Abstract
Pertussis is a highly infectious respiratory disease that has been on the rise in many countries worldwide over the past several years. The drivers of this increase in pertussis incidence remain hotly debated, with a central and long-standing hypothesis that questions the ability of vaccines to eliminate pertussis transmission rather than simply modulate the severity of disease. In this paper, we present age-structured case notification data from all provinces of Thailand between 1981 and 2014, a period during which vaccine uptake rose substantially, permitting an evaluation of the transmission impacts of vaccination. Our analyses demonstrate decreases in incidence across all ages with increased vaccine uptake — an observation that is at odds with pertussis case notification data in a number of other countries. To explore whether these observations are consistent with a rise in herd immunity and a reduction in bacterial transmission, we analyze an age-structured model that incorporates contrasting hypotheses concerning the immunological and transmission consequences of vaccines. Our results lead us to conclude that the most parsimonious explanation for the combined reduction in incidence and the shift to older age groups in the Thailand data is vaccine-induced herd immunity.
Introduction
Pertussis, or whooping cough, is a highly infectious respiratory disease that remains a major public health concern [43, 13]. Despite the initial success of paediatric vaccination programs, pertussis is currently responsible for an estimated 300,000 deaths per year worldwide [12]. In addition, it has undergone a resurgence in many countries – including the United States [38, 24] – despite maintaining high levels of vaccine uptake [43]. While many plausible mechanisms driving these dynamics has been proposed [20, 13], the protective immunity elicited by both whole cell (wP) and acellular (aP) vaccines has long been questioned [33, 9, 5]. The absence of a reliable serological marker for protection has meant that much of the discussion regarding the consequences of vaccination for immunity have focused on epidemiological patterns [23].
The ability of whole cell vaccines to prevent pertussis transmission has been discussed for more than fifty year [32] and was famously called into question by Fine & Clarkson in 1982 [16], who argued based on patterns of periodicity in pertussis incidence that whole cell vaccines likely prevented disease rather than transmission. If true, this would lead to significant silent circulation of pertussis [1, 22], posing a major threat to unvaccinated or partially immunized infants, who are at highest risk of pertussis-related complications and death. More recently, the debate regarding the mechanisms behind pertussis resurgence and the potential role played by has centered on acellular vaccines [4, 5, 14]. Two modeling studies have concluded that pertussis resurgence may be the natural consequence of widespread switch to the aP vaccine [17] and its inability to generate protective immunity [15, 2]. This conclusion is supported by animal transmission models, in baboons [42] and mice [40], where the experimental challenge of aP-vaccinated animals indicates the potential for a transmissible infection.
To better understand the extent of the cross-over between individual-level animal challenge experiments to population-level pertussis incidence in humans and the consistency between epidemiological evidence in different countries, we examined incidence reports from Thailand. Specifically, we scrutinized age-stratified incidence data from different provinces in Thailand from 1981 to 2000. During this period, vaccine uptake systematically increased from ∼26% in the early 1980s to greater than 95% by mid-1990s. We explored the epidemiological consequences of this vaccine ramp-up to assess the protective effects of wP vaccination. We paid particular attention to changes in incidence among “high risk” groups – such as unvaccinated infants – as uptake increased. Epidemiological theory predicts that vaccination programs that successfully protect vaccinees from infection should induce herd immunity, thereby minimizing transmission to unprotected high risk groups [18]. Tracking incidence in these age-groups is therefore an essential component to quantifying the success of vaccination in reducing circulation [21, 13]. For this reason, we also examined pertussis incidence reports from 2003-2014 in infants younger one month and those between 1 and 12 months.
The analyses of age-incidence patterns were allied to two age-structured transmission models, which were used to distinguish among competing hypotheses about the role of vaccine-protection as a driver of observed age-structured incidence (ex. [34, 23, 37]). We challenged these models to capture the qualitative epidemiological transition observed in Thailand when contrasting assumptions were made regarding the immunological impacts of infant immunization. In previous analyses, we reported that there is an increase in herd immunity with vaccine uptake in Thailand and we supported these claims through the use of statistical inference to select a best-fitting model of pertussis dynamics [8]. However, that analysis primarily considered the aggregate data independent of age. Here, we provide additional corroboration via a study of age-structured dynamics. Consistent with our prior analyses, we find empirical support for increased herd immunity with vaccine uptake in Thailand, a finding that is re-inforced by our transmission modeling.
Pertussis incidence data, 1981-2000
Thailand adopted the National Expanded Programme on Immunization (EPI) in 1977 for infant vaccination. Initially, two doses of the diptheria-tetanus-pertussis (DTP) whole cell vaccine was administered until a third dose was added to the schedule in 1982 [7]. Beginning in 1992, the schedule included 5 doses of DTP at 2, 4, 6, and 18 months with the final booster dose at 4-6 years. The whole cell vaccine was used exclusively until 1998. Since then, the DTP-HiB has been favoured in government clinics and the acellular vaccine (DTaP) in private clinics. We obtained the corresponding annual national vaccine uptake estimates for 1981-1996 and 1999 from the Vaccine Coverage Survey of Thailand. Missing years (1997-8, 2000) were assumed to have attained the same uptake as the most recently reported year.
