Abstract
Objective
To characterize ovarian cancers associated with endometriosis and to evaluate the prognostic impact of endometriosis.
Methods
Ovarian cancer cases from a single institution diagnosed between 2000 and 2013 were examined and specimens reviewed by two pathologists for the presence of endometriosis. Ovarian cancer cases with and without endometriosis were compared to determine the clinical factors associated with endometriosis and the prognostic significance of endometriosis. Two-sample T-tests, Chi-square tests, multivariable logistic regression, and Cox proportional hazards models were used for statistical analysis.
Results
Among 139 epithelial ovarian cancers diagnosed between 2000 and 2013, there were 49 (35%) with endometriosis and 90 (65%) without endometriosis. The distribution of histologies of ovarian cancers with endometriosis was 43% endometrioid, 23% clear cell, 20% mixed, 8% mucinous, and 6% serous. Endometriosis associated ovarian cancers were more likely to be confined to the pelvis (54% vs. 9%, p<0.0001) and of lower tumor grade (51% vs. 29%, p=0.014). Younger age and earlier stage independently predicted the presence of endometriosis (p=0.0011 and p<0.0001, respectively). Ovarian cancer patients with endometriosis had improved PFS and OS [(HR=0.20; 95% CI, 0.09–0.43), (HR=0.18; 95% CI, 0.04–0.81)], compared to patients without endometriosis. After controlling for tumor stage and age, endometriosis was not an independent predictor of survival.
Conclusions
Patients with ovarian cancer and endometriosis are younger with lower stage and grade, and disease confined to the pelvis compared to patients without endometriosis. Ovarian cancers with endometriosis have improved PFS and OS; however endometriosis had no independent prognostic significance in our study.
Introduction
Endometriosis is a benign gynecologic condition affecting 10% of women and characterized by the presence of extra-uterine endometrial tissue (1–3). The malignant potential of endometriosis is well-documented (4–6).
The clinical features and pathogenesis of endometriosis-associated ovarian cancer are topics of active investigation. Previous studies have documented an association between endometriosis-associated ovarian cancer and younger, premenopausal women who present with lower stage and tumor grade (5, 7, 8). Endometrioid and clear cell histologies are more common in endometriosis- associated ovarian cancer (5, 9, 10).
Prior studies have reached discrepant conclusions regarding the impact of endometriosis on the survival of patients with ovarian cancer (5, 9, 11, 12). In a meta-analysis including 444,255 patients, there was no difference in progression free or overall survival between ovarian cancer patients with and without endometriosis (5). While some studies support these findings, other studies report improved survival among patients with endometriosis-associated ovarian cancer (5, 7–9, 11–13). Given these conflicting findings, we sought to characterize ovarian cancers associated with endometriosis and to evaluate the prognostic impact of the presence of endometriosis on ovarian cancer in our institution.
Materials and Methods
Data Source
Ovarian cancer cases from Weill Cornell Medical College diagnosed between January 1, 2000 and December 31, 2013 were examined and specimens reviewed by two pathologists for the presence of endometriosis. Ovarian cancer cases were stratified by the presence or absence of endometriosis and were compared to determine the clinical factors associated with endometriosis and the prognostic significance of the presence of endometriosis. Institutional review board was obtained from Weill Cornell Medical College.
Covariates and outcomes
Clinical and pathologic data including year of diagnosis, age at diagnosis, tumor stage, tumor grade, pelvic or abdominal distribution of disease, and histology were collected. All patients underwent surgical evaluation. Adjuvant chemotherapy drugs and dates of administration were collected. Dates of recurrence and death were collected. Platinum free interval was calculated as the number of months from completion of platinum-based chemotherapy to recurrence. Progression free survival was calculated as the number of months from cancer diagnosis to recurrence, and overall survival was calculated as the number of months from cancer diagnosis to death. Patients who were alive at last follow-up or who had not recurred at last follow-up were censored.
Statistical Analysis
Each outcome was initially reported descriptively. Continuous variables were compared using two-sample T-tests. Frequency distributions between categorical variables were compared using χ2 tests. Pearson Correlation Coefficients were calculated to determine trends in endometriosis associated ovarian cancer over time. Logistic regression models described predictors of association with endometriosis, while controlling for other predictive variables. Kaplan Meier curves were generated for platinum free interval, progression free survival, and overall survival based on association with endometriosis. Cox proportional hazards models were used to examine survival, comparing ovarian cancer cases with and without endometriosis. Outcomes were reported as hazards ratios and 95% confidence intervals. All statistical tests were two-sided and a p<0.05 was considered statistically significant.
