: 10.1007/s10802-007-9183-7. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Parenting and child behavioral adjustment in early childhood: a quantitative genetic approach to studying family processes. Child Development. 2000;71:468–484. doi: 10.1111/1467-8624.00158. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–350. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. Retrieved from: http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Shuckit MA, Bierut L, Hinrichs A, Fox L, et al. Marital status, alcohol dependence, and GABRA2: evidence for gene-environment correlation and interaction. Journal of Studies on Alcohol. 2006;67:185–194. doi: 10.15288/jsa.2006.67.185. Retrieved from: http://www.jsad.com/ [DOI] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Riley B. Incorporating genetics into your studies: A guide for social scientists. Frontiers in Child and Neurodevelopmental Psychiatry. 2011;17:1–11. doi: 10.3389/fpsyt.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN, et al. Dopamine transporter gene conveys familial risk of attention-deficit/hyperactivity disorder through striatal activation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:61–67. doi: 10.1097/chi.0b013e31815a5f17. [DOI] [PubMed] [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K, et al. Teaching tasks administration and scoring manual. Minneapolis, MN: University of Minnesota Institute of Child Development; 1995. [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, et al. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Napolitano M, Lau JYF, Gregory AM. Does childhood anxiety evoke maternal control? A genetically informed study. Journal of Child Psychology and Psychiatry. 2010;51:772–779. doi: 10.1111/j.1469-7610.2010.02227.x. [DOI] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Impber SD, Sotsky SM, Collins JF, et al. NIMH Treatment of Depression Collaborative Research Program: General effectiveness of treatments. Archives of General Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. Retrieved from: http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Englund MM, Luckner AE, Whaley GJL, Egeland B. Children’s achievement in early elementary school: longitudinal effects of parental involvement, expectations, and quality of assistance. Journal of Educational Psychology. 2004;96:723–730. [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, Troughton E, et al. The developmental interface between nature and nurture: a mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1996;32:574–589. [Google Scholar]
- Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: expanded content, stronger genetic evidence, new evidence for the importance of the environment. Developmental Psychology. 1997;33:891–905. doi: 10.1037//0012-1649.33.6.891. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. On making behavioral genetics truly developmental. Human Development. 2003;46:337–355. [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Developmental Neuroscience. New York: Wiley Press; 2006. pp. 533–577. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW, et al. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene- environment interaction. Psychiatric Genetics. 2010;20:304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JD, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hill WG, Goddard ME, Visscher PM. Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 2008;4:e1000008. doi: 10.1371/journal.pgen.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Unpublished manuscript. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RMP, Sasvari-Szekely M, Johnson MH. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes, Brain and Behavior. 2011;10:513–522. doi: 10.1111/j.1601-183X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implication for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TD, Edwards L. Genes, interactions, and the development of behavior. Psychological Review. 2002;109:26–34. doi: 10.1037/0033-295x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Jones MB, Szatmari P. A risk-factor model of epistatic interaction, focusing on autism. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:558–565. doi: 10.1002/ajmg.10513. [DOI] [PubMed] [Google Scholar]
- Joyce PR, McHugh PC, Light KJ, Rowe S, Miller AL, Kennedy MA. Relationships between angry-impulsive personality traits and genetic polymorphisms of the dopamine transporter. Biological Psychiatry. 2009;66:717–721. doi: 10.1016/j.biopsych.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Kendler KS. “A gene for…”: the nature of gene action in psychiatric disorders. American Journal of Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Kolts RL, Brown WA. Severity of depressive symptoms and response to antidepressants and placebo in antidepressant trials. Journal of Psychiatric Research. 2005;39:145–150. doi: 10.1016/j.jpsychires.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Chronis-Tuscano A, Waldman ID, Lee SS, Pelham WE, et al. Interactions between early parenting and a polymorphism of the child’s dopamine transporter gene in predicting future child conduct disorder symptoms. Journal of Abnormal Psychology. 2011;120:33–45. doi: 10.1037/a0021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Calder AJ, McGowan SW, Grasby PM. Selective disruption of the recognition of facial expressions of anger. NeuroReport. 2002;13:881–884. doi: 10.1097/00001756-200205070-00029. Retrieved from http://journals.lww.com/neuroreport/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Lee SS, Chronis-Tuscano A, Keenan K, Pelham WE, Loney J, Van Hulle CA, et al. Association of maternal dopamine transporter genotype with negative parenting: evidence for gene x environment interaction with child disruptive behavior. Molecular Psychiatry. 2010;15:548–558. doi: 10.1038/mp.2008.102. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Waldman I, Van Hulle CA, Rathouz P, Pelham WE, et al. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. American Journal of Medical Genetics Part B(Neuropsychiatric Genetics) 2007;144B:310–317. doi: 10.1002/ajmg.b.30447. [DOI] [PubMed] [Google Scholar]
- Lengua LJ, Kovacs EA. Bidirectional associations between temperament and parenting and the prediction of adjustment problems in middle childhood. Applied Developmental Psychology. 2005;26:21–38. [Google Scholar]
- Lettre G, Lange C, Hirschhorn JN. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genetic Epidemiology. 2007;31:358–362. doi: 10.1002/gepi.20217. [DOI] [PubMed] [Google Scholar]