Annual case notification data from each of Thailand's 72 provinces between 1981 and 2000 were obtained from the Ministry of Public Health [10]. From 1981-2000, the case notification data were also available annually for the following age groups: under 1 year, 1–4, 5–9, 10–14, 15–24, 25–34, 35–44, 45–54, 55–64, and older than 65 years of age. In 1985 these data were also available for one year age groups up to age seven. In our analysis we assumed the age of cases were uniformly distributed between all ages within each age group. We obtained age-stratified population data for 1980, 1990, and 2000 from the National Statistical Office of Thailand censuses and log-linear fits between census years were used to estimate annual population sizes from 1981-2000 [29, 30, 31].
Age-structured incidence dynamics
In this section, we present the age-structured incidence data from Thailand during the time period 1981–2000. To paint a general picture of pertussis in Thailand, in Figure 1A we present aggregated monthly incidence in addition to the annual vaccine uptake. Three clear epidemics are observed in 1981, 1982, and 1983 followed by a rapid decline from 1984–1990 and stable low incidence throughout the 1990s. These dynamics coincide with the rapid rise in vaccine uptake, which consistently exceeds 95% beginning in 1996.
Figure 1.
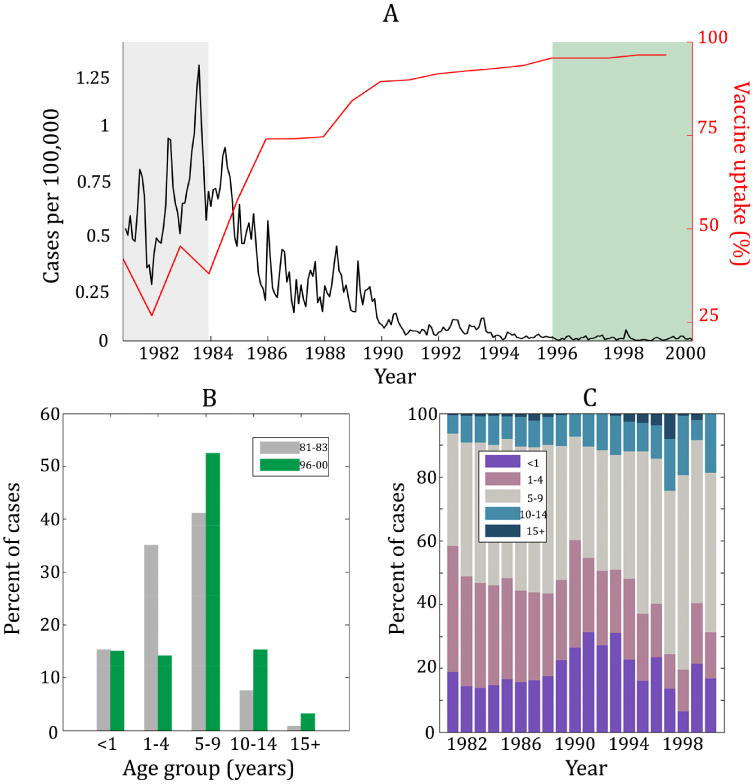
(A) Monthly incidence in Thailand (black) and the corresponding vaccine uptake (red) from 1981 through 2000. These colors match the data as shown in (B) of this figure. (B) The percent of cases in five different age groups during the time period 1981–1983 (gray bars) compared to the time period 1996–2000 (green bars). Note that the shading in (A) of this figure matches in bars in (B). Notice that between time periods, the distribution of cases shifts so that there is a greater percentage of cases in adolescents and adults. (C) Percent of annual cases per year in four different age groups. The age distribution stays relatively constant until 1988 and then the percent of cases in individuals ages 5+ tends to increase for the remainder of the time series.
Figure 1A also includes two shaded regions: 1981–1983 (gray) and 1996–2000 (green). These two time periods display dramatically different incidence patterns corresponding to changes in vaccine uptake. Namely, incidence was highest from 1981–1993 when vaccine uptake was the lowest, and incidence remained very low from 1996–2000 when vaccine uptake remained above 95%. A comparison of the age-specific incidence patterns during these two “eras” is presented in Figure 1B, depicting the percentage of reported cases attributed to five different age groups (under 1 year, 1–4, 5–9, 10–15, and 15+ years). The figure indicates a shift in incidence to older age groups in the later era.