Results
Among 139 epithelial ovarian cancers diagnosed between 2000 and 2013, there were 49 (35%) associated with endometriosis and 90 (65%) not associated with endometriosis. The demographic characteristics and clinical variables of the study population are outlined in Table 1. There was no trend in incidence of ovarian cancer associated with endometriosis over time (Pearson Correlation Coefficient = 0.087, p=0.77). Ovarian cancer patients with endometriosis were younger than those without endometriosis (p<0.0001), with a median age among those with endometriosis of 52 years (47–57 years) and without endometriosis of 61 years (55–70 years). A woman was 7% less likely to have ovarian cancer associated with endometriosis for each year she aged. The distribution of histology of endometriosis-associated cancers included 21 (43%) endometrioid, 11 (23%) clear cell, 10 (20%) mixed, 4 (8%) mucinous, and 3 (6%) serous.
Table 1.
Association between presence of endometriosis in ovarian cancer and demographic and clinical variables
| Ovarian cancer associated with endometriosis (n=49) |
Ovarian cancer not associated with endometriosis (n=90) |
||||
|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | P |
| Age (years) | <0.0001 | ||||
| Range | 47–57 | 55–70 | |||
| Mean | 52 | 62 | |||
| Median | 52 | 61 | |||
| Year of diagnosis | 0.77 | ||||
| 2000–2006 | 28 | 57 | 40 | 44 | |
| 2007–2013 | 21 | 43 | 50 | 56 | |
| Stage | <0.0001 | ||||
| Early stage | 37 | 76 | 27 | 30 | |
| Late stage | 10 | 20 | 62 | 69 | |
| Unknown | 2 | 4 | 1 | 1 | |
| Tumor grade | 0.014 | ||||
| Low grade | 20 | 41 | 19 | 21 | |
| High grade | 29 | 59 | 71 | 79 | |
| Distribution of Ovarian | <0.0001 | ||||
| Cancer | 39 | 80 | 33 | 37 | |
| Pelvis | 5 | 10 | 48 | 53 | |
| Abdomen and Pelvis | 5 | 10 | 9 | 10 | |
| Unknown | |||||
Optimal surgical debulking was performed in 90% of endometriosis-associated cancers and in 79% of cases without endometriosis. The distribution of surgical approach for ovarian cancer patients with endometriosis was 34 (70%) laparotomy, 4 (8%) laparoscopy, 6 (12%) robotic, and 5 (10%) unknown, compared to patients without endometriosis, which were 71 (79%) laparotomy, 6 (7%) laparoscopy, 5 (5%) robotic, and 8 (9%) unknown. Patients with endometriosis were significantly more likely to be of early surgical stage (I/II vs. III/IV) compared to cases without endometriosis (79% vs 30%, respectively, P<0.0001). The tumor was confined to the pelvis in 89% of endometriosis associated cancers compared with 41% of cancers without endometriosis, (P<0.0001) Younger age (P=0.0011), early stage (P<0.0001), lower grade (p=0.014), and tumor confinement to the pelvis (p<0.0001) were all found to be independent predictors of the presence of endometriosis. Ovarian cancers diagnosed at early stage were eight times more likely to be associated with endometriosis compared to late stage (OR 7.646; 95% CI, 3.199 to 18.277). Among ovarian cancer cases associated with endometriosis, 67% received adjuvant chemotherapy, in contrast to 76% of cases not associated with endometriosis that received adjuvant chemotherapy.