- Lucht M, Barnow S, Schroeder W, Joergen Grabe H, Finckh U, John U, et al. Negative perceived paternal parenting is associated with dopamine D2 receptor exon 8 and GABA(A) alpha 6 receptor variants: an explorative study. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:167–172. doi: 10.1002/ajmg.b.30255. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ. Current directions in mediation analysis. Current Directions in Psychological Science. 2009;18:16–20. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nikolas M, Jernigan K, Friderici K, Nigg JT. Personality mediation of genetic effects on attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2010;38:633–643. doi: 10.1007/s10802-010-9392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Weisz JR, Wood JJ. Examining the association between parenting and childhood depression: a meta-analysis. Clinical Psychology Review. 2007;27:986–1003. doi: 10.1016/j.cpr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mill J. Epigenetic effects on gene function and their role in mediating gene-environment interactions. In: Kendler KS, Jaffee SR, Romer D, editors. The Dynamic Genome and Mental Health. New York, NY: Oxford University Press; 2011. pp. 145–171. [Google Scholar]
- Miller GM, Madras BK. Polymorphisms in the 3’-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Molecular Psychiatry. 2002;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy J-L, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: The family system as the unit of analyses. Development and Psychopathology. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, et al. COMT genetic variation affects fear processing: psychophysiological evidence. Behavioral Neuroscience. 2008;122:901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Moore JH. The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Human Heredity. 2003;56:73–82. doi: 10.1159/000073735. [DOI] [PubMed] [Google Scholar]
- Moore PS, Whaley SE, Sigman M. Interactions between mothers and children: Impacts of maternal and child anxiety. Journal of Abnormal Psychology. 2004;113:471–476. doi: 10.1037/0021-843X.113.3.471. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit GS, Bates JE, Dodge KA. Supportive parenting, ecological context, and children’s adjustment: a seven-year longitudinal study. Child Development. 1997;68:908–923. doi: 10.1111/j.1467-8624.1997.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, and Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rothbaum F, Weisz JR. Parenting caregiving and child externalizing in nonclinical samples: a meta-analysis. Psychological Bulletin. 1994;116:55–74. doi: 10.1037/0033-2909.116.1.55. Retrieved from http://www.apa.org/pubs/journals/bul/index.aspx. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Stever C, Card JMC, Cleveland HH, Sanders ML, Abramowitz A, et al. The relation of the dopamine transporter gene (DAT1) to symptoms of internalizing disorders in children. Behavior Genetics. 1998;28:215–225. doi: 10.1023/a:1021427314941. [DOI] [PubMed] [Google Scholar]
- Rutter M. Nature, nurture, and psychopathology: a new look at an old topic. Development and Psychopathology. 1991;3:125–136. [Google Scholar]
- Rutter M. Commentary: nature-nurture interplay in emotional disorders. Journal of Child Psychology and Psychiatry. 2003;44:934–944. doi: 10.1111/1469-7610.00178. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene-environment interdependence. Developmental Science. 2007;10:12–18. doi: 10.1111/j.1467-7687.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Understanding and testing risk mechanisms for mental disorders. Journal of Child Psychology and Psychiatry. 2009;50:44–52. doi: 10.1111/j.1469-7610.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, et al. Association between the dopamine transporter gene and posttraumatic stress disorder. Molecular Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, et al. Emotion-based decision making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Sheffield Morris A, Silk JS, Steinberg L, Sessa FM, Avenevoli S, Essex MJ. Temperamental vulnerability and negative parenting as interacting predictors of child adjustment. Journal of Marriage and Family. 2002;64:461–471. [Google Scholar]
- Smith HJ, Sheikh HI, Dyson MW, Olino TM, Laptook RS, Durbin CE, Hayden EP, Singh SM, Klein DN. Parenting and child DRD4 genotype interact to predict children’s early emerging effortful control. Child Development. doi: 10.1111/j.1467-8624.2012.01818.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends in Neuroscience. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stormshak EA, Bierman KL, McMahon RJ, Lengua LJ. Parenting practices and chid disruptive behavior problems in early elementary school. Journal of Clinical Child Psychology. 2000;29:17–29. doi: 10.1207/S15374424jccp2901_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S. Genetic polymorphisms of serotonin and dopamine in mental disorders. Journal of Medical Investigation. 2003;50:25–31. Retrieved from http://medical.med.tokushima-u.ac.jp/jmi/index.html. [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- van de Giessen EM, de Win MML, Tanck MWT, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. Journal of Nuclear Medicine. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 Gene. Journal of Nuclear Medicine. 2005;46:745–751. Retrieved from http://jnm.snmjournals.org. [PubMed] [Google Scholar]
- Wacholder S, Rothman N, Caporaso N. Counterpoint: Bias from population stratification is not a major threat to the validity of conclusions from epidemiological studies of common polymorphisms and cancer. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:513–520. Retrieved from http://cebp.aacrjournals.org/ [PubMed] [Google Scholar]
- Wood D, Bruner JS, Ross G. The role of tutoring in problem solving. Journal of Child Psychology and Psychiatry. 1976;17:89–100. doi: 10.1111/j.1469-7610.1976.tb00381.x. Retrieved from http://www.wiley.com/bw/journal.asp?ref=0021-9630. [DOI] [PubMed] [Google Scholar]
- Young SE, Smolen A, Corley RP, Krauter KS, DeFries JC, Crowley TJ, et al. Dopamine transporter polymorphism associated with externalizing behavior problems in children. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:144–149. doi: 10.1002/ajmg.10155. [DOI] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa JR, Egeland B, et al. Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Developmental Psychology. 2006;42:27–37. doi: 10.1037/0012-1649.42.1.27. [DOI] [PubMed] [Google Scholar]