To observe the age-stratified dynamics at a finer temporal resolution, in Figure 1C we display the percentage of annual cases in four age classes. During the early through mid-1980s, the age distribution of cases remains relatively stable with between 13% and 23% of all cases attributed to infants. The percent of cases in infants then rises briefly and drops again in the early 1990s. The decline in the percent of cases in infants coincides with a dramatic increase in vaccine uptake. While this is indicative of reduced transmission to infants – who are more susceptible to disease because they are less likely to be fully immunized against pertussis – it should be noted that the incidence is very low throughout the 1990s. As a consequence, variability in the age-distribution is to be expected and such variation is indeed observed during the years post-1995.
To determine whether these observations are consistent at finer spatial resolutions, Figure 2B-G replicates Figure 1B for each of the six distinct geographic regions of Thailand (as developed by the National Geographical Committee in 1978). In Figure 2B-G, the percent of cases in each age group from 1981 through 1983 are represented by gray bars and the percent of cases in each age group from 1996–2000 are in color. The bars are colored by region, and Figure 2A provides a map of Thailand such that the provinces in each region are colored accordingly. These data are consistent with those for Thailand as a whole (Figure 1B), with a higher percentage of cases attributed to older age classes during 1996–2000. While the Western (Figure 2C, pink) region appears to contradict this observation, it only accounts for 5.3% of the total cases in Thailand and therefore has minimal impact on the overall age-structured dynamics in the country as a whole.
Figure 2.
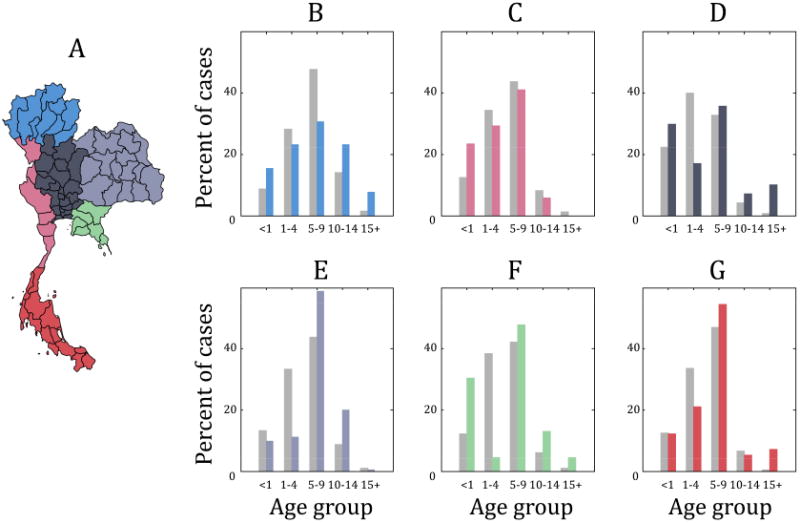
The percent of cases in age group from 1981–1983 (light gray bars) as compared to the percent of cases in each age group from 1996–2000 (colored bars). The color representing 1996–2000 in (B)-(G) correspond to one of the six distinct geographic regions of Thailand: (B) Northern, (C) Western, (D) Central, (E) Northeastern, (F) Eastern, and (G) Southern region. (A) provides a map of Thailand that is colored according to region. With the exception of the Western region (which only accounts for 5.3% of the total cases in Thailand), the percentage of cases in older age groups increases in all regions in the later time period.
Previous work has shown that an increase in the mean age of infection may correspond to decreasing transmission to infants (e.g. [39]). We therefore estimated the mean age of infection for the early (1981-1983) and late (1996-2000) vaccine eras, assuming that cases are uniformly distributed within age classes (see Supplementary Information for details). To test whether the mean age at infection significantly increased from the early to the late era, we bootstrapped the ages of the case reports. The resulting distribution of 10,000 bootstrap samples for each region revealed a significant increase of 4.31, 2.07, 1.93, and 2.87 years, respectively, for Regions 3–6. With the exception of Region 5, the respective 95% CI for each vaccine era were completely non-overlapping. In contrast, there was no increase in the mean ages of infection in Regions 1 and 2 (details and figures provided in Supplementary Information). However, we note that Regions 1 and 2 account for only 15% of the total cases reported in Thailand. For the purposes of comparison, we performed the same analysis on the aggregated data at the national level. We estimated that the nationwide mean age of infection increased from 5.44 years in 1981–1983 to 7.77 years in 1996–2000. The resulting distribution of 10,000 bootstrap samples displays a shift in the mean age of infection between the vaccine eras (Figure 4B). In fact, the 95% CIs are completely non-overlapping ((5.35, 5.52) yrs for the first vaccine era and (7.12, 8.65) for the later era). Therefore, there is a clear increase in the mean age of infection that is driven by the incidence dynamics in Regions 1–4.
Figure 4.