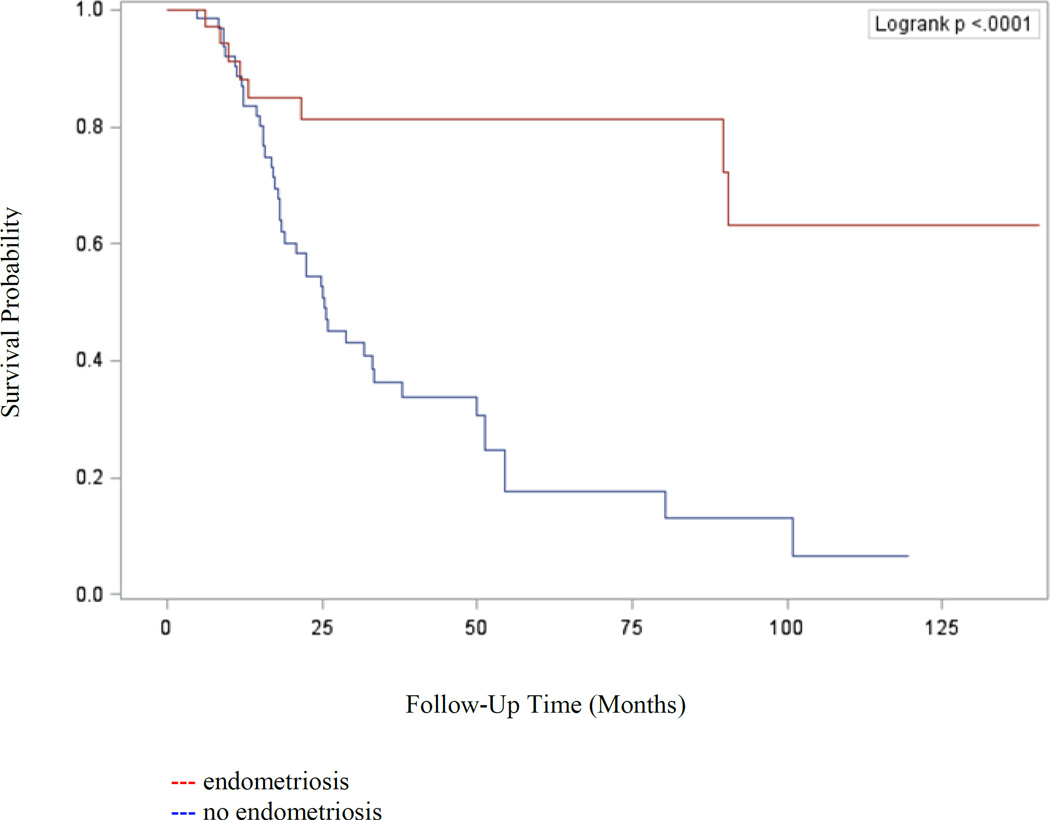
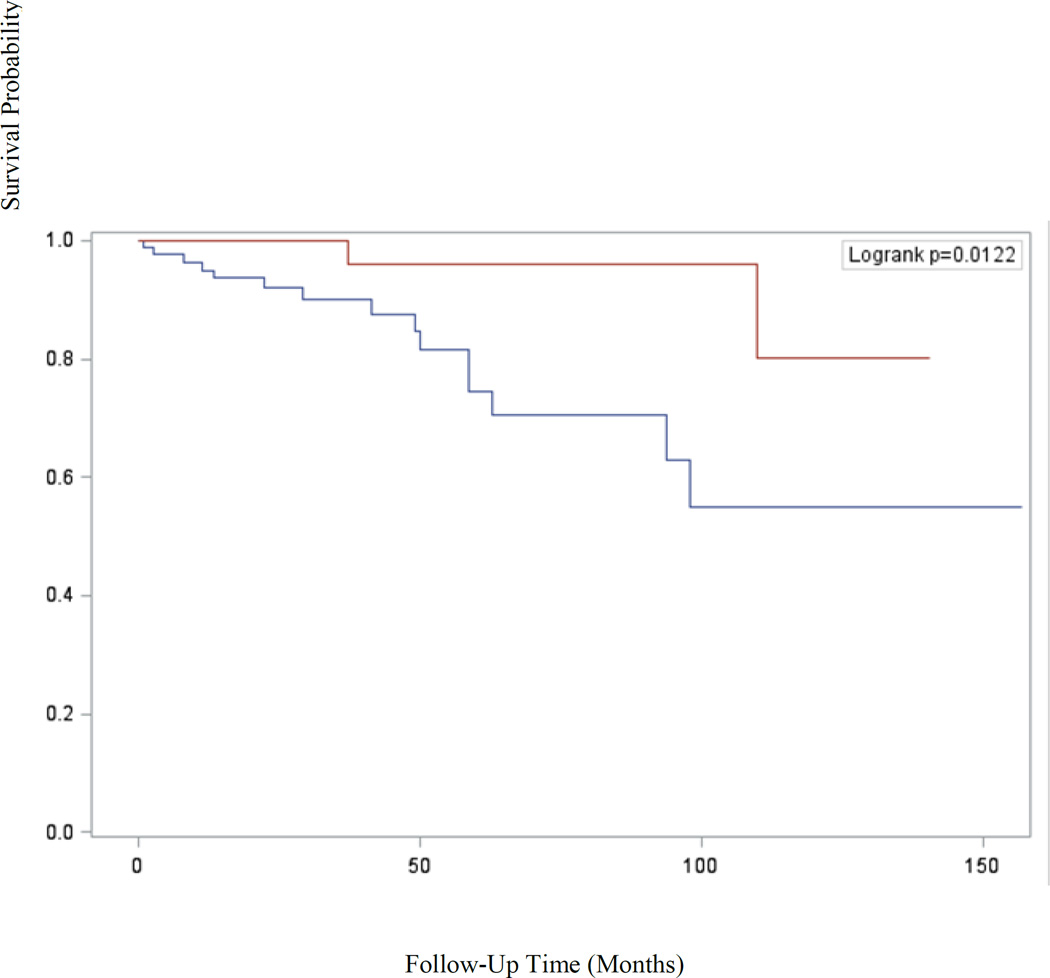
Table 2 displays Cox proportional hazards models of survival for ovarian cancer based on presence of endometriosis. Ovarian cancer patients with endometriosis had improved platinum free interval (HR=0.25; 95% CI, 0.12 to 0.55), progression free survival (HR=0.20; 95% CI, 0.09 to 0.43), and overall survival (HR=0.18; 95% CI, 0.04 to 0.81) compared to patients without endometriosis. Kaplan-Meier analysis confirmed the improved progression free (p<0.0001) and overall survival (p=0.012) experienced by patients with endometriosis-associated ovarian cancers and is shown in Figures 1 and 2, respectively. However, after controlling for tumor stage and age at diagnosis, endometriosis was not an independent predictor of survival.
Table 2.
Cox proportional hazards model of impact of presence of endometriosis on platinum free interval, progression free survival, and overall survival in epithelial ovarian cancer
| Platinum Free Interval |
Progression Free Survival |
Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | HR | 95% CI |
p | HR | 95% CI |
p | HR | 95% CI |
p |
| Ovarian cancer not associated with endometriosis |
Referent | Referent | Referent | ||||||
| Ovarian cancer associated with endometriosis |
0.25 | 0.12 to 0.55 |
0.001 | 0.20 | 0.09 to 0.43 |
<0.0001 | 0.18 | 0.04 to 0.81 |
0.012 |
Figure 1.
Kaplan-Meier analysis of progression free survival (p<0.0001) of patients with epithelial ovarian cancer stratified by presence of endometriosis versus no endometriosis
Figure 2.
Kaplan-Meier analysis of overall survival (p=0.012) of patients with epithelial ovarian cancer stratified by presence of endometriosis versus no endometriosis