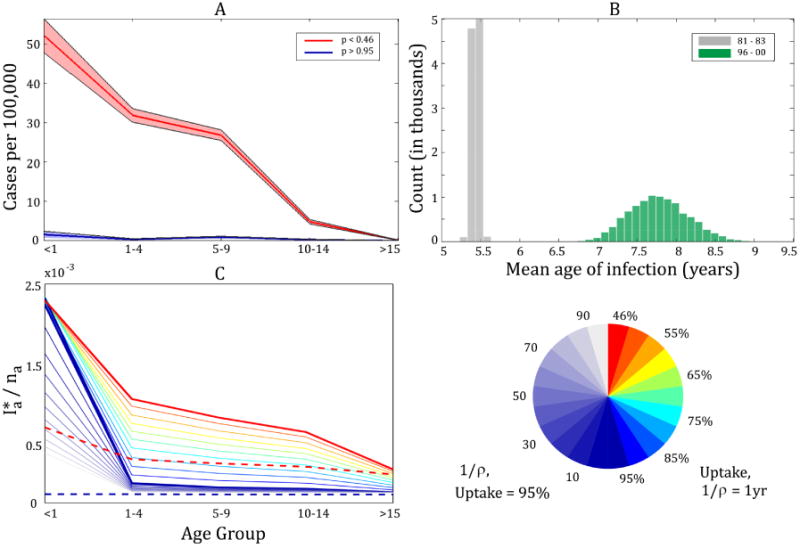
(A) Mean age structure of aggregated annual case notifications in Thailand with vaccine uptake of <46% (years 1981-1983, red) and high vaccine uptake of >95% (years 1996-2000, blue). Under the assumption that case reports are drawn from a binomial distribution, shading indicates 95% CI using the Fisher information. (B) displays the distribution of 10,000 bootstrap samples of the mean age of infection (p < 46% in gray, p > 95% in green). The sample means are 5.44 and 7.77 yrs, respectively with 95% CI of (5.35, 5.52) and (7.12, 8.65). Results from the SIVIR model assuming homogeneous mixing are displayed in (C) as the equilibrium prevalence by age group, , where ni is the fraction of the total population in age group i. Prevalence is corrected to account for the difference between the modeled infectious period (21 d) versus actual case notifications which are reported monthly. This is shown for various levels of vaccine uptake while holding the duration of immunity 1/ρ at one year (right side of colormap circle) and various values 1/ρ while holding vaccine uptake at 95% (left side of colormap circle). The dashed lines display the results from the age-structured SIR model for low (red) and high (blue) vaccine uptake.
Finally, an alternative way to visualize the impact of vaccination on age-structured incidence is presented in Figure 4A. Here, the age-specific incidence is shown when vaccine uptake is relatively low (< 46%, in red) as well as when it is high (> 95%, in blue). This figure displays the average of the aggregate incidence across Thailand for each vaccine era. Under the assumption that case reports were drawn from a binomial distribution, 95% CIs are found using the Fisher information. Here, the increase in vaccine uptake clearly corresponds to a decline incidence across all ages, especially in infants. In particular, there were an average of 571 cases in infants per year during 1981–1983 compared to an average of 13.2 cases per year during 1996–2000. While these averages are computed at the national level, we demonstrate that the decrease in cases in infants is consistent across the provinces of Thailand (see Supplementary Information). Figure 4A is consistent with declining pertussis transmission following vaccination. We assume that reporting is consistent across all ages, so while biases in reporting may contribute to the dynamics, we argue that it is very unlikely that reporting alone accounts for the observed dramatic shift in age structure.
Modeling age-structured pertussis dynamics
While the age stratified incidence data are strongly suggestive of decreased transmission with increased vaccination, they do not eliminate the possibility that vaccines prevent symptomatic disease but not transmission [16, 1]. Ideally, this issue would be resolved by statistical inference methods applied to a mechanistic transmission model (eg, [8, 17]). However, the absence of age-stratified data more highly resolved than annual incidence precludes such an analysis. We therefore formulated a simple age-structured mathematical model [37] for use as a heuristic device: to explore the dynamical consequences of alternative assumptions regarding vaccine impact and their parsimony with the Thai data. For the purposes of direct comparison with the Thai data (Figure 4A), we explored five age classes: <1,1-4, 5-9,10-14, and >15 years old.
Our compartmental transmission models were constructed using the SIR (Susceptible - Infected - Recovered) paradigm [3, 21]. Consistent with estimates obtained via likelihood-based statistical inference of the Thai data [8], we assumed naturally acquired immunity to be lifelong. We assumed newborns (<1 yr) to be susceptible to pertussis. Upon entering the next age class, with probability p, an infant may be immunized. In the first model, we assumed that both immunization and naturally-acquired infections provide lifelong protection against pertussis infection, yielding a simple SIR model. In the second model, we assumed that only naturally-acquired infections results in lifelong immunity. To examine the consequences of vaccine-induced protection against infection versus disease, we assumed immunization to provide a temporary period of protection against infection after which indviduals experience reduced susceptibility to disease, but not infection. Specifically, this model included three additional classes: a vaccinated and fully protected class V(with mean duration of protection 1/ρ years), a vaccinated but susceptible to infection class SV, and a vaccinated infected class (IV) in which individuals were assumed to be asymptomatic but otherwise function identically to infectious individuals with no history of vaccination. In this model, only symptomatic infections (I individuals) were reported which allowed us to directly examine whether subclinical infections can explain the shift in the age structure of case notifications. We refer to this model as SIVIR.
For all age classes and both models, the force of infection – the per capita hazard of transmission experienced by susceptibles – due to individuals in the infected (I) class is
| (1) |
where βij represents the transmission rate from individuals in the jth age class to members of the ith age class, and Ii is the proportion of the population infected in each age group i, as specified above. The SIVIR additionally had an associated force of infection arising from individuals in the infected class that can transmit without being observed. We denoted this by :
| (2) |
where is the proportion of individuals who are infected but were previously vaccinated in each age group j.
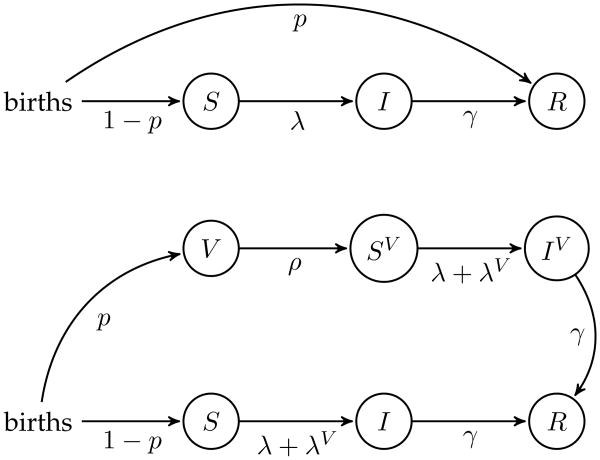
To analyze the SIR and SIVIR models, we assumed two contrasting patterns of age-specific contacts: (i) homogeneous mixing and (ii) age-assortative contact structure, as reported from the POLYMOD study of several European countries [28]. The results for the two different assumptions on contact structure were similar, so the results using the POLYMOD contact matrix are presented in the Supplementary Information. The basic reproduction ratio, R0, set to equal 16 in both models. The models were simulated for levels of vaccine uptake ranging from 46% to 95%, as observed in Thailand. To quantify the age-stratification of transmission, we obtained the equilibrium prevalence in each age class (i.e. , where ni is the proportion of the population in age class i). A schematic of each model is provided in Figure 3 and the models are fully described in the Supplementary Information.
Figure 3.
Schematic representation of the age-structured models: the SIR model is the schematic on the upper panel and the SIVIR model is on the lower panel. Full model descriptions are provided in the Supplementary Information.
The age distribution of incidence predicted from the SIR model and the SIVIR model are displayed in Figure 4C. The observed changes in the Thailand data were well-captured by the SIR model (dashed lines) with a incidence dramatically reduced across all age classes when vaccine uptake is increased to 95%. In contrast, under the assumption that vaccine prevents disease but not transmission (the SIVIR model), incidence is lowered in older age groups but remains high in unvaccinated infants.
To determine the sensitivity of the dynamics of the SIVIR model to vaccine uptake, we ran the model for several vaccine uptake levels. As shown in Figure 4C, incidence in all age classes decreased as vaccine uptake increased, except incidence in the infant class which remains unchanged. To determine whether a longer duration of temporary immunity following vaccination can qualitatively produce changes in age incidence consistent with the observed case notification data, we also varied the number of years spent in the protected vaccinated class (1/ρ) while holding vaccine uptake at 95% (indicated by blue on the colormap). While a long duration of immunity (up to 1/ρ = 100 years) yields lower levels of incidence among infants, these reductions do not achieve comparable levels of the decline observed in data. This qualitative analysis is consistent with the interpretation that vaccination effectively reduces pertussis transmission [8], with the SIR model providing the most parsimonious explanation of the data. The details of age-specific patterns of contacts did not affect our qualitative conclusions.
Contemporary pertussis incidence: 2003-2014
Our analyses of incidence data from 1981-2000 support the conclusion that vaccination in Thailand has reduced the transmission of pertussis. However, in many countries, a honeymoon effect has been observed whereby the initial impact of high vaccine coverage has been to markedly reduce incidence only for a rebound at a later date [27, 38, 35, 26, 25]. To examine the contemporary transmission of pertussis in Thailand, we depict, in Figure 5, age-stratified pertussis incidence from 2003-2014. These data indicate that pertussis incidence remains very low, at levels comparable to those displayed in Figure 4A corresponding to vaccine uptake >95%. The contemporary data are especially informative regarding transmission dynamics since they include incidence among infants <28 days old, who are too young to be immunized. Hence, any resurgence in pertussis transmission would be expected to result in an increasing trend in incidence among this age group. Indeed, incidence remains very low and in half of the years from 2002–2014 there are no reported cases in this age class. We submit that the absence of any rising trend, together with the rarity of pertussis in young infants, may be taken as additional support for vaccine-induced herd immunity in Thailand.
Figure 5.