Conclusions
Our findings indicate that endometriosis is frequently associated with ovarian cancer and that this association is strongest in younger women with low tumor stage and grade. While an improved progression-free and overall survival was found in our patients with endometriosis-associated ovarian cancer, this benefit was related to the favorable pathologic features of the ovarian cancers and the presence of endometriosis had no independent impact on patient outcome. These findings are consistent with results of prior studies in this area.
Similarly to our findings, multiple studies report endometriosis associated ovarian cancer more commonly in younger women or premenopausal compared to postmenopausal women (8). Scarfone et al noted that compared with clear cell ovarian cancers without endometriosis, those with endometriosis occurred in younger women 51.4 years old vs. 58.4 years old (p=0.02) (13). In agreement with our study, in a meta-analysis, compared to ovarian cancers without endometriosis, those with endometriosis were more likely to be stage I-II (RR 1.959, 95% CI 1.367 to 2.807) and grade 1 (RR 1.319, 95% CI 1.149 to 1.514) (5). While many studies report that endometriosis associated ovarian cancers are most often clear cell or endometrioid histology (5, 11, 14), some studies also report the presence of endometriosis in low-grade serous (14) and mixed tumors (11). In an analysis of 15 published reports, the distribution of histologies among endometriosis associated ovarian cancers were clear cell (39.2%), endometrioid (21.2%), serous (3.3%), and mucinous (3.0%) (10).