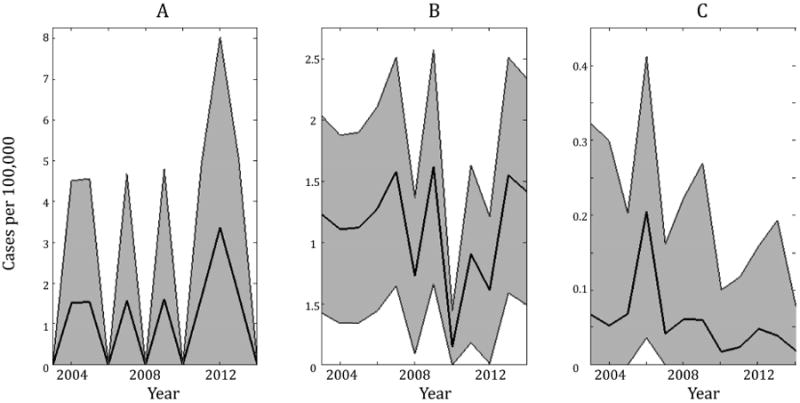
Age-stratified annual incidence from 2003-2014. Under the assumption that case reports are drawn from a binomial distribution, shading indicates 95% CI using the Fisher information. (A) displays incidence in infants <28d, (B) is 28d - 1 yr, and (C) is the mean incidence across all older age groups. In (C), 95% CIs are displayed as the bounds that fall farthest from the mean across all age groups in each year.
Discussion
Pertussis incidence has continued to rise over the last couple of decades in many developed countries in spite of longstanding vaccination programs, some of which began as early as the 1940s [6, 19]. Understanding the drivers of this resurgence is becoming increasingly critical as case reports continue to rise. For example, in 2012 the United States had 48,277 cases reported to the World Health Organization — the most cases in a given year since 1959 and substantially higher than the number of cases reported in the epidemic of 2010 (27,550) [11, 44]. A central question that has persisted for more than three decades concerns the transmission consequences of vaccines, and specifically whether they merely protect against disease and not infection. These differences have significant impacts on the design of successful booster vaccination programmes [36]. In this paper, we returned to this issue by taking advantage of long-term age-stratified pertussis incidence records in Thailand allied to contrasting hypotheses regarding vaccine impacts formulated within mathematical transmission models.
Much of the recent pertussis vaccines literature has focused on the role of the acellular vaccines (DTaP) [4, 5, 14, 42, 40]. In contrast, in Thailand the whole cell vaccine (DTwP) was exclusively used until 1998 after which a combination of the acellular and whole cell vaccines have been in use. Our examination of age-structured case notification data from different regions of Thailand over the period 1981-2000 indicated a substantial decrease in incidence as vaccine uptake increased. Although much of these data correspond to the period in which DTwP was the only vaccine used (1981-1998), incidence remained very low in Thailand from 2003-2014 – with only 14 cases reported in 2014.
While the data appear to paint a clear picture of a rise in herd immunity, it is plausible that alternative mechanisms may yield a similar age-specific profile of cases. If vaccination simply prevents clinical symptoms rather than transmission, the decrease in incidence could be the natural result of a greater number of undiagnosed asymptomatic cases. To parse these alternatives, we analyzed two age-structured transmission models. The first model assumed that vaccination protects from infection (the age-structured SIR model) whereas the second, and more complex, model assumed that following temporary vaccine-derived immunity, individuals can acquire an asymptomatic infection that goes unreported (the SIVIR model). The resulting age distribution of incidence in each of these models is found for both high and low levels of vaccine uptake and compared to the corresponding age-structure of the data. While the SIVIR model accurately captured lowered incidence in older age groups following high levels of vaccine uptake, it failed to capture the substantial decline in incidence in infants as observed in the data. In contrast, the simple age-structured SIR model accurately predicted substantially lowered incidence across all age groups, including infants. To assess the robustness of our conclusions, we determined the sensitivity to the SIVIR model to various levels of vaccine uptake ranging from 46% to 95%. Further, we allowed the duration of temporary immunity provided by vaccination to range from one to 100 years. While increasing the duration of temporary immunity caused a decrease in incidence in infants with high levels of vaccine uptake, all parameter values failed to reproduce the dramatic decline in incidence in infants as observed in the data. Further, this analysis was performed for both homogeneous mixing of contacts and non-homogeneous mixing; both analyses yielded qualitatively similar results.
Through combining age-structured incidence data and mathematical models, our findings point to a rise in herd immunity as a result of a decline in transmission following vaccination in Thailand. We submit that these findings provide a useful counterpoint to the increasing reliance on serology. For instance, Wanlapakorn et al. [41] have recently presented age-stratified serological data from Thailand indicating decling antibody levels with age. Despite the uncertainty associated with the interpretation of pertussis toxin antibodies as a correlate of protection, the authors recommend “a booster dose during adolescence should be considered in order to reduce the incidence of pertussis disease”. Our examination of pertussis incidence reports lead us to question this inference. Substantial pertussis circulation among adults and adolescents would inevitably lead to increased incidence among infants, especially those too young to be immunized [18, 13]. In contrast, the very low incidence in infants in high-vaccine coverage periods is a strong indicator of herd immunity.
Supplementary Material
Highlights.
Analyzed age-structured case notification data of pertussis in Thailand
Observed substantial decreases in incidence in all age classes following increased vaccine uptake.
Used mathematical models to demonstrate the changes in age-structure are likely a result of reduced transmission following vaccination.
Acknowledgments
We thank an anonymous reviewer for their helpful feedback on earlier drafts of this manuscript. This work is supported by the Research and Policy in Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security, the Fogarty International Center, National Institutes of Health and by a research grant from the National Institutes of Health (1R01AI101155) and by MIDAS, National Institute of General Medical Sciences U54-GM111274.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Águas R, Gonçalves G, Gomes MGM. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infect Dis. 2006;6:112–117. doi: 10.1016/S1473-3099(06)70384-X. [DOI] [PubMed] [Google Scholar]
- 2.Althouse Benjamin M, Scarpino Samuel V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Medicine. 2015 Jun;:1–12. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RM, May RM. Infectious diseases of humans. Oxford University Press; New York: 1991. [Google Scholar]
- 4.Aoyama T, Murase Y, Kato T, Iwata T. Efficacy of an acellular pertussis vaccine in Japan. The Journal of pediatrics. 1985;107(2):180–183. doi: 10.1016/s0022-3476(85)80121-9. [DOI] [PubMed] [Google Scholar]
- 5.Ausiello CM, Cassone A. Acellular Pertussis Vaccines and Pertussis Resurgence: Revise or Replace? mBio. 2014 Apr;5(3):e01339–14–e01339–14. doi: 10.1128/mBio.01339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass JW, Stephenson SR. The return of pertussis. Pediatr Infect Dis J. 1987;6:141–144. doi: 10.1097/00006454-198702000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bhunbhu T. Expanded programme on immunization in Thailand. Rev Infect Dis. 1989;11(Supplement 3):S514–S517. doi: 10.1093/clinids/11.supplement_3.s514. [DOI] [PubMed] [Google Scholar]
- 8.Blackwood JC, Cummings DAT, Broutin H, Iamsirithaworn S, Rohani P. Deciphering the impacts of vaccination, demographic transition and immunity on pertussis epidemiology in thailand. 2013:–––. doi: 10.1073/pnas.1220908110. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blennow M, Olin P, Granström M, Bernier RH. Protective efficacy of a whole cell pertussis vaccine. British medical journal (Clinical research ed) 1988 Jun;296(6636):1570–1572. doi: 10.1136/bmj.296.6636.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bureau of Epidemiology. Annual epidemiological surveillance report. Ministry of Public Health of Thailand; Nonthaburi, Thailand: 1981-2000. [Google Scholar]
- 11.Clark Thomas A. Changing pertussis epidemiology: Everything old is new again. Journal of Infectious Diseases. 2014;209(7):978–981. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 12.Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006;367:1926–1936. doi: 10.1016/S0140-6736(06)68848-X. [DOI] [PubMed] [Google Scholar]
- 13.domenech de Celles M, magpantay FMG, king AA, rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proceedings of the Royal Society of London - Biological Sciences. 2016 Jan;283:1–10. doi: 10.1098/rspb.2015.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domenech de Celles M, Riolo MA, magpantay FMG, Rohani P, King AA. Epidemiological evidence for herd immunity induced by acellular pertussis vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2014 Feb; doi: 10.1073/pnas.1323795111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards Kathryn M. Unraveling the challenges of pertussis. Proceedings of the National Academy of Sciences. 2014;111(2):575–576. doi: 10.1073/pnas.1321360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine PEM, Clarkson JA. The recurrence of whooping cough: possible implications for assessment of vaccine efficacy. Lancet. 1982;1:666–669. doi: 10.1016/s0140-6736(82)92214-0. [DOI] [PubMed] [Google Scholar]
- 17.Gambhir Manoj, Clark Thomas A, Cauchemez Simon, Tartof Sara Y, Swerdlow David L, Ferguson Neil M. A Change in Vaccine Efficacy and Duration of Protection Explains Recent Rises in Pertussis Incidence in the United States. PLoS Computational Biology. 2015 Apr;11(4):e1004138. doi: 10.1371/journal.pcbi.1004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay NJ, Miller Elizabeth. Pertussis transmission in England and Wales. The Lancet. 2000 Apr;355(9214):1553–1554. doi: 10.1016/S0140-6736(05)74603-1. [DOI] [PubMed] [Google Scholar]
- 19.Güris¸ D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. Changing epidemiology of pertussis in the Unites States: increasing reported incidence among adolescents and adults, 1990-1996. Clin Infect Dis. 1999;28:1230–1237. doi: 10.1086/514776. [DOI] [PubMed] [Google Scholar]