Overall prior studies comparing ovarian cancers with and without endometriosis show either no difference in survival, or improved either progression free or overall survival among cancers with endometriosis that is not statistically significant in multivariate analysis. Among 144 ovarian cancer patients, Cuff et al found no difference in progression free survival (p=0.7) (11), a finding similar to that of Scarfone et al who showed no difference in overall survival between these groups in a cohort of 73 patients (13). In a study of 201 patients, Davis et al demonstrated that compared to ovarian cancer patients without endometriosis, those with endometriosis had an improved 5-year progression free survival 75% vs. 55% (p=0.03) but no difference in overall survival 85% vs. 77% (p=0.2) (9). Noli et al and Garrett et al both demonstrated improved survival in univariate analysis that was not statistically significant in multivariate analysis (8, 12). In a meta-analysis of 444,255 patients, in crude analysis endometriosis associated ovarian cancer was associated with an improved overall survival (HR 0.778; 95%CI, 0.655–0.925) but not progression free survival (HR 1.023, 95% CI 0.712–1.470) compared to non-endometriosis associated ovarian cancer. In subgroup analyses, progression free survival and overall survival were not different between the groups (15).
The increased risk of ovarian cancer in women with endometriosis has been previously demonstrated. In a study of 20,686 women, endometriosis increased ovarian cancer risk (HR 1.9; 95% CI, 1.3 to 2.8); the increased risk of ovarian cancer was most notable in women with long-standing endometriosis greater than ten years (16). Similarly, in a pooled analysis of thirteen case control studies, including 13,226 controls and 7,911 ovarian cancer cases, the Ovarian Cancer Association Consortium noted that women with a reported history of endometriosis had an increased risk of clear cell ovarian cancer (OR 3.05, 95% CI 2.43–3.84), low grade serous ovarian cancer (OR=2.11; 95% CI, 1.39 to 3.20), and endometrioid ovarian cancer (OR=2.04; 95% CI, 1.67 to 2.48) (14). These findings call into question the safety of expectant management in long-standing ovarian endometriomas, particularly in perimenopausal women.
The favorable phenotype of endometriosis-associated ovarian cancer seen in the current study suggests a unique pathogenesis of these tumors. Recent advances in gene expression profiling and analysis have expanded our understanding of the genetic alterations associated with the various histologic types of ovarian cancer. Ovarian cancers that originate from malignant transformation of endometriosis appear to be part of type I ovarian cancers, which are typically of endometrioid or clear cell histology, with defects in ARID1A. These tumors are slower growing and more indolent compared to type II ovarian cancers (17–21). This genetic difference may explain the tendency for early stage and confinement to the pelvis in cancers associated with endometriosis in the current study. Both endometriosis and ovarian cancer have also been associated with alterations in the PIK3/AKT pathway (2, 22). Early clinical trials of novel inhibitors of PIK3 and its downstream effector, mTOR, in recurrent endometrial cancer have shown promising results and this may prove to be an effective strategy in endometriosis-associated ovarian cancer as well (23, 24). The relative resistance to platinum-based chemotherapy in low-grade serous and clear cell carcinoma, which are both associated with the presence of endometriosis, has been previously described (25–29). Future studies are needed to further elucidate the hormonal, inflammatory, and molecular pathways leading to the malignant transformation of endometriosis to ovarian cancer. Understanding of these pathways could identify precursor lesions in pelvic endometriosisthat provide a possible means for cancer risk stratification and identify a population that benefit from risk-reducing interventions.
Limitations of the current study include the relatively small number of endometriosis-associated ovarian cancers, its retrospective study design, and the collection of all cases from a single institution. Larger, multi-institutional studies in this area are necessary to confirm our findings. While our study did not identify an independent impact of endometriosis on ovarian cancer survival, it adds to the literature supporting the role of endometriosis in identifying a specific phenotype of ovarian cancer defined by clear cell, and endometrioid histology, lower tumor grade, early onset, and confinement to the pelvis. As the progression toward a more individualized approach to ovarian cancer treatment continues, this information may prove useful in tailoring the surgical and medical approach to these tumors.
Research Highlights.
We compare ovarian cancer cases with and without endometriosis.
Ovarian cancers with endometriosis occur in younger women with low stage and grade.
Endometriosis-associated ovarian cancers have improved PFS and OS.
The presence of endometriosis has no independent impact on patient outcome.