- 20.Jackson D, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their causes. Epidemiology and Infection. 2014 doi: 10.1017/S0950268812003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton University Press; Princeton: 2008. [Google Scholar]
- 22.Kretzschmar Mirjam, Teunis Peter FM, Pebody Richard G. Incidence and reproduction numbers of pertussis: Estimates from serological and social contact data in five european countries. PLoS Med. 2010 Jun;7(6):e1000291. doi: 10.1371/journal.pmed.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1014394108. page. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.magpantay FMG, rohani P. Dynamics of Pertussis Transmission in the United States. American Journal of Epidemiology. 2015 Jun;181(12):921–931. doi: 10.1093/aje/kwv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.magpantay FMG, Rohani P. Dynamics of Pertussis Transmission in the United States. American Journal of Epidemiology. 2015 Jun;181(12):921–931. doi: 10.1093/aje/kwv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magpantay FG, Riolo MA, Domenech de Celles M, King AA, Rohani P. Population-level model of imperfect vaccines. SIAM Applied Mathematics. 2014 doi: 10.1137/140956695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean AR, Anderson RM. Measles in developing countries. part ii. the predicted impact of mass vaccination. Epidemiology and Infection. 1988 doi: 10.1017/s0950268800067170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossong Jol, Hens Niel, Jit Mark, Beutels Philippe, Auranen Kari, Mikolajczyk Rafael, Massari Marco, Salmaso Stefania, Tomba Gianpaolo Scalia, Wallinga Jacco, Heijne Janneke, Sadkowska-Todys Malgorzata, Rosinska Magdalena, Edmunds W John. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008 Mar;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Statistical Office of Thailand. 1980 census Technical report. Bangkok: National Statistical Office of Thailand; 1980. [Google Scholar]
- 30.National Statistical Office of Thailand. 1990 census Technical report. Bangkok: National Statistical Office of Thailand; 1991. [Google Scholar]
- 31.National Statistical Office of Thailand. 2000 census Technical report. Bangkok: National Statistical Office of Thailand; 2001. [Google Scholar]
- 32.Preston NW. Effectiveness of pertussis vaccines. Br Med J. 1965;2(5452):11. doi: 10.1136/bmj.2.5452.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preston NW, Stanbridge TN. Efficacy of pertussis vaccines: a brighter horizon. British Medical Journal. 1972;3(5824):448. doi: 10.1136/bmj.3.5824.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riolo MA, King AA, Rohani P. Can vaccine legacy explain the british pertussis resurgence? Vaccine. 2013;32:5903–5908. doi: 10.1016/j.vaccine.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riolo Maria A, King Aaron A, Rohani Pejman. Article In Press. Vaccine. 2013 Oct;31(49):5903–5908. doi: 10.1016/j.vaccine.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riolo Maria A, Rohani Pejman. Combating pertussis resurgence: One booster vaccination schedule does not fit all. Proceedings of the National Academy of Sciences of the United States of America. 2015 Jan;112(5):E472–E477. doi: 10.1073/pnas.1415573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science. 2010;330:982–985. doi: 10.1126/science.1194134. [DOI] [PubMed] [Google Scholar]
- 38.Rohani Pejman, Drake John M. The decline and resurgence of pertussis in the US. Epidemics. 2011 Sep;3(3-4):183–188. doi: 10.1016/j.epidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Danuta Skowronski M, De Serres Gaston, MacDonald Diane, Wu Wrency, Shaw Carol, Macnabb Jane, Champagne Sylvie, Patrick David M, Halperin Scott A. The changing age and seasonal profile of pertussis in canada. Journal of Infectious Diseases. 2002;185(10):1448–1453. doi: 10.1086/340280. [DOI] [PubMed] [Google Scholar]
- 40.Smallridge William E, Rolin Olivier Y, Jacobs Nathan T, Harvill Eric T. Different effects of whole-cell and acellular vaccines on bordetella transmission. Journal of Infectious Diseases. 2014;209(12):1981–1988. doi: 10.1093/infdis/jiu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanlapakorn Nasamon, Ngaovithunvong Varisara, Thongmee Thanunrat, Vichaiwattana Preeyaporn, Vongpunsawad Sompong, Poovorawan Yong. Seroprevalence of Antibodies to Pertussis Toxin among Different Age Groups in Thailand after 37 Years of Universal Whole-Cell Pertussis Vaccination. PLoS ONE. 2016 Feb;11(2):e0148338. doi: 10.1371/journal.pone.0148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warfel Jason M, Zimmerman Lindsey I, Merkel Tod J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proceedings of the National Academy of Sciences. 2014;111(2):787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008;9:201–212. doi: 10.1016/j.prrv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. [Accessed 10 July 2014];WHO Vaccine Preventable Diseases Monitoring System: 2014 Global summary. 2014 Available: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencepertussis.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.