References
- 1.Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328(24):1759–1769. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 2.Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci. 2013;14(9):18824–18849. doi: 10.3390/ijms140918824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The relation between endometriosis and ovarian cancer - a review. Acta Obstet Gynecol Scand. 2014;93(1):20–31. doi: 10.1111/aogs.12255. [DOI] [PubMed] [Google Scholar]
- 4.Gadducci A, Lanfredini N, Tana R. Novel insights on the malignant transformation of endometriosis into ovarian carcinoma. Gynecol Endocrinol. 2014:1–6. doi: 10.3109/09513590.2014.926325. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Kim TH, Chung HH, Song YS. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br J Cancer. 2014 doi: 10.1038/bjc.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101(2):331–341. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Lyttle B, Bernardi L, Pavone ME. Ovarian cancer in endometriosis: clinical and molecular aspects. Minerva Ginecol. 2014;66(2):155–164. [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett LA, Growdon WB, Goodman A, Boruta DM, Schorge JO, del Carmen MG. Endometriosis-associated ovarian malignancy: a retrospective analysis of presentation, treatment, and outcome. J Reprod Med. 2013;58(11–12):469–476. [PubMed] [Google Scholar]
- 9.Davis M, Rauh-Hain JA, Andrade C, Boruta DM, 2nd, Schorge JO, Horowitz NS, et al. Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa H, Jimbo H, Okada S, Matsumoto K, Onda T, Yasugi T, et al. Prevalence of endometriosis in ovarian cancer. Gynecol Obstet Invest. 2000;50(Suppl 1):11–17. doi: 10.1159/000052873. [DOI] [PubMed] [Google Scholar]
- 11.Cuff J, Longacre TA. Endometriosis does not confer improved prognosis in ovarian carcinoma of uniform cell type. Am J Surg Pathol. 2012;36(5):688–695. doi: 10.1097/PAS.0b013e31824b6eed. [DOI] [PubMed] [Google Scholar]
- 12.Noli S, Cipriani S, Scarfone G, Villa A, Grossi E, Monti E, et al. Long term survival of ovarian endometriosis associated clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2013;23(2):244–228. doi: 10.1097/IGC.0b013e31827aa0bb. [DOI] [PubMed] [Google Scholar]
- 13.Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, et al. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol. 2014;133(3):480–484. doi: 10.1016/j.ygyno.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MY, Lee S, Van K, Kim TH, Jeong SC, Choi IY, et al. Whole-genome sequencing and intensive analysis of the undomesticated soybean (Glycine soja Sieb. and Zucc.) genome. Proc Natl Acad Sci U S A. 2010;107(51):22032–22037. doi: 10.1073/pnas.1009526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176(3):572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 17.Herzog TJ. The current treatment of recurrent ovarian cancer. Curr Oncol Rep. 2006;8(6):448–454. doi: 10.1007/s11912-006-0074-9. [DOI] [PubMed] [Google Scholar]
- 18.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourley C. Link between endometriosis and ovarian-cancer subtypes. Lancet Oncol. 2012;13(4):326–328. doi: 10.1016/S1470-2045(12)70029-3. [DOI] [PubMed] [Google Scholar]
- 21.Herzog TJ, Dinkelspiel HE. Fallopian tube removal: "stic-ing" it to ovarian cancer: what is the utility of prophylactic tubal removal? Curr Oncol. 2013;20(3):148–151. doi: 10.3747/co.20.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabert B, Pinto L, Hartge P, Kemp T, Black A, Sherman ME, et al. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecol Oncol. 2014 doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markman B, Tabernero J, Krop I, Shapiro GI, Siu L, Chen LC, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012;23(9):2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 24.Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30(8):777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan DS, Miller RE, Kaye SB. New perspectives on molecular targeted therapy in ovarian clear cell carcinoma. Br J Cancer. 2013;108(8):1553–1559. doi: 10.1038/bjc.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katagiri A, Nakayama K, Rahman MT, Rahman M, Katagiri H, Nakayama N, et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol. 2012;25(2):282–288. doi: 10.1038/modpathol.2011.161. [DOI] [PubMed] [Google Scholar]
- 27.Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60(3):412–417. doi: 10.1006/gyno.1996.0065. [DOI] [PubMed] [Google Scholar]
- 28.Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Gourley C, Farley J, Provencher DM, Pignata S, Mileshkin L, Harter P, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian and primary peritoneal low-grade serous carcinomas. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S9–S13. doi: 10.1097/IGC.0000000000000257. [DOI] [PubMed] [Google Scholar]